Research Article, J Diagn Tech Biomed Anal Vol: 9 Issue: 1
A Comparison of Three Simple, Rapid Methods for Red Blood Cell Removal from Whole Blood, Which are Suitable for Measuring Biomarkers at the Point of Care
Frederic Bedin* and Agnes Rubens
Innovation Department, BioMerieux SA, Chemin de l’Orme, 69280 Marcy l’Etoile, France
*Corresponding Author : Bedin F, Innovation Department, BioMerieux SA
Chemin de l’Orme, 69280 Marcy l’Etoile, France, Tel: +33478873685; E-mail: Frederic.bedin@biomerieux.com
Received date: January 30, 2020; Accepted date: February 12, 2020; Published date: February 20, 2020
Citation: Frederic B, Rubens A (2020) A Comparison of Three Simple, Rapid Methods for Red Blood Cell Removal from Whole Blood, Which are Suitable for Measuring Biomarkers at the Point of Care. J Diagn Tech Biomed Anal 9:1. doi: 10.37532/jdtba.2020.9(1).140
Abstract
Objective: Plasma is often used to assess the concentration of clinical markers, as the presence of red blood cells (RBCs) can interfere with the correct measurement of parameters. The objective was to select a simple, rapid and efficient method for RBCs removal from a small volume of whole blood usable at the point of care.
Methods: Three alternative RBC removal methods were assessed from 50 µL of whole blood and compared with the centrifugation method. Two methods involved agglutination molecules, such as lectins or antibodies, whilst the third method used lateral filtration on a glass fiber paper strip.
Results: In each case, the RBCs were efficiently separated from whole blood in less than five min at room temperature. In terms of marker recovery and residual hemoglobin concentration, the method that used antibodies performed the best and could be integrated into a process whereby plasma markers are measured from a small volume of whole blood at the point-of-care.
Keywords: Red blood cells; Plasma; Whole Blood; Biomarkers
Introduction
Today, many clinical diagnostic assays that involve blood specimens require the use of plasma or serum samples to prevent interference from red blood cells [1-3]. Conventional methods for plasma or serum separation from whole blood, such as centrifugation, are often impractical in remote settings or in physician’s offices because they take time, require trained technical personnel and specific equipment, such as a benchtop refrigerated centrifuge and electricity to operate [4].
Cells and plasma are the main constituents of whole blood and on average account for approximately 45% and 55% volume fractions, respectively. The serum is the fluid and solute component of blood after clotting. Of the 45% blood cell components, more than 99% are RBCs [5]. Removal of these cells, for use in downstream disease diagnosis and biochemical analysis, is necessary for most clinical assays that involve blood specimens. This is mainly to prevent the large volume of cellular inclusion, cell lysis and contamination from lysates from potentially affecting the accuracy and reproducibility of diagnostics and analytical measurements [5,6]. For example, hemoglobin found in erythrocytes, has been identified as a PCR inhibitor [7].
The hematocrit is the relative volume occupied by blood cells (99% of which are RBCs), when compared to the total volume of blood. Hematocrit is expressed as a percentage. The normal range is 47% ± 5 for men and 42% ± 5 for women [8,9]. As the hematocrit is variable from person to person [10] and a marker concentration is generally given as a plasmatic dose, it is important to perform measurements on a blood product from which the RBCs have been discarded.
In an attempt to overcome some of the limitations associated with the traditional techniques for plasma separation, strategies have been developed using various sophisticated approaches, such as hydrodynamic filtration [11,12], the Zweifach-Fung effect [13], dielectrophoresis [14], magnetic interactions [15], acoustic transducers [15] and hydrogen peroxide-powered pumping [16,17]. However, to date, there are few methods for removal of RBCs that are equipment free, take less than five min and are simple enough to be performed with minimal training. Effective but unsophisticated methods for RBC removal include simple sedimentation, agglutination or passive filtration, where RBC sedimentation occurs through gravity. These methods are very simple but take time. Molecules that can agglutinate RBCs are mainly plant lectins and RBC-specific antibodies. Agglutination occurs when these molecules come into contact with RBCs [18,19].
Technical paper, such as VIVID paper, is able to filter blood cells from whole blood but is expensive and requires the application of a vacuum to recover the plasma. Glass fiber paper is traditionally used in commercial lateral flow assays to perform the sample preparation, which is upstream from marker detection on an adjacent nitrocellulose strip [20,21]. Filtration using glass fiber paper is passive and does not require any equipment. In order to improve the efficiency of separation, agglutination can be combined with sedimentation or filtration [22].
In this study, methods that use sedimentation, agglutination and filtration for the removal of RBCs from whole blood were compared in terms of simplicity, efficiency, rapidity and biomarker recovery.
Materials and Methods
Materials and specimens
The anti-RBC monoclonal antibody (3B9A6D5) was an Immunoglobulin M (IgM) that was directed against a RBC surface protein and was produced by bioMerieux SA (Lyon, France). Lectins were obtained from Vector Laboratories (Burlingame, CA USA) or Sigma-Aldrich (Saint Louis MO USA).
Procalcitonin (PCT) and Prostate Specific Antigen (TPSA) recombinant proteins were developed and produced by bioMerieux SA (Lyon, France) using expression in prokaryotic (PCT) or eukaryotic cells (TPSA).
Whole blood specimens were obtained from healthy donors from the French National Blood Bank (Establishment Francais du Sang, Lyon, France). Informed consent was obtained for any experimentation. All experiments were performed in compliance with the relevant laws and institutional guidelines and in accordance with the ethical standards of the Declaration of Helsinki.
Red blood cell agglutination using lectins or specific antibodies
Fifty μL of fresh whole blood (heparinized or citrated) were mixed with various concentrations of lectins or antibody 3B9A6D5, diluted in PBS1x (Euromedex, France). After five min of incubation at 22°C ± 1.5°C (room temperature, RT), the supernatant was recovered for further analysis. A mix of antibodies that contained both 3B9A6D5 and a donkey IgG directed against mouse IgM (Jackson Immunoresearch, Baltmore Pike, PA USA) was also used. Agglutination was observed using an inversed microscope (AXIO Imager M1m, Zeiss, Oberkochen, Germany) as follows: 25 μL of blood was mixed with 25 μL of PBS1x. The diluted sample was dropped onto a glass slide and the reading was performed under the microscope, using 40 x magnifications.
Blood cell filtration on glass fiber paper
Fifty microliters of fresh whole blood were loaded onto a laser-cut strip of MF1 glass fiber paper (GE Healthcare, Velizy, France). When the blood had been completely absorbed into the paper and the plasma was completely separated from the RBCs, the section of paper corresponding to the “plasma” (in the present study “plasma” refers to whole blood without RBCs) was cut out and punched ten min in 25 μL of PBS1x before analysis.
Blood centrifugation
Blood centrifugation was used as a reference method for plasma separation. Fresh blood was centrifuged for five min at 20 000 g, at 4°C, using a refrigerated microcentrifuge 5424R (Eppendorf, Montesson, France). After centrifugation, the supernatant, which corresponds to the plasma, was recovered for further analysis.
Measurement of the amount of residual hemoglobin and blood markers
After removal of the RBCs, the residual hemoglobin concentration was estimated using the hemoglobin assay kit (Abnova, Tapei, Taiwan), following the manufacturer’s instructions. For the measurement of blood markers before and after RBC removal, the fresh whole blood was first spiked with a precise amount of purified recombinant protein. The quantity of the protein was then measured in 25 μL of the sample using a VIDAS kit on a VIDAS automate, in accordance with the manufacturer’s instructions (bioMerieux SA, Marcy l’Etoile, France).
Results
Development of a method for RBC agglutination using lectins
In order to determine the most suitable type of lectin for RBC agglutination, six plant lectins were evaluated. These originated from Ulex europeaus (gorce), Phaseolus vulgaris (red bean), Lens culinaris (lentil), Solanum tuberosum (potato) and Triticum sativum (wheat).
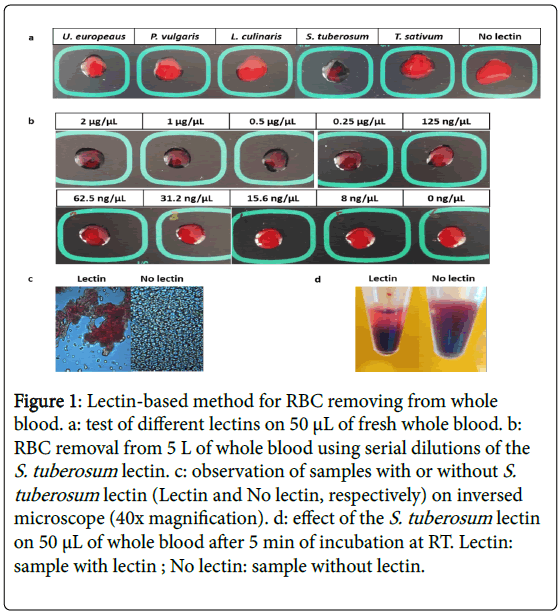
Ten μL of each lectin were mixed with 50 μL of fresh blood (final lectin concentration: 1 μg/mL), spread on black waterproof cardboard and incubated for five min at RT. As illustrated in Figure 1a, a strong partitioning of the RBCs was observed when S. tuberosum lectin was added. The results were much less pronounced with lectins from U. europeaus , P. vulgaris and T. sativum . No difference was observed with L. culinaris when compared to the control without lectin. Agglutination was visible within a few sec from blood contact when using S. tuberosum lectin.
Figure 1: Lectin-based method for RBC removing from whole blood. a: test of different lectins on 50 μL of fresh whole blood. b: RBC removal from 5 L of whole blood using serial dilutions of the S. tuberosum lectin. c: observation of samples with or without S. tuberosum lectin (Lectin and No lectin, respectively) on inversed microscope (40x magnification). d: effect of the S. tuberosum lectin on 50 μL of whole blood after 5 min of incubation at RT. Lectin: sample with lectin ; No lectin: sample without lectin.
Fifty μL of blood samples from eight patients with different serogroups (five group O, two group A and one group B) and different hematocrit rates (mean: 42.5%, range: 40-48%) were mixed with S. tuberosum lectin (1 μg/mL). The female to male ratio was 6/2 and the average age was 44.6 (range: 19-66). All the samples showed strong agglutination (data not shown), which indicates that this method can be reproducibly used for blood samples, regardless of the serogroup and the hematocrit rates.
In order to determine the minimal concentration of S. tuberosum lectin required for efficient RBC agglutination, serial dilutions of the lectin, from 2 μg/mL to 8 ng/mL, were assessed using 50 μL of fresh blood (Figure 1b). Although agglutination tended to decrease below 250 ng/mL, it could still be observed for a lectin concentration of 31.2 ng/mL. Below this value, the effects of lectin were not visible.
The agglutination of RBCs was observed under an inversed microscope, after the addition of lectin (1 μg/μL). No agglutination was observed in the sample without lectin (Figure 1c).
To demonstrate the role of lectins in RBC separation by gravity in a vertical container, 100 μL of blood and 20 μL of S. tuberosum lectin (500 ng/μL) were mixed in a polypropylene Eppendorf tube. After five min of incubation at RT, the lectin-agglutinated RBC sediment could be observed at the bottom of the tube (Figure 1d). As a control, when 100 μL of blood was mixed with 20 μL of PBS1x, little sedimentation was observed after five min of incubation.
In conclusion, it is possible to efficiently separate the RBCs from 50 μL of whole blood, using S. tuberosum lectin at 250 ng/mL, in less than five min.
Development of a method for RBC agglutination using antibodies
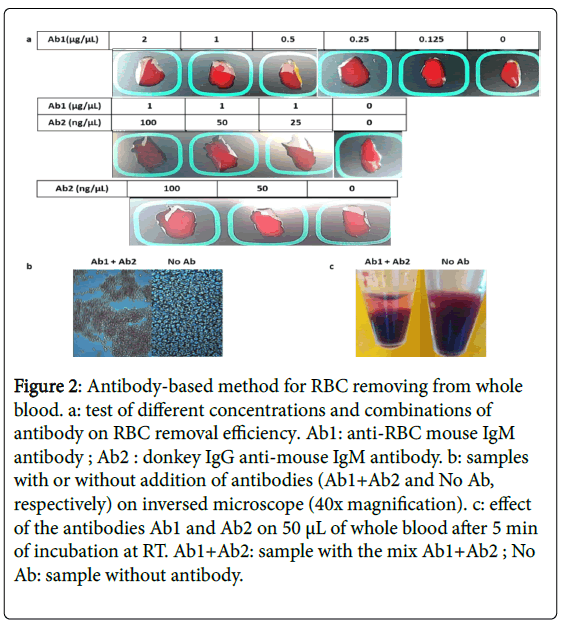
We evaluated an IgM monoclonal antibody (Ab1), which was directed against a surface protein of the RBC. Ten μL of serial dilutions of the antibody, from 2 μg/μL to 0.125 μg/mL, were mixed with 50 μL of fresh blood, spread on black waterproof cardboard and incubated for five min at RT. As illustrated in Figure 2a, no agglutination of the RBCs was observed five min after addition of the antibody, at any concentration. A small effect was observed 10 min after addition of the antibody (data not shown).
Figure 2: Antibody-based method for RBC removing from whole blood. a: test of different concentrations and combinations of antibody on RBC removal efficiency. Ab1: anti-RBC mouse IgM antibody ; Ab2 : donkey IgG anti-mouse IgM antibody. b: samples with or without addition of antibodies (Ab1+Ab2 and No Ab, respectively) on inversed microscope (40x magnification). c: effect of the antibodies Ab1 and Ab2 on 50 μL of whole blood after 5 min of incubation at RT. Ab1+Ab2: sample with the mix Ab1+Ab2 ; No Ab: sample without antibody.
In order to accelerate the agglutination rate, a second antibody, which was directed against the μ chain of the mouse IgM (Ab2, range of concentration; 100 ng/mL to 25 ng/mL), was tested in association with the anti-RBC antibody (Ab1, 1 μg/μL). As illustrated in Figure 2a, when Ab1 was mixed with Ab2 (100 ng/μL), a partitioning of the RBCs was clearly noticeable after five min of incubation at RT. When 50 ng/μL were used, the effect was less visible and disappeared at lower concentrations of Ab2.
No agglutination was observed when Ab2 was tested alone (range of concentration: 100 ng/μl to 50 ng/μL), even after 10 min of incubation (Figure 2a).
Agglutination of the RBCs was observed under an inversed microscope when Ab1 and Ab2 were added (1 and 0.1 μg/μL, respectively). No agglutination was observed in a sample without antibodies (Figure 2b).
To demonstrate RBC separation by gravity, in a vertical container, blood and the two antibodies (Ab1 and Ab2) were mixed in a polypropylene Eppendorf microtube. After five min of incubation at RT, sedimentation of the RBCs could be observed at the bottom of the tube (Figure 2c). When fresh blood was mixed with 10 μL of PBS1x, as a control, no sedimentation was observed, even after 15 min of incubation.
In conclusion, it was possible to separate out the RBCs from 50 μL of whole blood in five min using a mixture of two antibodies, with the first directed against RBCs (1 μg/μL) and the second directed against mouse IgM (100 ng/μL).
Development of a method for RBC removal using glass fiber paper
Glass fiber paper has already been shown to be efficient at RBC removal from whole blood [20]. Here, a method was optimized for separation using 50 μL of fresh blood. We also measured a plasmatic marker on a simple strip of laser-cut MF1 glass fiber paper.
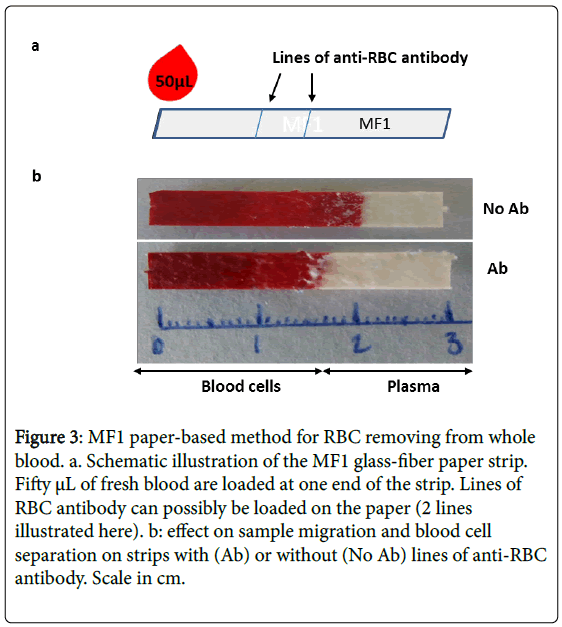
We estimated the minimum size of the MF1 strip required for optimal separation of the RBCs. To compare the relative migration distance of RBCs and “plasma”, MF1 strips of 10, 8, 5 or 4 mm widths were tested (see supplementary data S1). The length of the strip was always 45 mm. We found that the greater the width, the shorter the migration distance. For example, on a 10 mm wide strip the RBCs migrated 14 mm and the plasma migrated 5 mm. On a 4 mm wide strip, the distances of migration for RBCs and plasma were 25 and 16 mm, respectively. On a 5 mm wide strip, 29 to 30 mm of length was found to be the minimum size covered by 50 μL of blood. When using 50 μL of blood, RBCs migrated 21 mm and plasma migrated 7.7 mm (average of eight samples). Thereafter, 50 μL of blood were loaded onto 5 × 30 mm laser-cut strips. Under these conditions, the separation of the RBCs from the plasma was effective within two min from deposition of the blood at one extremity of the strip (Figure 3b, No Ab).
Figure 3: MF1 paper-based method for RBC removing from whole blood. a. Schematic illustration of the MF1 glass-fiber paper strip. Fifty μL of fresh blood are loaded at one end of the strip. Lines of RBC antibody can possibly be loaded on the paper (2 lines illustrated here). b: effect on sample migration and blood cell separation on strips with (Ab) or without (No Ab) lines of anti-RBC antibody. Scale in cm.
| Migration distance (mm) | ||||
|---|---|---|---|---|
| Width (mm) | RBC (mean) | SD | plasma (mean) | SD |
| 11 | 14 | 1.8 | 19 | 1.1 |
| 8 | 19 | 2.2 | 25.5 | 1.4 |
| 5 | 21 | 2.5 | 28.7 | 1.5 |
| 4 | 25 | 3.3 | 41 | 2.1 |
Supplementary data S1: migration distance of RBC and “plasma” using different widths of MF1 glass fiber paper. SD: standard deviation, mean of 8 experiments.
The effect of the addition of anti-RBC antibody on RBC separation was then evaluated. The 3B9A6D5 antibody was loaded onto different locations of the test strip. For each deposit, 2.5 μL of antibody, diluted in PBS1x, were coated onto the strip at a concentration of 4 mg/mL. After deposition of lines over the width of the strip, the antibodies were fixed by a three min incubation at 60°C. One to three deposits and different distances from the end of the strip were evaluated. The first line of antibody was placed 10 mm from the edge of the MF1 strip where the sample was loaded. The second and the third lines were applied at 15 and 20 mm from the edge, respectively. The results obtained after three min of migration, using eight blood samples, are summarized in Table 1.
| Ab1, 1 line | Ab1, 2 lines | Ab1, 3 lines | No Ab1 | |||||
|---|---|---|---|---|---|---|---|---|
| RBC | "Plasma" | RBC | "Plasma" | RBC | "Plasma" | RBC | "Plasma" | |
| Migration (mean), mm | 19.2 | 29.4 | 17.1 | 29.7 | 17.4 | 29.6 | 21 | 28.7 |
| (Plasma - RBC), mm | - | 10.2 | - | 12.6 | - | 12.2 | - | 7.7 |
| SD | 1.14 | 0.97 | 0.63 | 0.8 | 0.83 | 0.4 | 2.61 | 1.55 |
Table 1: MF1 strips- Effect of RBC antibody on sample migration. One to three lines of anti-RBC antibody were loaded on MF1 glass-fiber paper. No Ab1: no RBC antibody loaded. The distance of migration of the RBC (RBC) and the distance of migration of the “plasma” (“plasma”) on the strip, were measured. (Plasma-RBC): distance of migration of plasma minus distance of migration of RBC. SD: Standard Deviation. Results correspond to the mean of 8 samples.
The results showed that adding antibodies to the MF1 strip reduced the migration distance of the RBCs and increased the length of paper soaked by the plasma (Figure 3). In addition, the presence of antibodies minimized variations in RBC migration, which depends on RBC rate (hematocrit) and is variable from patient to patient.
Two antibody deposits (10 and 15 mm from the end of the MF1 strip) gave the best results in terms of RBC migration, plasma migration and robustness of data. Consequently, this combination will be used for later experiments.
In conclusion, it was possible to separate RBCs from 50 μL of whole blood, in less than three min, using a strip of MF1 glass fiber paper. The addition of two lines of antibodies directed against RBCs improved both the separation efficiency and robustness.
Comparison of marker recovery and hemoglobin contamination after RBC removal
As the three methods used for removing RBCs may have an impact on plasma protein concentrations, a comparison of these methods was performed in terms of marker recovery.
The two markers that were evaluated were procalcitonin (PCT) and total prostatic specific antigen (TPSA). The recombinant proteins that correspond to these antigens were spiked into fresh whole blood at a concentration of 5 ng/mL (PCT) or 7 ng/mL (TPSA). These concentrations correspond to high physiological levels. The marker concentration was estimated on a VIDAS using the BRAHMS PCT and TPSA VIDAS kits (bioMérieux SA). The experiments were conducted twice on three fresh blood samples (two females and one male; average age 48; average hematocrit 41%). Each method was compared with the reference method (i.e. blood centrifugation at 20 000 g at 4°C for five min). A mean and percentage of marker recovery were calculated. The percentage corresponded to the ratio of the concentration of a marker, using a specific RBC removal method, against the concentration of the same marker using the reference method (centrifugation).
As illustrated in Table 2, for methods using lectins or antibodies, the PCT concentrations were equivalent to those obtained after blood centrifugation (99.2% to 100% of the reference method). However, for the MF1 paper, the PCT ratio represented 63% of the concentration obtained with the reference method. For TPSA, the method using antibodies provided a concentration close to that obtained with the reference method (96%). However, methods using lectin or MF1 paper performed less well (67.4% and 60%, respectively).
| Lectin | Ab1+Ab2 | MF1 paper | Centrifugation | |
|---|---|---|---|---|
| PCT | 99.2a | 100 | 63.4 | 100 |
| TPSA | 67.4 | 96 | 60 | 100 |
Table 2: Impact of the method of RBC depletion on PCT and TPSA plasmatic concentration. a: Results expressed in percentage of recovery/reference method (Centrifugation).PCT: procalcitonin ; TPSA: Prostate Specific Antigen. The methods of RBC removing are: lectin of S. tuberosum (Lectin), mix of antibodies (Ab1+Ab2), MF1 glass fiber paper (MF1 paper) and centrifugation.
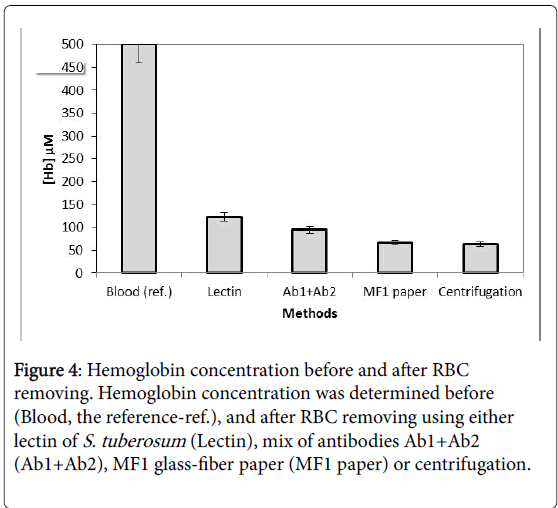
In order to check for the presence of hemoglobin residues in the preparations, the hemoglobin concentration was measured in plasma using a specific commercial kit. As shown in Figure 4, the results indicated a low level of hemoglobin, close to that obtained with the centrifugation method, particularly for the MF1 paper ([Hb]=63 μM) and, to a lesser extent, for the antibodies ([Hb]=97 μM). The highest concentration of hemoglobin was found for the method using S. tuberosum lectin ([Hb]=133 μM). As a reference, unpurified blood gave a saturating hemoglobin signal of over 800 μM. Interestingly, Figures 1c and 2c show supernatants with a slight red coloring, which confirmed the presence of residual hemoglobin in preparations using lectin or antibodies.
Figure 4: Hemoglobin concentration before and after RBC removing. Hemoglobin concentration was determined before (Blood, the reference-ref.), and after RBC removing using either lectin of S. tuberosum (Lectin), mix of antibodies Ab1+Ab2 (Ab1+Ab2), MF1 glass-fiber paper (MF1 paper) or centrifugation.
Taken together, these comparative results showed that the method using antibodies performed best in terms of PCT/TPSA recovery and residual hemoglobin concentration.
Discussion
In this study, different methods for removing RBCs from whole blood, using sedimentation, agglutination and lateral filtration on glass fiber paper, were compared in terms of simplicity, efficiency, rapidity and marker recovery.
It was possible to easily and reproducibly separate the RBCs from 50 μL of whole blood, in less than five min, using either S. tuberosum lectin at 0.5 μg/mL, a mix of two antibodies (the first being directed against RBCs and the second against mouse IgM) or a strip of MF1 glass fiber paper that had been functionalized with anti-RBC antibodies.
The method that used the mix of antibodies was the best in terms of marker recovery and residual hemoglobin concentration. The MF1 paper-based method was the most rapid.
The use of two monoclonal antibodies, one IgM directed against RBCs and the other against the mouse IgM antibody, demonstrated an improvement in RBC agglutination when compared with the RBC antibody alone. Previous studies have discussed the use of two antibodies (RBC antibody+anti-species antibody) for the efficient agglutination and separation of RBCs from plasma. The Coombs antibody test [23] traditionally used for the detection of plasma immunoglobulins that can opsonize red blood cells and involved in diseases uses also these two kinds of antibody. In the experiment presented here, it was hypothesized that the binding of the second antibody on the anti-RBC IgM increases the formation of a network that accelerates the agglutination and the sedimentation of RBCs.
Plant lectins, particularly those from S tuberosum , have strong clumping properties on different types of RBCs. The combination of two or three different lectins (S. tuberosum /U. europeaus/P. vulgaris lectins) has been tested but did not improve the agglutination rate. Results from samples that contained TPSA showed that the use of lectins may have consequences for the recovery of markers in the plasma. The TPSA is a glycoprotein that harbors N-glycans on its surface [24]. Solanum tuberosum lectins bind to polysaccharides that contain N-Acetyl glucosamine and galactose, which are all sugar components of N-glycans. Consequently, lectins may also bind to the sugars on the surface of TPSA and, more generally, on the surface of all glycoproteins. For TPSAs that have at least one N-glycan, the percent recovery after using the lectin method was lower than the technique that uses antibodies. In contrast, there are no N-glycans on the surface of PCT [25] and the percent recovery was equivalent to the method using antibodies, which indicates that the lectin method has no impact on PCT recovery.
The use of glass fiber paper, such as MF1, is a potential alternative in terms of time to result and purity. However, part of the plasma is probably retained in the fibers of the paper, which impacts the marker recovery. The marker recovery rate can be increased by washing the MF1 strip with small volumes (10-20 μL) of a buffer, such as PBS1X or TBS1X, supplemented with trehalose [26]. The disadvantages of this additional step (deposition of the elution buffer) are that it makes the method more complex and doubles the time required to obtain plasma. Moreover, the marker concentration is diluted when compared with the others methods.
The recurring problem with these methods is the purity level. Indeed, it is important to obtain samples with a highly reduced hemoglobin level as it is known to interfere with immunoassays [27] and is also an inhibitor of gene amplification [3]. For immunoassays, working with hemolyzed samples is generally not recommended (www.biomerieux.com/techlib). In this study, all techniques gave a low level of hemoglobin, under 150 μM. The level of residual hemoglobin for each method was compatible with the implementation of an efficient immunoassay. For gene amplification, the inhibitory concentration of hemoglobin is 16 μM. All methods assessed in this study, including centrifugation, were above this threshold. To bring the concentration below the threshold, it will be necessary to dilute the samples (e.g. six fold dilutions for the method using antibodies) before the application of amplification techniques.
The volume of blood used for RBC removal is small and for an initial sample of 50 μL it is possible to recover a volume of between 20 and 25 μL, after purification. This small volume may be difficult to handle in an automated environment, which may need to be addressed during product development. In the case of the MF1 test strip, the sample volume is integrated into the paper support and can be handled more easily. However, a sample elution from the glass fiber paper may be necessary, which increases the time to result for this technique.
When we considered the cost of goods for the different techniques, the glass fiber paper method was the most expensive. If the cost is based on one hundred tests, this method is approximately two times more expensive than the antibodies method and 12 times more expensive than the lectins method. If MF1 paper is functionalized with lines of RBC antibody, the cost of this method increases dramatically and is 22 times more expensive than lectins.
Conclusion
Three simple methods have been successfully tested for the rapid removal of RBCs from whole blood. The method that used a combination of a mouse RBC monoclonal antibody and a mouse IgM antibody, allowed clear plasma to be obtained in five min. This plasma was suitable for the measurement of biomarkers, particularly in the context of an immunoassay. For molecular biology techniques, it may be necessary to dilute the plasma before the measurement of markers. These methods may be integrated into an automated process, potentially at the point-of care.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
All the authors except are employed by bioMérieux SA.
Authors’ Contributions
All of the authors contributed to the study conception. FB, AR performed research. FB, wrote the manuscript. All of the authors analyzed data, read, corrected and approved the final manuscript.
Acknowledgement
Authors wish to thank Celine Roesch, Michèle Guillote (Immunoassays department of bioMérieux) for antibody productions ; Florence Bettworth, Blandine Le Levreur and Marie-Claire Cavaud (Immunoassays department of bioMérieux) for antibody purification ; Frederic Foucault for MF1 strip manufacturing ; Nadia Piga, Anne- Sophie Suchel-Jambon (BioBank, BioMerieux) for blood samples management. The final manuscript has been red and corrected by Dr Nicky Thelwell (UK).
References
- Tarkkinen P, Palenius T, Lovgren T (2002) Ultrarapid ultrasensitive one-step kinetic immunoassay for C-reactive protein (CRP) in whole blood samples: measurement of the entire CRP concentration range with a single sample dilution. Clin Chem 48: 269-277.
- von Lode P, Rainaho J, Pettersson K (2004) Quantitative, wide-range, 5-minute point-of-care immunoassay for total human chorionic gonadotropin in whole blood. Clin Chem 50: 1026-1035.
- Sidstedt M, Hedman J, Romsos EL, Waitara L, Wadso L, et al. (2018) Inhibition mechanisms of hemoglobin, immunoglobulin G, and whole blood in digital and real-time PCR. Anal Bioanal Chem 410: 2569-2583.
- Nabatiyan A, Parpia ZA, Elghanian R, Kelso DM (2011) Membrane-based plasma collection device for point-of-care diagnosis of HIV. J Virol Methods 173: 37-42.
- Hou HW, Bhagat AA, Chong AG, Mao P, Tan KS, et al. (2010) Deformability based cell margination-a simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip 10: 2605-2613.
- Al-Soud WA, Radstrom P (2001) Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol 39: 485-493.
- Akane A, Matsubara K, Nakamura H, Takahashi S, Kimura K (1994) Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. Journal of Forensic Sciences 39: 362-372.
- Mole RH (1945) Diurnal and sampling variations in the determination of haemoglobin. J Physiol 104: 1-5.
- Stengle JM, Schade AL (1957) Diurnalnocturnal variations of certain blood constituents in normal human subjects: plasma iron, siderophilin, bilirubin, copper, total serum protein and albumin, haemoglobin and haematocrit. Br J Haematol 3: 117-124.
- Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, et al. (1999) Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest 59: 491-500.
- Kersaudy-Kerhoas M, Sollier E (2013) Micro-scale blood plasma separation: from acoustophoresis to egg-beaters. Lab Chip 13: 3323-3346.
- Yamada M, Seki M(2006) Microfluidic particle sorter employing flow splitting and recombining. Anal Chem 78: 1357-1362.
- Yang S, Undar A, Zahn JD (2006) A microfluidic device for continuous, real time blood plasma separation. Lab Chip 6: 871-880.
- Voldman J (2006) Electrical forces for microscale cell manipulation. Annu Rev Biomed Eng 8: 425-454.
- Melville D (1975) Direct magnetic separation of red cells from whole blood. Nature 255: 706.
- Dimov IK, Basabe-Desmonts L, Garcia-Cordero JL, Ross BM, Park Y, et al. (2011) Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS). Lab Chip 11: 845-850.
- Qin L, Vermesh O, Shi Q, Heath JR (2009) Self-powered microfluidic chips for multiplexed protein assays from whole blood. Lab Chip 9: 2016-2020.
- Karpova IS (2016) Specific interactions between lectins and red blood cells of Chornobyl cleanup workers as indicator of some late radiation effects. Exp Oncol 38: 261-266.
- Sawierucha J, Posset M, Hahnel V, Johnson CL, Hutchinson JA, et al. (2018) Comparison of two column agglutination tests for red blood cell antibody testing. PloS One 13: e0210099.
- Bedin F, Boulet L, Voilin E, Theillet G, Rubens A, et al. (2017) Paper-based point-of-care testing for cost-effective diagnosis of acute flavivirus infections. J Med Virol 89: 1520-1527.
- Theillet G, Rubens A, Foucault F, Dalbon P, Rozand C, et al. (2018) Laser-cut paper-based device for the detection of dengue non-structural NS1 protein and specific IgM in human samples. Arch Virol 163: 1757-1767.
- Jarujamrus P, Tian J, Li X, Siripinyanond A, Shiowatana J, et al. (2012) Mechanisms of red blood cells agglutination in antibody-treated paper. Analyst 137: 2205-2210.
- Segel GB, Simon W, Lichtman MA (2011) Should we still be focused on red cell hemoglobin F as the principal explanation for the salutary effect of hydroxyurea in sickle cell disease? Pediatr Blood Cancer 57: 8-9.
- Scott E, Munkley J (2019) Glycans as Biomarkers in Prostate Cancer. Int J Mol Sci 20: E1389.
- Taylor R, Jones A, Kelly S, Simpson M, Mabey J (2017) A Review of the Value of Procalcitonin as a Marker of Infection. Cureus 9: e1148.
- Neo SH, Chung KY, Quek JM, Too HP (2017) Trehalose significantly enhances the recovery of serum and serum exosomal miRNA from a paper-based matrix. Scientific Reports 7: 16686.
- Steen G, Klerk A, Laan K, Eppens EF (2011) Evaluation of the interference due to haemoglobin, bilirubin and lipids on Immulite 2500 assays: A practical approach. Ann Clin Biochem 48: 170-175.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi