Review Article, J Virol Antivir Res Vol: 14 Issue: 1
A Comprehensive Review on Phage Therapy
Calmly M Koshy, Sruthi Sekar and Shobana Sugumar*
Department of Genetic Engineering, School of Bioengineering, SRM Institute of
Science and Technology, Chennai, India
*Corresponding Author:Shobana Sugumar
Department of Genetic Engineering, School of Bioengineering, SRM Institute of Science and Technology, Chennai, India
E-mail: shobanas@srmist.edu.in
Received date: 15 December, 2023, Manuscript No. JVA-23-122901;
Editor assigned date: 19 December, 2023, PreQC No. JVA-23-122901 (PQ);
Reviewed date: 02 January, 2024, QC No. JVA-23-122901;
Revised date: 16 January, 2025, Manuscript No. JVA-23-122901 (R);
Published date: 23 January, 2025, DOI: 10.4172/2324-8955.1000698
Citation: Koshy CM, Sekar S, Sugumar S (2025) A Comprehensive Review on Phage Therapy. J Virol Antivir Res 14:1.
Abstract
Bacteriophages are a particular kind of virus that only have the ability to infect and kill the cells of prokaryotic organisms, not mammalian cells. They are abundantly found in nature and are effective against drug-resistant bacteria. The emergence of pathogenic microbes immune to the majority, if not all, currently accessible antimicrobial medicines has turned into a serious problem in contemporary healthcare due to the concomitant increase in patients who are immunosuppressed. The quest for new anti-infection techniques has been prioritized by modern medicine and biotechnology due to the grave concern that humanity is once again entering the "preantibiotics" era. Since 1919, bacteriophages have been tested to treat bacterial contaminations brought by Staphylococcus species, Pseudomonas species, Vibrio cholerae, and other bacteria, with varying degrees of success. These bacterial contaminations include diarrhoea caused by Shigella dysenteriae. This survey presents extensive information about, phage science fundamentals, early investigations of bacteriophage therapy, prevention and treatment of bacterial diseases in people, advancement and use of phage-determined lytic proteins, and phage treatment versus anti-infection treatment. In India, phage therapy is practiced in clinical trials where the effectiveness of bacteriophage treatment was marked at 92% in clinical improvements and 84% in bacteriological clearance. This survey intends to give an effective point of view on the verifiable setting of bacteriophage treatment, to feature current advances in bacteriophage exploration and developments in the field.
Keywords: Bacteriophage therapy, Phage treatment, Antibiotics, Infectious diseases, Clinical trials
Abbreviations
MDR: Multidrug Resistance; WHO: World Health Organisation; IBMV: Institute of Bacteriophage Microbiology and Virology; GLP: Good Laboratory Practices; GMP: Good Manufacturing Practices
Introduction
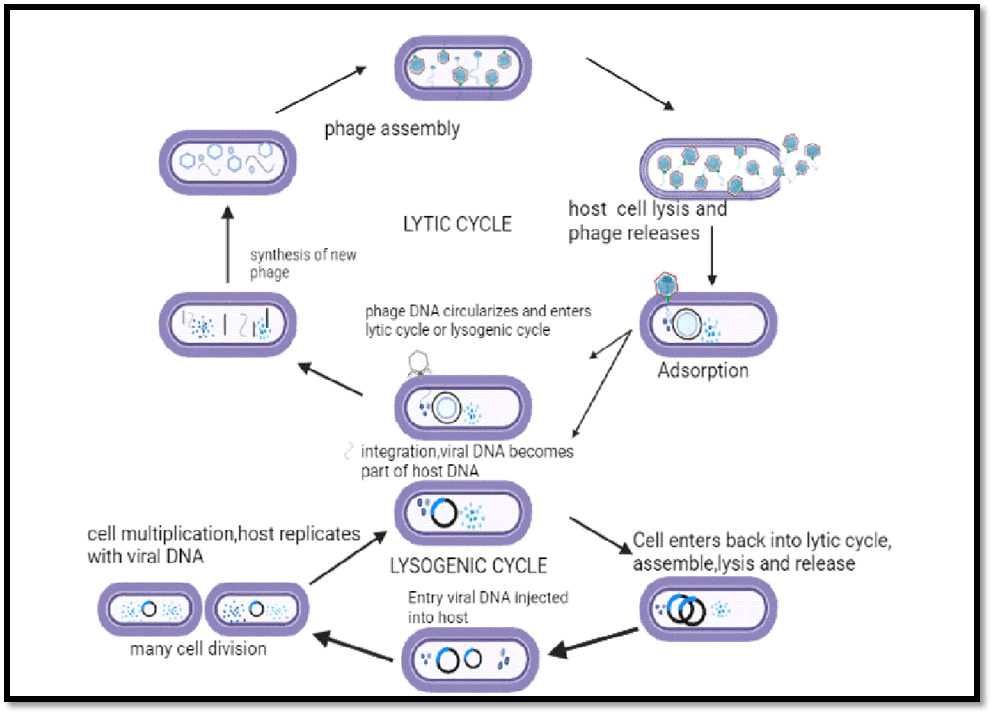
Bacteriophages, also referred to as phages, are infectious bacterial viruses that penetrate microbial cells and, in the case of lytic viruses, disrupt the metabolic process of the infected bacteria, causing the bacterium to lyse [1]. Long-running arguments have surrounded the discovery of bacteriophages, including a dispute over claims of precedence (Figure 1).
Figure 1: Steps involved in the lytic and lysogenic cycle.
Felix d'Herelle, a French microbiologist, was the one who initially coined the term "bacteriophages" and came up with the notion of employing them therapeutically to treat bacterial infections. Felix d'Herelle finally founded the Eliava Institute of Bacteriophage Microbiology and Virology (IBMV) in Tbilisi, Georgia, in 1933, in conjunction with Georgian scientist George Eliava. This institution has grown into one of the world's most respected phage therapy research facilities. Patients with antibiotic-resistant diseases travelled from all over the world to the center to attain tailored bacteriophage treatments [2]. Despite their apparent efficacy in treating antibiotic-resistant illnesses, there remain significant limitations to their safe and effective clinical application, and phage therapy is still regarded as an experimental treatment. Before the discovery of antimicrobial drugs in the 1940’s, bacterial infections seemed among the leading causes of fatalities and disabilities in the world's population. Phage therapy was a treatment for these illnesses in the early 1900’s. Bacteriophages have a major advantage over antibiotics because they are naturally occurring predators of bacteria. They tend to be highly specific, only harming their host bacteria, suggesting a more gentle method of treating specific microbiota [3].
The strategy of phage therapy in clinical practice is studied here to assist in the earliest possible introduction of phage therapy into the clinic. The World Health Organization (WHO) published a paper in 2017 that defined deadly antibiotic-resistant bacteria, known as the ESKAPE group. Resistant strains of Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter species, Acinetobacter baumannii, Klebsiella pneumonia, and Enterococcus faecium. These bacteria cause deadly infections in the community and come from hospital-acquired infections [4-6]. Phage treatment, in which phages are used against bacterial infections, is one of the most promising techniques. One of the most compelling justifications for using therapeutic bacteriophages as therapies for antibiotic-resistant bacterial illnesses is their general safety when prepared under Good Laboratory Practices (GLP)/Good Manufacturing Practices (GMP) settings. The absence of new medicines, combined with their misuse and overuse, has resulted in what has been dubbed the "post-antibiotic" period.
Bacteriophage therapy is a new technique for treating MDRO infections that are regarded as one of the top ten promising therapeutics in the antimicrobial field that needs more research.
Contrarily, due to the antibacterial market's lower profitability than that of other pharmaceutical products, pharmaceutical corporations are demonstrating less interest in researching and producing new antimicrobial medications [7]. Given the situation, the development of chemotherapeutic drugs that are antimicrobial substitutes could be highly beneficial in the fight against antibiotic resistance. Scientists are reconsidering phages as therapeutic agents due to issues in treating many deadly bacterial diseases. One of the best alternatives to antibiotic resistance may be bacterial phage and bacteriophage-based products. This review breaks down on the state, constraints, and future of phage therapy, including the emergence of bacteriophage as an intriguing therapeutic.
Literature Review
Mechanism of bacterial resistance to bacteriophage
Bacterial resistance might create regardless of the utilization of different phages, which may likewise restrict broad use. However advanced bacteriophages might be discovered in the case of objective microscopic organisms in a customized bacteriophage treatment. Infectious hosts safeguard instruments against phage. Momentarily, each stage of bacteriophage connection, disguise hereditary substance, and replication series may be influenced by the advancement of opposition to the infectious host. The initial phase of bacteriophage disease to microscopic organisms attached to the bacteriophage in the pathogenic surface molecule. A typical obstruction technique avoids bacteriophage nucleation or change in the explicit virus. One more recognized interference is limitation adjustment (R-M) frameworks that end attacking. A quality transformation imperative for phage replication or gathering can likewise prompt bacterial opposition. However, one bacteriophage may be utilized, mixed drinks, mixes of bacteriophages, and many wanted preventive medicine. The perspective behind using phage mixed drinks is that the mix of phages expands the range of movement against explicit life forms and along these lines, exemplifies the capacity to diminish the development of phage safe microorganisms. A chance that the living being creates protection from one of the phages in the mixed drink. The dynamic phages can contaminate the microorganisms, keeping up with the antimicrobial impact [1].
Furthermore, specific phage-safe bacterial strains may be less harmful or have broader antimicrobial helplessness designs. Prior phage contamination with microbial cells, for example, utilising calm viruses, could hinder sections that contain different phages via "superinfection prohibition frameworks”. Because of the innate particularity, the bacteriophage-have bacterium collaboration, and business arrangements of phage may not be dynamic against all organic entities inside a specific animal type. A fundamental capability is that there must be the accessibility of phages dynamic against the patient segregate. B.T. will probably not trade anti-infection agents for most contaminations, however, might be utilized in the setting of MDRO or other anti-toxin headstrong diseases, as noted before. Advancement of examinations that quickly distinguish bacterial weakness to phage is of fundamental significance to work with the fast arrangement of phage treatment when required and is a functioning space of examination. A distinctive trademark is that every bacteriophage is explicit to one or a couple of related strains/types of microscopic organisms. Along these lines, when bacteriophage therapy is utilized restoratively, it straightforwardly attacks infectious microscopic organisms. Different qualities of bacteriophage therapy that make it an alluring remedy are a potential for biofilm entrance, collective energy with antioxidants, and collaboration with other phage species.
Bacteriophages likewise identify duplicating and restricting as they can just increase within sight of a defenseless bacterial host. Bacteriophages focus on their particular bacterial cells, and it does not contaminate eukaryotic cells in this way, restricting the potential for unfavorable occasions. These components make B.T. an appealing possible choice to work in the treatment of human bacterial contaminations. Microscopic organisms can oppose phage assault through various features, including unconstrained changes, and limitation adjustment frameworks. Unconstrained changes are the primary systems driving both phage opposition and phage–bacterial coevolution. Lytic bacteriophages can eliminate antimicrobial safe microorganisms that are about to finalize the bacteriophage contamination. In any case, most of them use 2 gatherings to kill the bacterium. Hollins utilizes the particular subsequent endolysins to cause reproduction [9]. Phage endolysins are enzymatic proteins liable for cell divider corruption. Phage therapy analyzes the mucopeptide of the harmful microbes.
Regarding bacteriophage replication, endolysins advance the arrival of offspring virions. Phages have gained instruments for opposing countermeasures displayed by microscopic organisms. It should be noticed that the "unavoidable" advancement of phage-safe microbes might be bio-medical worthwhile whether the change caused to repel bacteriophage and direct a wellness decrease that may be utilized through auxiliary antibacterial therapy. The development of phage-safe microbes should be recognized as a specific result of human phage treatment. The present known components by which microorganisms can resist phage.
Phage interactions with the human body
Bacteriophage treatment in human diseases is crucial to profound comprehension utilizing phages communicating with microscopic organisms that alter human safe framework. Connections between the phages, microscopic organisms, and insusceptible frameworks are mind-boggling, taking into account that the bacteriophages usually are available in all aspects of the human body. It is common knowledge that the most complex and diverse phages can be found in the human digestive tract. The understanding of the relationship between tiny organisms and phages in relation to the microbiome in the gut has been established by a number of investigations. Undoubtedly, microorganism populace and bacteriophage variety are firmly connected, both opposing in manners. The temperate phages go through lysogenic change and upgrade poison creation in microbes. On the outer layer of the erythrocyte, bacteriophages are likewise well known to increase the colonization of microorganisms. As bacteriophages can be controlled relatedly, one more significant inquiry to determine is the movement or dissemination of bacteriophages from the intestine placenta to different pieces of the human body, like lymph hubs and inner organs. Phages tie to the outer layer of layers utilizing explicit acknowledgment of receptors, which thus initiates signal transductions. A non-explicit acknowledgment of bacteriophages likewise prompts different instruments of endocytosis using vesicles in the cells. The connection of phages with the intrinsic invulnerable reaction has been affirmed.
Phagocytic cells in the intrinsic insusceptible framework, like macrophages, neutrophils, and dendritic cells, can figure [10].
Association of microbes-phages-invulnerable framework; (A) Microbes and phages are linked. Bacterial death of microscopic organisms by covering the phones improves the resistance enactment. (B) Phage-microorganism-insusceptible framework collaboration. (1) Phages are determinate explicit antibodies and explicit receptors, resulting in macrophage endocytosis; (2) Delivered phages, along with bacterial particles (which contain LPS), are recognized by TLRs and endocytosed by macrophages (3) Opsonized microorganisms have to improve affectability and the actuation of immune reactions; and (4) Macrophages can induce endocytosis free phages and microscopic organisms without phage or phage particle receptor take-up. Green Fluorescent Protein (GFP) incorporated in the phage genome allowed researchers to witness the phage by cells. The phage particles were efficiently swallowed by the dendritic cells, according to photographic visualisation of the cells carrying phage fragments. The dendritic cells were a touchy and had an endless supply of virion particles than manufactured particles. Following the ingestion of the phages, another review utilizing macrophages affirmed phage debasement inside splenocytes, which wound up in macrophage compartments. The discoveries propose the safe framework can't be contaminated by and can dispose of the phage particles. Bacteriophages establish colonies on every part of the body's specialties, such as the skin, oral palate, lungs, gastrointestinal tract, and the urinary pathway. Notwithstanding, phages are habitually disregarded in the microbiome, and metagenomic considerations, and their job are frequently hazy. The majority of the phages found in viruses that infect bacteria are calm phages capable of incorporating their genomes (prophage), and as such, can modify the aggregate of the host microscopic organisms by lysogenic transformation of the multitude of microbial networks inside the body, the local digestive area is the most perplexing and thick by a wide margin [11]. Around ninety percent of the gut, virome comprises bacteriophages, assessed at 109 infections for every gram of dung. As new individuals from the bacterial local area are presented, the phage populations in the digestive system broaden, proposing that bacteriophage and bacterial variety are connected. Besides, this relationship is unique in newborn children and settles in grown-ups [12].
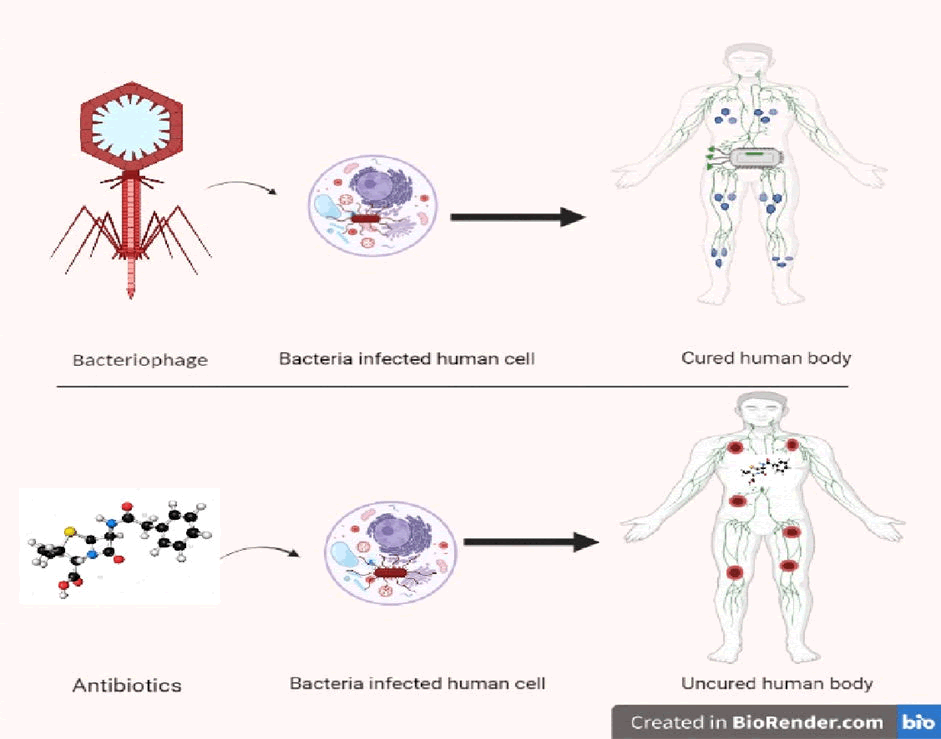
Even though there is less variety of gastrointestinal phage populaces inside people over the long run, there is abundant variety between people, in any event, when those people have comparable bacterial local area structures. The information explored here shows that bacteriophages can cooperate with the resistant framework in an assortment of immediate and aberrant ways. As of now, the accessible information proposes that these associations will, in general, be calming. Concerning the mitigating of phages can additionally be approved, it is possible that phages could impact both our associations with our commensal verdure just as the result of helpful phage mediations (Figure 2).
Figure 2: Phage interaction with human body.
Antibiotic therapy vs. phage therapy
Antibiotics have a nearly century-long clinical history. The global population's stress of transmissible diseases has decreased significantly over the last century, owing in part to the growing prevalence of antibacterial drugs. The use of antibiotics drastically reduced transmissible disease-related casualties, yet they additionally caused new problems with antibacterial resistance. A significant number of antibiotics were identified during this time period by isolating them from natural sources. These antibiotics were primarily supplied by, fungi, actinomycetes, and other microbes were the principal suppliers of these antibiotics. This was accompanied by a significant reduction in infectious disease-related deaths. During the twentieth century, the primary cause of death in the United States shifted from infectious diseases like pneumonia and tuberculosis to non-communicable illnesses conditions like cancer and myocardial ischemia. The disease was responsible for 46.4 percent of deaths at the turn of the twentieth century. Infectious diseases caused only 4.5 percent of death by the turn of the century [13]. The fight against infectious diseases, on the other hand, is far from over.
Antibiotic resistance mechanisms can evolve in bacteria, preventing antibiotics from interfering with cellular functions. Modifying the antibiotic target, efflux pumps, and the production of antibiotic degrading enzymes are examples of these methods. When the structure of an antibiotic target changes, it is referred to as modification. Mutations in the genome are frequently in charge of these structural alterations. Because of the structural change, the antibiotics can no longer bind to the target, and the medication has become less effective. The antibiotic is unable to reach a critical concentration within the cell itself. Antibiotic resistance is a severe danger to global health and the global economy. The economic cost of a "worst-case scenario" for the AMR issue was assessed in this study. In this scenario, all bacteria eventually develop MDR, rendering antibiotics. To solve this dilemma, alternative treatments for microbial diseases may be required. One option is phage therapy, which involves using viruses to treat bacterial illnesses. Because phage treatment is a viable answer to antibiotic resistance, it is being investigated and tried with fresh vigor [14].
Although d'Herelle did not fully comprehend the nature of bacteriophages, his scientific acumen enabled him to carry out rigorous procedures that incorporated many of the critical prerequisites for phage therapy to be successful. D'Herelle was so concerned about the consequences of having inactive phage preparations that he established a laboratory to research therapeutic phages. He intended to communicate his findings with any formal laboratory interested in phage therapy research. He realized that each therapeutic phage needed to be well described, which would take years to do. A commercial laboratory, according to d'Herelle, would not be able to take on such a task and profit from it. This could have been an early attempt at individualized medicine, according to specific theories. We now know that bacteriophages must possess several essential properties before they can be considered therapeutic candidates. These include: Only using obligately lytic bacteriophage incapable of transferring genes (specifically those encoding for toxins) from one bacterium to another; applying appropriately lytic bacteriophage to the target bacterial infection while remaining stable at relevant pH and temperatures for an extended period (e.g. 2-4 years). The attributes that, in our assessment, may considerably enhance the therapeutic value of phage therapy are examined in this section. Microorganisms that have been tainted with lytic phages can't recuperate their suitability. Certain antioxidants, like antibiotic medication, are bacteriostatic, and accordingly, they might permit microscopic organisms to develop obstruction all the more without any problem. Numerous harmful microscopic organisms are handily recognized by phages, commonly found in sewage with high bacterial densities [15].
Notwithstanding, if the host microbes are hard to culture, and microscopic organisms might contrast as far as the phage types to which they are allowed, confinement can be all the more, in fact, challenging. Unlike expansive range compound antimicrobials, disposed of remedial phages, which are mostly made of nucleic acids and proteins and have moderately slender host ranges, will just minorly affect a minuscule subset of ecological microorganisms. Phages that aren't accustomed to degradative ecological conditions like daylight or temperature limits can likewise be inactivated quickly (Table 1) [16].
| Criteria | Antibiotics therapy | Bacteriophage therapy |
| Action specificity | A wide range of actions is available. | A highly specific range of action. |
| Penetration of biofilms | Primarily in high dosages, it can penetrate. | Ability to penetrate effectively. |
| Possibility of opposition | Resistance is a significant chance. | Resistance is significantly less. |
| Toxicity | Toxicity levels can range from minor to severe. | Almost completely non-toxic. |
| The expense of treatment | Potentially costlier. | Potentially less expensive. |
| Ease of administration | Requires multiple doses. | Do not necessitate multiple doses. |
Table 1: Comparison between antibiotics therapy and bacteriophage therapy.
A synopsis of bacteriophage therapy's history
Bacteriophages were once assumed to be bacteriolytic organisms capable of attacking bacterial cultures when they were introduced in the early 1900’s. D'Herelle began the phage therapy trial for adult patients with acute dysentery at the hospital des Enfants-Malades in Paris in 1917 after demonstrating preliminary study safety by ingesting the therapy himself. According to Bruynoghe and Maisin's report, the initial clinical study associated with the administration of phages into and around lesions of the skin brought on by infections caused by staphylococcal bacteria and observation of the infection's remission in 24-48 hours happened in France in 1921. In India, thousands of patients were treated with phage preparations in the 1920’s for a variety of diseases, including bubonic plague and cholera. A multitude of phage therapy trials were also carried out in Soviet Russia and East Europe. An important step that it enabled the reassessment of phage therapy in the West was the publication of regulated animal trials in the language of English scientific journals in the decade of the eighties, which introduced an entirely new generation of researchers to their potential. For a while now, phage materials have been approved for usage in the US for preventing bacterial contamination during food processing.' However, doctors' euphoric enthusiasm and unrealistic expectations resulted in their misuse and abuse before the repercussions became clear, resulting in the resurfacing of antibiotic resistant microorganisms. As the challenge of antibiotic resistance intensified, Western scientists rediscovered phage treatment. Bacteriophages are very host-specific, with the majority of known phages infecting and replicating in a small variety of host strains). This specialization makes it more challenging to use bacteriophages as broad-spectrum antibacterial medicines; yet, phage treatment has several benefits. The co-evolution of phages and their host has permitted bacteria to develop ways to fight off phage infections, like uptake blocks, limitation, and abortive infections. It has also allowed the phage community to become even more infective to a wider variety of populations through mutations that make their binding site less specific. This coevolution is likely driven by directional selection rather than spontaneous mutations, which happen by accident. Antibiotics don't have a way to choose which bacteria to kill. This means that their indiscriminate attacking of bacteria is much more likely to lead to resistance. Bacteria have a chance to become resistant to a broad-spectrum antibiotic if they are under a lot of pressure from the antibiotic. In addition, plasmid transmission can spread the resistance to all the bacteria in the world. Selective pressure is put on a much smaller group of bacteria by very specific bacteriophages. Bacteriophages make it much less likely that phage-resistant bacteria will be formed because they make the population smaller [17]. Antibiotics have turned out to be too good, and antibiotic-resistant infectious diseases have become a huge burden. Phage therapy is viewed as a cure for antibiotic resistance, and a few early-phase clinical trials have yielded promising results (Table 2). However, phage biologists should be careful not to make the same overconfidence mistakes as the early advocates of antibiotics.
| Infectious Host | Clinical signs and symptoms | Bacteriophage | Family |
| Acinetobacter baumannii P | Urinary tract infection pneumonia and meningitis | vB_AbaM_Acibel004 | Myoviridae |
| Escherichia coli | Urinary tract infection, meningitis, and Diarrhoea | EC200PP | Podoviridae |
| Klebsiella pneumoniae | Pneumonia and lung infections | NK5 | Podoviridae |
| Mycobacterium tuberculosis | Tuberculosis | TM4, DS-6A | Siphoviridae |
| Streptococcus pyogenes | skin disorder and sore throat | A25 | Podoviridae |
| Staphylococcus aureus | flesh-eating disease and pneumonia | MSa | Myoviridae |
| Clostridium difficile | Diarrhea | CDHM 1-6 | Myoviridae |
| Enterococcus faecium | endocarditis, wound infection, bacteremia, and urinary tract infection | IME-EFm5 | Siphoviridae |
| Pseudomonas aeruginosa | Pneumonia | M4 | Myoviridae |
Table 2: Bacteriophages are viewed as potent against the effective microbes, are likewise connected with antimicrobial resistance as seen in the centers.
Application of phage therapy
As antimicrobial agents, phages offer several advantages over antibiotics, but some of those advantages might be limiting in specific applications. The benefit of phages is that they have a lesser tendency to cause resistance and do not cross-resistance to antibiotics. Phages are an excellent treatment against multidrug-resistant bacteria and biofilms. In juxtaposition with antibiotics, phages have fewer expenses for production and a wider range of production and utilization options, which makes them more advantageous for rapid isolation. Even though it is simple to obtain specifically lytic phages for prevalent pathogenic bacteria like E. coli, Campylobacter, Pseudomonas aeruginosa and Staphylococcus aureus, isolation for specific bacteria like Clostridium difficile or Mycobacterium tuberculosis has proven challenging. The few several hundred phages that are presently available in publicly accessible databases represent the estimated 1031 virions that can be found in nature. Because of their variety, they have the potential to be one of the most promising treatment techniques discovered to date. As a result, it is believed that as additional phages are found, the use of phage treatment will grow increasingly successful [18].
Successful case studies using phage therapy
Case study 1: A 41-year-old woman who has had plastic surgery for over 7 years, her gender transition treatments 15 years ago included an injection of industrial silicone oil into her buttocks and lower back. She had many infections in the afflicted places in the prior seven years, and she had multiple extensive hospitalizations to treat and manage these infections. Because the silicone oil was diffusely infiltrated in muscles involving the thigh, gluteus maximus, and lower back, it was impossible to debride the tissue fully; much of the soft tissue in the gluteal and thigh areas had already been debrided. Pseudomonas aeruginosa was the cause of her past illnesses, which developed resistance to every antibiotic treatment over the period. Over six months, six distinct morphotypes were gathered, each with its antimicrobial susceptibility pattern. Sensitivity testing demonstrated symbiosis between P. aeruginosa phage and Meropenem against these six isolates, which were all present at the start of phage therapy. After 17 days of IV phage BID, the patient got a single P. aeruginos bacteriophage with a frequency of (1 × 1011 PFU/dose) and meropenem. On 1st Febuary 2021, wound improvement and discomfort decreased, and granulated tissue was noted with lower secretions on 9th Febuary 2021. The first round of therapy finished on February 10. On February 25, 2021, the patient had another debridement due to sustained wound improvement following treatment. However, on February 28, 2021, more secretions were discovered at the surgical site, prompting the need for further phage. A total of 8 vials were delivered to continue the treatment. The patient is currently undergoing the second course of therapy, which includes 2 weeks of intravenous bacteriophage BID and locally QD at a titer of 1 × 1010 PFU/dose.
Case study 2: A 7-year-old girl was injured in a motor vehicle accident in 2018. This injury resulted in a lengthy hospital stay and many surgical operations (bilaterally, from the hips down), resulting in chronic multidrug-resistant P. aeruginosa osteomyelitis of the left foot. Further surgery might weaken the ankle joint, culminating in amputation. Thus, her doctors sought phage treatment. With just one phage, the patient could complete two weeks of treatment. Her physician noted healing at the infection site and enhanced mobility two weeks following discharge.
Proteins of phage
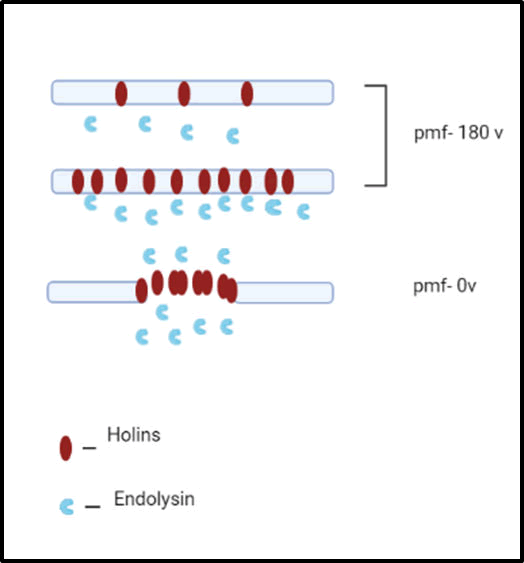
Two phage proteins the holin and the endolysin were specifically mentioned in a 1992 general theory for phage lysis. Just a few of these enzymes turn out to have the activity of conventional lysozyme, therefore the latter word was coined to describe the phage muralytic activity necessary for lysis. Small membrane proteins that were suggested to regulate the timing of lysis were given the name "holin" when they were first created. The main source of inspiration for the model came from genetic and molecular research on phage lambda. In general, it was thought that while the pathways for virion assembly were active, the holin, encoded by lambda S, accumulated in the membrane, and the endolysin, encoded by lambda R, did the same in the cytoplasm during the morphogenesis phase of the infection cycle. The holin would create non-specific channels, or holes, in the cytoplasmic membrane at a predetermined period (totally independent of the course of morphogenesis), allowing the endolysin to escape and assault the peptidoglycan. Since then, evidence from genetics, biochemistry, structural biology, and most recently genomics has been used to convincingly support the holin-endolysin concept (Figure 3).
Figure 3: Canonical holins-endolysin.
Discussion
At least six out of ten recognized infectious diseases in humans are thought to have originated in animals. Furthermore, the pressure of phytobacteria will encourage the evolution of a wide range of the defense mechanism, which can lead to enhanced virulence in humans, particularly those who are elderly, immunocompromised, or have cancer [19]. The one health idea recognizes that human and animal health and the health of our environment are all intertwined. Under the one health perspective, novel alternative therapies are required to combat the spread of antibiotic resistance. Phages have been used to limit bacterial proliferation in various microbiomes, including people (as discussed above), animals, and various environmental settings. The fitness is caused against the resistance of many variables; a combination of treatments leads to the limiting of developed resistance to multiple modes of action. Phage therapy in the twenty-first century antibiotic resistance, a significant problem in the twenty-first century, has rekindled interest in phage therapy in the western world. The World Health Organization (WHO) announced a susceptible situation in 2012 because of the quick spread of Multi-Drug Safe (MDR) microbes, cautioning of the risk of entering a time where anti-infection agents lose their viability against bacterial illnesses [20]. In animal models of both extraordinary and determined pollution, such as recollecting abscesses and subcutaneous illnesses in mice and cystic fibrosis lung sickness in mice. Pseudomonas aeruginosa has been used to treat long-term, purposeful, or microbial infections in human clinical practice. People with abscesses, prostatitis, skin ulcers, venous leg, and diabetic foot ulcers, suppurative fistulas, and cystic fibrosis seems to be treated using bacteriophagesss. These days, a fifteen-year old cystic fibrosis young adult with an inevitable Mycobacterium abscess tainting following two-sided lung motions stands out as truly newsworthy. This infection was treated using intravenous phage implantation. A sixty-eight-year-old diabetic patient with necrotizing pancreatitis caused by a multi-drug resistance Acinetobacter baumannii infection was also treated with bacteriophage. Lytic bacteriophages have also been helpful in the treatment and prevention of bacterial biofilm development.
Conclusion
Bacteriophage therapy is an effective alternative for multi-drug resistant organisms. Antibiotic-resistant bacterial infections kill more than seven lakh fifty thousand people each year worldwide, which estimated that this number will rise to almost 10 million by 2050. Bacteriophages can be given with a combination of antibiotics to cure illness. Bacteriophages can be utilized as therapeutic involvement in treating microorganisms that are impermeable to antimicrobials. Several restrictions must be overcome before bacteriophage therapy can become a standard treatment option in clinical trials. Pharmaceutical phage products must meet several requirements, including long-term stability and compatibility for delivery. New pharmaceutical formulations for therapeutic phages may be required for more fragile biological entities, particularly when compared to small-molecule medicines. A variety of alternatives for phage encapsulation and distribution are now widely available. The current post-antibiotic age has resulted in an increased search for alternative treatments due to the rising frequency of multidrug-resistant organism infections and the absence of new antibiotic development.
Ethical Approval
Not applicable.
Competing Interests
The authors declare no conflict of interest, financial or otherwise.
Funding
No funding.
Availability of Data and Materials
All datasets generated or analysed during this study are included in the manuscript and/or the Supplementary Files.
References
- Kutateladze M, Adamia R (2010) Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol 28: 591-595.
[Crossref] [Google Scholar] [PubMed]
- Fernández L, Gutiérrez D, García P, Rodríguez A (2019) The perfect bacteriophage for therapeutic applications—A quick guide. Antibiotics 8: 126.
[Crossref] [Google Scholar] [PubMed]
- Grose JH, Casjens SR (2014) Understanding the enormous diversity of bacteriophages: The tailed phages that infect the bacterial family Enterobacteriaceae. Virology 468: 421-443.
[Crossref] [Google Scholar] [PubMed]
- O'flaherty S, Ross RP, Meaney W, Fitzgerald GF, Elbreki MF, et al. (2005) Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl Environ Microbiol 71: 1836-1842.
[Crossref] [Google Scholar] [PubMed]
- Hung CH, Kuo CF, Wang CH, Wu CM, Tsao N (2011) Experimental phage therapy in treating Klebsiella pneumoniae-mediated liver abscesses and bacteremia in mice. Antimicrob Agents Chemother 55: 1358-1365.
[Crossref] [Google Scholar] [PubMed]
- Tunjungputri RN, Mobegi FM, Cremers AJ, van der Gaast-de Jongh CE, Ferwerda G (2017) Phage-derived protein induces increased platelet activation and is associated with mortality in patients with invasive pneumococcal disease. mBio 8: 10-128.
[Crossref] [Google Scholar] [PubMed]
- Aminov RI (2010) A brief history of the antibiotic era: Lessons learned and challenges for the future. Front Microbiol 1: 134.
[Crossref] [Google Scholar] [PubMed]
- Castillo DE, Nanda S, Keri JE (2019) Propionibacterium (Cutibacterium) acnes bacteriophage therapy in acne: Current evidence and future perspectives. Dermatol Ther 9: 19-31.
[Crossref] [Google Scholar] [PubMed]
- Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P (2017) Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis 215: 703-712.
[Crossref] [Google Scholar] [PubMed]
- Pires DP, Cleto S, Sillankorva S, Azeredo J, Lu TK (2016) Genetically engineered phages: A review of advances over the last decade. Microbiol Mol Biol Rev 80: 523-543.
[Crossref] [Google Scholar] [PubMed]
- Chegini Z, Khoshbayan A, Vesal S, Moradabadi A, Hashemi A (2021) Bacteriophage therapy for inhibition of multi drugâ?ÂÂresistant uropathogenic bacteria: A narrative review. Ann Clin Microbiol Antimicrob 20: 30.
[Crossref] [Google Scholar] [PubMed]
- Röttjers L, Faust K (2018) From hairballs to hypotheses–biological insights from microbial networks. FEMS Microbiol Rev 42: 761-780.
[Crossref] [Google Scholar] [PubMed]
- Serwer P, Wright ET, Lee JC (2019) High murine blood persistence of phage T3 and suggested strategy for phage therapy. BMC Res Notes 12: 560.
[Crossref] [Google Scholar] [PubMed]
- Levin BR, Bull JJ (2004) Population and evolutionary dynamics of phage therapy. Nat Rev Microbiol 2: 166-173.
[Crossref] [Google Scholar] [PubMed]
- Burrowes B, Harper DR, Anderson J, McConville M, Enright MC (2011) Bacteriophage therapy: Potential uses in the control of antibiotic-resistant pathogens. Expert Rev Anti Infect Ther 9: 775-785.
[Crossref] [Google Scholar] [PubMed]
- Betts A, Kaltz O, Hochberg ME (2014) Contrasted coevolutionary dynamics between a bacterial pathogen and its bacteriophages. Proc Natl Acad Sci 111: 11109-11114.
[Crossref] [Google Scholar] [PubMed]
- Morozova VV, Vlassov VV, Tikunova NV (2018) Applications of bacteriophages in the treatment of localized infections in humans. Front Microbiol 9: 1696.
[Crossref] [Google Scholar] [PubMed]
- Monteiro R, Pires DP, Costa AR, Azeredo J (2019) Phage therapy: Going temperate?. Trends Microbiol 27: 368-378.
[Crossref] [Google Scholar] [PubMed]
- Sulakvelidze A, Alavidze Z, Morris Jr JG (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45: 649-659.
[Crossref] [Google Scholar] [PubMed]
- Payne RJ, Jansen VA (2000) Phage therapy: The peculiar kinetics of selfâ?ÂÂreplicating pharmaceuticals. Clin Pharmacol Ther 68: 225-230.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi