Research Article, J Pulm Med Vol: 5 Issue: 5
A CROSS SECTIONAL DESCRIPTIVE STUDY OF THE FACTORS ASSOCIATED WITH CHANGES OF LUNG FUNCTION IN PULMONARY TUBERCULOSIS SEQUELAE PATIENTS IN A TERTIARY CARE CENTRE
Sreekaanth Sundarraj*
Department of Medicine, India
*Corresponding author: Sreekaanth S, Department of Medicine, India
Tel: +9176792965;
E-mail: sreemac55@gmail.com
Abstract
Tuberculosis is a communicable disease and remains the number one cause of death by infectious disease. Evidence of tuberculosis dates back to 700 AD. Robert Koch, a German microbiologist and physician discovered the tuberculosis bacilli in 1882 for which he received the nobel prize in 1905.2 Tuberculosis is caused by Mycobacterium tuberculosis, a slender rod shaped bacilli which is intracellular, non-sporing, non-motile, noncapsulated, facultative, catalase negative, gram positive and acid and alcohol fast. The bacilli are obligate aerobes.3 Increased CO2 tension improves growth and the temperature optimum for growth is 37? and no growth occurs below 25? & also above 40?.4
Keywords: Tuberculosis, Pulmonary Medicine
Introduction
I“AS A DESTROYER OF MANKIND, TUBERCULOSIS HAS NO EQUAL- VA MOORE”1
‘Tubercula’ in latin = small lump
Tuberculosis is a communicable disease and remains the number one cause of death by infectious disease. Evidence of tuberculosis dates back to 700 AD. Robert Koch, a German microbiologist and physician discovered the tuberculosis bacilli in 1882 for which he received the nobel prize in 1905.2
Tuberculosis is caused by Mycobacterium tuberculosis, a slender rod shaped bacilli which is intracellular, non-sporing, non-motile, noncapsulated, facultative, catalase negative, gram positive and acid and alcohol fast. The bacilli are obligate aerobes.3
Increased CO2 tension improves growth and the temperature optimum for growth is 37°c and no growth occurs below 25°c;& also above 40°.4
The emergence of HIV infection has increased the risk for tuberculosis. Poverty, smoking and illiteracy also contribute to the incidence of tuberculosis. Approximately, 10 million people fell ill with tuberculosis with 1.2 million deaths in HIV negative individuals and 2,51,000 deaths in HIV positive individuals in 2018. India contributed to 27 % of the worldwide cases.5
Transmission occurs through inhalation of droplet nuclei which are released into the environment by the infected person. Droplet nuclei are dried residua of larger respiratory droplets, <5 μm in size, remain suspended in air for long periods of time and travel for a distance of > 1 metre. Risk factors for transmission of bacilli include poor socioeconomic status, poor health, poor sanitation and overcrowding, diseases like silicosis, malignancy, pulmonary alveolar proteinosis and immunocompromised conditions.
Tuberculosis has a chronic course of illness. Symptoms of tuberculosis include cough >2 weeks, evening rise of temperature, loss of weight and hemoptysis. Tiredness, loss of appetite, headache and night sweats are other non specific constitutional symptoms. Sputum can be mucoid, mucopurulent, purulent or blood tinged and is usually scanty in quantity. Shortness of breath is usually seen only in extensive disease with bronchial obstruction, or with associated pleural effusion and pneumothorax3
Pulmonary Tuberculosis patients who have taken full course of Anti- tuberculosis treatment (ATT), with chest radiograph revealing inactive lesions are left with Respiratory disability because of impairment and decline in pulmonary function. 27% of Global Tuberculosis burden is accounted by India, hence post Tuberculosis sequelae causes significant morbidity and increase in DALYs (Disability-adjusted life year).6]
Many studies show that almost 50 % of cured patients live with permanent impairment of lung function and decreased life expectancy7
Immune response of the host plays important role in pathology of post TB sequelae lung. Neutrophils and their inflammatory mediators also contribute to poor lung function and lung damage. Socioeconomic factors like occupational loss and contribution of income towards poor treatment outcomes leads to drug resistant MTB strains, high risk of infection and progression which in turn raises risk of lung function impairment and sequelae 7
PTB causes destruction of lung parenchyma by up-regulating several processes and also by dysregulating protease control. Histopathological abnormalities after completion of treatment for PTB include cavitation, fibrosis, bronchiectasis and calcification. Lung function impairment is related to long term Respiratory symptoms, in turn affecting quality of life 8
Pulmonary function tests (PFTs) play an important role in identifying lung function derangement and also provides vital information regarding the large and small airways, lung parenchymal abnormalities and integrity and size of the pulmonary capillary bed. Despite not providing a diagnosis per se, PFTs show different patterns of abnormalities which are helpful in establishing the diagnosis of various respiratory diseases. The most frequently used lung function measure is Spirometry which measures lung volumes against time. Spirometry is a highly reproducible and simple test where in patients are advised to take maximal inspiration followed by quick forceful expiration - (FVC) forced vital capacity manoeuvre. Measurements made include FEV1- Forced expiratory volume in first second, FVC- Forced vital capacity, FEV1/FVC- ratio of the two volumes, MEF 25- 75%- Maximal expiratory flow between 25% and 75% of forced vital capacity 9
NEED FOR THE STUDY:
Although there are several studies in literature about the functional impairment occurring in post tuberculosis sequelae, there is paucity in studies assessing the relationship of the severity of decline in lung function to associated factors like lag time, number of ATT courses and duration after treatment.
A delay in initiation of treatment contributes to progression of lung lesions and thereby disease which leads to increase in lung function impairment.10
Duration after completion of PTB treatment and Number of episodes of PTB were also found to correlate with the severity of pulmonary tuberculosis sequelae.11
In this study, we intend to assess the lung function impairment along with their relationship with the above mentioned factors which are associated with the decline in lung function in pulmonary tuberculosis sequelae patients.
AIM AND OBJECTIVES
Aim:
To assess the lung function and the factors associated with change in lung function among patients who were previously treated for pulmonary tuberculosis.
Primary objective:
To assess the lung function abnormality in patients with Pulmonary Tuberculosis sequelae and to assess the factors affecting severity of lung function.
Secondary objective:
- To differentiate the pattern of lung function – obstructive, restrictive or mixed, occurring in pulmonary tuberculosis sequelae
- To compare the relationship between Lag Time (interval between onset of PTB symptoms and diagnosis of disease), Number of PTB episodes and Duration After Completion of PTB Treatment with the Severity of lung function
REVIEW OF LITERATURE
- Early diagnosis with treatment of pulmonary tuberculosis reduces number of cases along with reduction of incidence of sequelae in turn increasing the quality of life12,13
- Neeta Singla et found that many number of cured tuberculosis patients develop clinical, functional and radiological sequelae which leads to significant morbidity. Pulmonary rehabilitation post treatment should be an essential part of DOTS programme which can improve the programme’s impact in the community14
- Effective early diagnosis and treatment of PTB decreases the likelihood of developing obstructive lung Lung function deterioration was seen predominantly with inappropriate regimens and seldom during successful treatment completion15
- In a study by Manji et al., among pulmonary tuberculosis sequelae patients, abnormal lung function was present in most of the The pattern was mostly obstructive followed by mixed and restrictive. The factors associated with lung function abnormalities included male sex, recurrent pulmonary tuberculosis and age more than 40 years. The prevalence of lung function abnormalities among tuberculosis patients after completion of treatment is alarmingly high. The morbidity and quality of life impairment produced by sequelae is also similarly high.16
- On evaluating the trends and predictors of lung function changes, the derangement of lung function developed approximately 18 months post completion of Risk factors related to lung function deterioration included extensive lung involvement prior to ATT, smear-positive tuberculosis, prolonged duration of anti-TB treatment and reduced radiologic improvement post treatment7
- During the early stages of sequelae, obstructive pattern was the most common abnormality but as duration after treatment added up, restrictive and mixed pattern became more Tuberculosis causes chronic lung function impairment which incrementally increases with total number of tuberculosis episodes17
- Risk factors for decreased lung function include previous culture- positive PTB, age more than 50 years, low literacy, and recurrent All previously treated PTB patients should be evaluated with pulmonary function testing. After treatment, greater than 40% of patients evolve to lung function impairment, particularly obstructive airway disorders. This underscores the importance of pulmonary function testing in treated PTB patients18
- Pulmonary tuberculosis remains an important cause of obstructive airways It is proved that FEV1 is inversely proportional to the extent of disease in the chest radiograph. Further, this relationship stands even for patients with minimal disease and without cavitations at presentation19
- Many lung function values reflect severity of fibrosis, FVC and DLCO being the most There is no standardised definition, but baseline FVC % predicted (forced vital capacity) is graded as, normal more than or equal to 80 %, mild 66-79 %, moderate 51 to 65 % and severe less than or equal to 50 %20
- Obstructive airway disease might be due to involvement of the bronchial tree by the tuberculous Submucosal nonspecific inflammation has been frequently demonstrated in bronchi from resected specimens of tuberculosis patients along with bronchiectatic lesions. Bronchiectasis in sequelae cases was localized to a lobe or segment, similar in location to the original infection. This suggests a discrete original insult which is superseded by static or nonprogressive ectasia21
- Guliani al stated that increase in the extent of radiological sequelae suggests severe lung function impairment. Convincing positive correlation exists between severity of lung function and the radiological score. Chest X-ray and if possible, HRCT chest should be done post treatment to predict the severity of residual pulmonary function impairment7
- Among tuberculosis sequelae, patients who had resolution of the initial inflammatory changes had better lung function and patients who had cicatricial lung tissue transformation had worse lung Like smoking, tuberculosis also increases matrix metalloproteinase (MMP) activity contributing to lung damage. Functional changes occur by fibrosis and hyperinflation, disorders of bronchial patency, change in elasticity and disorders of gas exchange.22
- TB related pathology can result from various processes, like direct cellular toxicity by infection, matrix metallo-proteinases causing destruction of matrix and T-cell driven Targeting MMP activity may generate interventions that can be clinically useful23
- Filley et stressed the importance of TNF-α (tumor necrosis factor-α) in the pathogenesis of tuberculosis sequelae. Mycobacterium is able to alter the usual pattern of response by the cells to TNF-α. TNF-α induced damage occurs to the endothelial cells, promotes extravasation of inflammatory cells through heightened expression of adhesion molecules24
- Keertan Dheda et stated that additional research into the molecular, genetic, cellular and immunological pathways that drive fibrosis and cavitation could provide clinical improvement and can interrupt transmission. Like anti-TNF-α drugs, other immunotherapeutic drugs could target pathways related to apoptosis, cytokine signaling, extracellular matrix turnover and protease activity25
- Significant elevation of C-Reactive Protein (CRP) is reported in active tuberculosis patients, which normalizes over weeks on therapy, correlating with the clinical There is also a direct association between higher CRP levels and increased severity of tuberculosis and poor prognosis. Patients who had severe lung destruction, like extensive lung cavitation, had markedly elevated CRP levels compared to patients without cavitation. Further, CRP levels reduced rapidly after the start of treatment26
- Destruction of extracellular matrix (ECM) is the basis for the success of tuberculosis because it allows cavitation and creates an immunoprivileged site inside which the organism can multiply and spread to new hosts. Since collagen and elastin degradation must be there for cavity formation, Matrix Metalloproteinases (MMPs) are most likely involved in tuberculosis pathology. Increased MMP-1 and MMP-9 gene expression is brought about by Mycobacterial lipoarabinomannan (LAM), which is a major antigenic cell wall component. Circulating MMPâ?9 levels also correlate with the severity of disease27
- One year after successful completion of treatment, under RNTCP, health related quality of life (HRQoL) for most of the patients was It was also associated with literacy, age, income, employment, alcoholism, smoking and persistence of symptoms28
- Early detection and treatment helps in preventing tuberculosis from having its effect on pulmonary Intervention on risk factors like silica dust exposure, HIV, and socioeconomic factors also helps in the reduction of risk. Increasing loss of pulmonary function also corresponded with increased number of episodes of infection with tuberculosis11
MATERIALS AND METHODS
STUDY DESIGN:
CROSS SECTIONAL DESCRIPTIVE STUDY
STUDY AREA:
Shri Sathya Sai Medical College & Research Institute, a tertiary care hospital, Ammapettai in South India.
STUDY POPULATION:
Patients presenting in Respiratory Medicine OPD with clinical and radiological features of pulmonary tuberculosis sequelae
STUDY DURATION:
2 Years
INCLUSION CRITERIA:
- Both male & female patients
- Patients who have completed treatment atleast 18 months prior to study
- Age >18 years
- Patients who have stopped smoking after diagnosis of Pulmonary TB
- X-ray chest showing features suggestive of fibrosis, cavity, calcifications, bronchiectasis
EXCLUSION CRITERIA:
- Active pulmonary tuberculosis patients
- Extra-pulmonary tuberculosis patients
- Severe Respiratory distress
- Any pre-existing airway, parenchymal or interstitial lung diseases
- Patients not fit for spirometry
SAMPLE SIZE:
SAMPLE SIZE: n=85
Based on previous study, prevalence value is 62.5% (1) n=4pq/L2 where p=62.5, q=100-62.5= 37.5
L is precision error = 11% (absolute precision error) n = 4 x 62.5 x 37.5/112
= 9375/121
= 77.47 + (10% non-response error)
= 77.47 + 7.74
= 85.21 ≈ 85
STATISTICAL ANALYSIS:
Data entered in MS-Excel and Statistical Analysis done by SPSS 23 software. The results will be presented in descriptive statistics and appropriate test of significance will be applied with 5% level of significance and 95% confidence interval.
STUDY VARIABLES:
- Socio-demographic variables: Name, Age, Sex, Height, Weight, Occupation, Socio-economic status
- Spirometry variables: FEV1, FVC, FEV1/FVC ratio, MEF 25-75%, DLCO
- Other study variables: Lag Time, Duration after Treatment, Number of PTB Episodes
METHODOLOGY:
Patients were selected as per inclusion and exclusion criteria, written informed consent obtained and then patients were subjected to spirometry and DLCO as per ATS/ERS Guidelines
Ethical consideration:
1.Institutional Ethical committee clearance was obtained prior to the study.
2.Informed consent was obtained from all the patients recruited prior to the start of the study.
RESULTS
TABLE 1: DISTRIBUTION OF AGE AMONG THE STUDY POPULATION
Among the study population with Age distribution, most of the subjects - 24 (28.24%) were in 41 - 50 years age group followed by 22 (25.88%) in 31 - 40 years and least 5 (5.88%) were in 21 - 30 years. More than half of the subjects belonged to the 31 to 50 years age group.
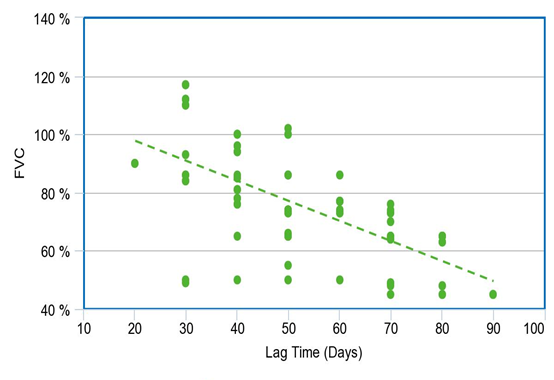
Among the study population, 50 subjects had decreased FVC
Lag Time (days) has a negative correlation with FVC with a correlation coefficient of -0.69
FVC decreases by -0.81 times for each unit increase in Lag Time (days)
The correlation between FVC and Lag Time (days) was statistically significant.
CORRELATION BETWEEN LAG TIME AND FEV1 (OBSTRUCTIVE PATTERN) IN THE STUDY POPULATION
Among the study population, 20 subjects (23.5%) had small airway obstruction.
Among the study population, 55 subjects had decreased FEV1
Lag Time (days) has a negative correlation with FEV1 with a
correlation coefficient of -0.83
FEV1 decreases by -0.98 times for each unit increase in Lag Time (days)
The correlation between FEV1 and Lag Time (days) was statistically significant
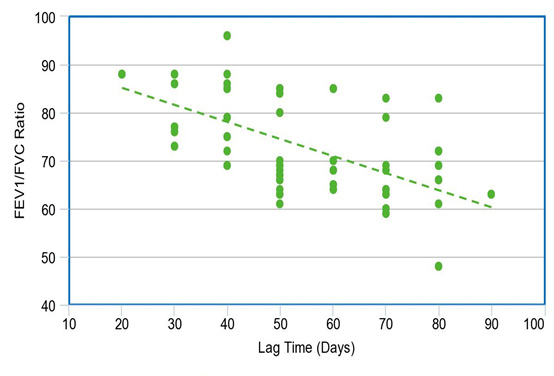
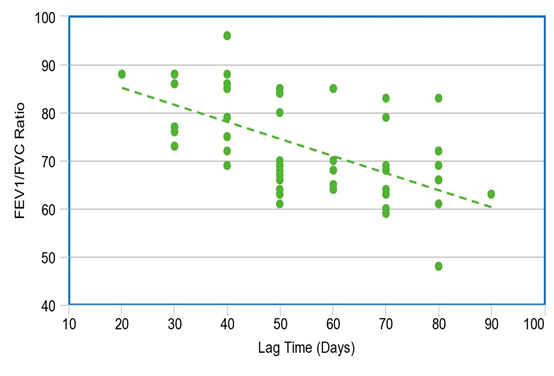
CORRELATION BETWEEN LAG TIME AND FEV1 / FVC IN THE STUDY POPULATION
Among the study population, 55 subjects had decreased FEV1/FVC ratio
Lag Time (days) has a negative correlation with FEV1/FVC with a
correlation coefficient of -0.51.
FEV1/FVC decreases by -0.31 times for each unit increase in Lag Time (days)
The correlation between FEV1/FVC and Lag Time (days) was statistically significant.
CORRELATION BETWEEN LAG TIME AND MEF 25 - 75% (SMALL AIRWAYS OBSTRUCTION) IN THE STUDY POPULATION
Among the study population, 66 subjects had decreased MEF 25-75%
Lag Time (days) has a negative correlation with MEF 25-75% with a correlation coefficient of -0.65.
MEF 25-75% decreases by -0.9 times for each unit increase in Lag Time (days)
The correlation between MEF 25-75% and Lag Time (days) was statistically significant.
CORRELATION BETWEEN LAG TIME AND DLCO IN THE STUDY POPULATION
Among the study population, 73 patients had decreased DLCO
Lag Time (days) has a negative correlation with DLCO with a correlation coefficient of -0.61
DLCO decreases by -0.58 times for each unit increase in Lag Time (days)
The correlation between DLCO and Lag Time (days) was statistically significant
CORRELATION BETWEEN DURATION AFTER TREATMENT AND FVC (RESTRICTIVE PATTERN) IN THE STUDY POPULATION
Among the study population, 50 subjects had decreased FVC
Duration After Treatment (years) has a negative correlation with FVC with a correlation coefficient of -0.08. FVC decreases by -0.22 times for each unit increase in Duration After Treatment (years). The correlation between FVC and Duration After Treatment (years) was not statistically significant
CORRELATION BETWEEN DURATION AFTER TREATMENT AND FEV1 (OBSTRUCTIVE PATTERN) IN THE STUDY POPULATION
Among the study population, 55 subjects had decreased FEV1
Duration After Treatment (years) has a negative correlation with FEV1 with a correlation coefficient of -0.16. FEV1 decreases by -0.45 times for each unit increase in Duration After Treatment (years). The correlation between FEV1 and Duration After Treatment (years) was not statistically significant
CORRELATION BETWEEN DURATION AFTER TREATMENT AND FEV1/FVC IN THE STUDY POPULATION
Among the study population, 55 subjects had decreased FEV1/FVC ratio
Duration After Treatment (years) has a negative correlation with FEV1/FVC with a correlation coefficient of -0.34. FEV1/FVC decreases by -0.49 times for each unit increase in Duration After
Treatment (years). The correlation between FEV1/FVC and Duration After Treatment (years) was statistically significant.
CORRELATION BETWEEN DURATION AFTER TREATMENT AND MEF 25 - 75% (SMALL AIRWAYS OBSTRUCTION) IN THE STUDY POPULATION
Among the study population, 66 subjects had decreased MEF 25-75%
Duration After Treatment (years) has a negative correlation with MEF 25-75% with a correlation coefficient of -0.32. MEF 25-75% decreases by -1.05 times for each unit increase in Duration After Treatment (years). The correlation between MEF 25-75% and Duration After Treatment (years) was statistically significant.
CORRELATION BETWEEN DURATION AFTER TREATMENT AND DLCO IN THE STUDY POPULATION
Among the study population, 73 patients had decreased DLCO
Duration After Treatment (years) has a negative correlation with DLCO with a correlation coefficient of -0.4
DLCO decreases by -0.89 times for each unit increase in Duration After Treatment (years)
The correlation between DLCO and Duration After Treatment (years) was statistically significant
COMPARISON BETWEEN NEW AND RECURRENT PTB CASES BASED ON SPIROMETRY PATTERN
Among the study population, Obstructive spirometry pattern was predominant in new cases and none of the recurrent cases had a normal spirometry, Restriction and Mixed spirometry pattern were predominant among recurrent cases compared to new cases
COMPARISON OF THE NUMBER OF NEW AND RECURRENT PTB CASES WITH REDUCTION IN LUNG FUNCTION24 (100%)
On comparing new and recurrent PTB cases based on spirometry parameters, more number of recurrent cases had a fall in FEV1, FVC, FEV1/FVC ratio and MEF 25-75%.
Restriction and mixed spirometry patterns were predominant among recurrent cases compared to new cases and obstruction was predominant among new cases. No recurrent cases had a normal or an obstructive spirometry pattern.
CORRELATION OF NEW AND RECURRENT PTB CASES WITH DLCO IN THE STUDY POPULATION
Among the study population, 73 subjects had decreased DLCO
No. of Courses of Anti-TB Treatment has a negative correlation with DLCO with a correlation coefficient of -0.41
DLCO decreases by -15.17 times for each unit increase in No. of Courses of Anti-TB Treatment
The correlation between DLCO and No. of Courses of Anti-TB Treatment was statistically significant
Majority of recurrent cases were found to have severe restriction compared to new cases.
CORRELATION OF MEAN LUNG FUNCTIONS BETWEEN NEW AND RECURRENT PTB CASES
The mean FVC among new cases was 80.03 (± 19.33) which is higher than mean FVC among recurrent cases which was 53.58 (± 10.58) and the difference was statistically significant
The mean FEV1 among new cases was 72.74 (± 19.83) which is higher than mean FEV1 among recurrent cases which was 51.33 (± 15.46) and the difference was
statistically significant
The mean FEV1/FVC among new cases was 73.89 (± 9.32) which is higher than mean FEV1/FVC among recurrent cases which was 69.25 (± 14.07) and the difference was not statistically significant
The mean MEF 25 - 75% among new cases was 48.97 (± 24.7) which is higher than mean MEF 25 - 75% among recurrent cases which was 33 (± 21.22) and the difference was statistically significant
DISCUSSION
Tuberculosis remains a public health problem worldwide with increased mortality and morbidity among all chronic infections. 24% of the global TB burden is accounted by India29
In the present study it was observed that most of the patients fall in the age group of 41-50 years (28.24%) and almost 54% fall in the age group of 31-50 years. The reason for this can be because there is a gradual loss of lung function normally after 30 years of age.30
Normal fall in FEV1 per year is around 30 ml in non smokers and around 50 ml in smokers.There is an accelerated decline in pulmonary function in elderly individuals affected with TB especially over 40 years of age.31
Male subjects were predominant in our study compared to females (53 males and 32 females). Global TB report 2014 states that 60% of new cases reported belong to the male gender every year.29 The gender discrepancy may be because women approach medical aid less frequently compared to men especially in rural areas. Other factors which can contribute to TB infection in males are risk of exposure to infection, tobacco use and passive smoking in and around the working environment.32
In our study, smoking was not considered as a significant predictor of pulmonary function impairment. This is because males who took part in the study quit smoking after diagnosis of tuberculosis and the women did not have any smoking history before and after infection with Tuberculosis.
In our study, around 86% of the subjects had abnormal lung functions. Such a huge impact is really alarming and requires assessment. A study conducted by Pasipanodya et al. in the United States showed that 59% of TB treated patients had abnormal pulmonary function. More than half of the treated PTB patients evolved to have significantly impaired lung function33
Symptom presence is not a specific and sensitive indicator of airflow limitation, and using a symptom questionnaire is an ineffective method of identifying lung impairment34 Spirometry remains the gold standard for identifying airflow limitation.
SPIROMETRY
The pattern of lung function impairment observed in the study was mostly mixed (38.8%) followed by obstructive (23.5%) and restrictive pattern (22.4%).
Almost the whole of the Respiratory system including lung parenchyma, bronchi, bronchioles and lymph nodes were involved in PTB. Dysregulation of protease control and upregulation of proteases like matrix metalloproteinases causes lung remodeling. Histopathological abnormalities can occur after successful treatment causing sequelae like cavity formation, fibrosis, bronchiectasis and bronchial & bronchiolar obstruction etc. Sequelae changes can cause restrictive, obstructive or mixed pulmonary function impairment.7
A study conducted by Agarwala et al. in India observed that 52.7% of PTB treated patients had an obstructive defect on spirometry.35
Brashier et al. conducted a study in India which reported a prevalence of 46% for airflow obstruction which also increased with the duration post treatment completion36
Most of the studies have shown that pulmonary TB sequelae patients have obstructive pattern of lung impairment. But more recent studies have revealed that mixed and restrictive patterns were more common compared to obstructive pattern7
A Korean study observed a mean decline in FEV1 of 38.2±8 mL/year in cured post TB patients which was consistent with decline in FEV1 of 33±2 mL/year in COPD patients without PTB.37
A study by Eun Jo Lee et al observed that there is negative correlation between FEV1 and FVC values and the intensity of lung damage, ie more the severity of sequelae, more severe will be the fall in FEV1 and FVC38
A study by Venkateshiah et al noted the versatility of FEV1 and FVC values in assessing the intensity of lung destruction. According to them, FEV1 and FVC values decline as severity of lung damage increases.39
FEV1 decline may be connected to multiple underlying pathological processes. Cavitation may distort or obliterate airways, leading to airway obstruction. Patients with lung cavities had considerably lower baseline FEV1 and at 1 month after start of treatment compared to patients with no cavities.40
Furthermore, loss of muscular and elastic bronchial wall components results in bronchiectasis, which causes associated airflow obstruction. This was observed more frequently in TB patients with cavities (64%) compared to those without cavities (11%)41
Bronchogenic spread is also a hallmark of pulmonary TB, during which, caseous material is released into the bronchial lumen and walls during cavitary disintegration.42-45
Even though post TB airflow obstruction received the greatest attention, Restrictive ventilatory defects/mixed pattern were the most common form of ventilatory abnormality in a review of studies conducted in South Africa.46 The severity of Restrictive ventilatory pattern was evaluated based on FVC value.30
Luiz Carlos D’Aquino et al observed that interpretation of Restrictive pattern can be made more accurately by integrating the degree of FVC reduction and increased ratio.47
The severity varied from mild to severe restriction in the present study, the most common being mild restriction.
Aberrant lung tissue repair causing lung structural changes (e.g. fibrotic bands, bronchovascular distortion and pleural thickening) 37, 48, 49 can explain restriction in post PTB patients
Eric Walter PefuraYone et al proposed that MEF 25-75% <65% can be useful in determining small airway obstruction in post PTB patients who had a normal FEV1/FVC ratio (restrictive/mixed ventilatory abnormality). Persisting chronic respiratory signs following TB treatment are very common and they are frequently associated with small airway obstruction50
The pathogenesis involved in small airway obstruction is fibrotic destruction of
parenchyma which is surrounding the small airways causing loss of
airway radial traction thereby causing narrowing and distortion of airways.51
During the early course of bronchiectasis, patients had obstructive disorder and small airways were particularly involved52
In our study, few subjects (12 out of 85) had a normal spirometry pattern.
This may be because lung parenchyma and airways were less extensively involved due to early diagnosis, treatment. According to a study by Mohammed Saleh Al Hajjaj, the process of healing continues even after completion of TB treatment. This may be another reason for the normal spirometry pattern observed in some patients.31
DLCO
In our study, majority of the subjects had mild DLCO followed by moderate and severe DLCO and very few subjects had a normal DLCO. There was a significant negative correlation between lag time, duration after treatment, number of PTB episodes and DLCO.
A study showed that at the time of diagnosis, patients with non- cavitary disease had virtually normal lung function, while patients with cavitary disease had very mild restriction (DLco averaging 85% of predicted, respectively) and trivial hypoxemia (PaO2 averaging 85 mm Hg)49
In 1963, Marcus et al reported an average vital capacity of 67% and an average DLco of 63% of predicted in a group of 73 patients with cavitary disease (with 94 cavities ) and 27 patients with non-cavitary disease53
In a study, DLCO of the Post TB Emphysema group decreased significantly. There was also an approximately 1 L difference in alveolar volume, residual volume, functional residual capacity and total lung capacity between the two groups. These findings can be interpreted as patients with Post TB Emphysema have a significant lower diffusing area, and they suggest that the ‘sclerosed’ portions of the lung have both low ventilation and low perfusion under baseline conditions. After exercise, the impairment of gas exchange was significantly aggravated54
DLCO decrease is due to the reduction in gas exchange surface area following alveolitis or “lipoid pneumonia”, occurring during cavitation. DLCO improves with treatment, but few individuals have everlasting impairment of diffusion and ventilation/perfusion mismatch, causing chronic hypoxaemia49,54
LAG TIME:
The Onset of Symptom. It is the day when the patient first becomes aware of the symptom or symptoms.
Patient Delay. Patient delay is the time interval from the first onset of symptom to the first visit to health center or hospital or private clinics, and time longer than 21 days was considered indicative of patient delay
Health Care System Delay. Health care system delay is the time interval from the patient’s first visit to the health center, hospital, or private clinic to the commencement of treatment, and time longer than 7 days is considered indicative of patient delay
Total Treatment Delay. Total treatment delay is the time interval from the first onset of symptom to the commencement of treatment or the sum of patient delay and health care system delay55-58
Onset of symptom Contact to health facility Commencement of treatment
Patient Delay Healthcare Delay Total Treatment Delay
After investigating the factors associated to lung function impairment, this study found that increase in time spent between the onset of disease symptoms and the diagnosis of TB was independently associated with lung function impairment. A negative correlation was observed between lag time and lung function parameters.
Minimum lag time in our study was 20 days and maximum was 90 days. The mean duration of lag time in our study was 53 days (â?7 to 8 weeks).
It is estimated that a patient with untreated smear-positive pulmonary TB may infect on average more than 10 persons per year. The treatment delay affects an individual, the community, and a country’s health and economy.59
Lag time also increases the extent of lung damage which in turn results in increased incidence of developing multi-drug resistant strains (MDR-TB).
Ngahane et al., found that the median duration of symptoms prior to TB diagnosis was greater in patients who were found to have lung function impairment (LFI) than in patients without LFI. The median duration of symptoms before the diagnosis of TB was 4 weeks (3 – 8). This duration was significantly higher in patients who had LFI (median 8) than that of patients without any LFI (median 4)60
In another study, which had information collected from the TB registry during December 2012 to May 2013 showed that the mean lag period for PTB was 7.05 ± 6.16 weeks. Delay in diagnosis of smear positive patients has adverse impact on the general population as during this period a patient can infect the community. This in turn affects the prevalence of TB locally and globally.61
A study by Bello et al. showed that the mean total delay was 87.6 days and mean health system delay was 39.3 days.62
Cause for delay in our study may be that most of the subjects who participated had a lack of adequate health education and they were from rural villages in a poor socioeconomical status which hampers them to seek medical aid as quickly as possible. This increased Lag time may be the cause for extensive pulmonary function derangement resulting in restrictive or mixed pattern.
According to Muniyandi et al., factors for longer delay included individual and provider/system levels. Reported individual-level barriers included physical, financial, stigma, health literacy and socio- cultural factors such as living habits and religious belief.26 These findings are in agreement with previous reports49,63
According to WHO, TB-associated stigma was one of the major factors associated with a patient delay. Patients with a high level of TB-associated stigma were more likely to delay when compared to the patients with low TB-associated stigma. TB-associated stigma may have played an important role in hindering patients from seeking early health care due to fear of being diagnosed with TB64
Adenager et al. found that immunosuppressive diseases like HIV often produce atypical presentations of TB which weakens the clinician’s and patient’s ability to notice TB, which in turn results in diagnostic delay65
But, in our study, we didn’t have any HIV infected subjects. Hence, HIV was not taken into consideration just like smoking.
IMPACT OF LAG TIME ON LUNG FUNCTIONS
In our study, FVC decreased by 0.81 %, FEV1 decreased by 0.98 %, FEV1/FVC ratio decreased by 0.31 %, MEF 25-75% decreased by 0.9
%, DLCO decreased by 0.58 % for each day delay.
Renata Báez-Saldaña et al showed that for each day delay in starting of treatment there is a fall of 0.01 to 0.07% in spirometry parameters.63 This shows that even a single day delay in starting treatment can have a huge impact.
Even though TB is a notifiable disease and comes under the National Tuberculosis Elimination Program, there is still a mean delay of 53 days. In addition to patient delay, healthcare delay is an important factor which has to considered. Improper management and delayed evaluation by the General Practitioners, improper or lack of health education to the public regarding tuberculosis may also be causes for delay in initiation of treatment. General Practitioners should have a high level of suspicion for diagnosing tuberculosis and aggressive screening should be carried out for any patient with symptoms and signs of tuberculosis (for eg. Cough with or without expectoration for > 2 weeks). Early identification and referral to a Specialist, when essential, is to be carried out by the General Practitioners. In our study, we haven’t studied these factors responsible for diagnostic and treatment delay.
A study by Yodi Mahendradhata et al. showed that although General Practitioners were well educated during their course of training and with continued medical education, significant delay in diagnosis occurred at their level. Continuous awareness and high degree of suspicion of TB is essential among the General Practitioners and the Doctors in Private Hospitals66
IMPACT OF DURATION AFTER TREATMENT ON LUNG FUNCTIONS
In our study more than half of the subjects 48 (54.2%) belonged to the 7 to 16 year time duration. The mean duration after treatment in our study was 15.45 years. Minimum duration was 2 years and maximum duration was 42 years. FVC decreased by 0.22 times, FEV1 decreased by 0.45 times, FEV1/FVC ratio decreased by 0.49 times, MEF 25-75% decreased by 1.05 times and DLCO decreased by 0.89 times for each year increase in duration after treatment.
Vargha .G et al also confirmed this in a fifteen year follow up study where there is considerable annual lung function decline even after completion of treatment. There was a fall in FEV1 of 35.3 mL/year within 15 years of completion of anti-tuberculosis treatment 67
In the present study, negative correlation was observed between FEV1/FVC ratio, MEF 25-75% and DLCO but there was no significant negative correlation between FEV1, FVC and duration after treatment. Studies also had controversial statements along with discrepancies between studies regarding the correlation between duration post ATT completion and lung function decline. Since this was a cross sectional study, follow up of patients could not be carried out.
The median interval between the end of anti-tuberculosis treatment and the pulmonary function test was 16 months. Some studies say that the derangement of pulmonary function impairment occurs at around 18 months after completion of treatment and as duration after treatment increases thereafter the severity of damage also increases. Few studies say that lung function falls until 18 months after treatment and then stabilizes. In this study by Chung et al, the estimated monthly reduction in FEV1 was 18.83 mL 8
At 1 year, 19.3% (59/305) and 14.1% (43/305) of patients had experienced fall in FEV1 or FVC of ≥100 mL respectively. The lowest spirometry volumes at 1 year were seen in those with the most extensive parenchymal pathology or bronchiectasis on HRCT, or the presence of symptoms at TB treatment completion68
IMPACT OF NUMBER OF PTB EPISODES ON LUNG FUNCTIONS
In our study, there was Negative correlation between spirometry parameters and number of episodes of ATT.
In our study, Restriction and Mixed pattern were found to be predominant among recurrent cases compared to new cases and Obstructive pattern was predominant among new cases. Majority of recurrent cases had severe restriction compared to new cases.
One or several active TB episodes may precede the development of respiratory symptoms and chronic airflow obstruction. This is more likely post extensive and severe infection, radiologically involving large and multiple areas of lung parenchyma69
A study by Eva Hnizdo et al showed that the increase in loss of lung function was associated with increase in number of episodes of treatment.17
This is consistent with our study as increase in PTB episodes were associated with fall in spirometry parameters and DLCO. Mean values of FEV1, FVC, FEV1/FVC ratio and MEF 25-75% were found to be lower among recurrent cases than new cases. DLCO decreased by
15.17 times for every single increase in the number of ATT courses.
This shows that prevention of Tuberculosis and regular follow up of patients should be carried out for early identification and initiation of treatment.
A study done in South Africa, showed that approximately 27,000 miners showed cumulative rise in abnormalities of lung function with increase in number of episodes of TB (18%, 27% and 35 % among 1st, 2nd and 3rd time sufferers respectively). FEV1 decreased by a mean value of 180 ml in patients who had one episode, 362 ml in those who had two episodes, 462 ml in those who had three episodes, and 964 ml in those who had four or more episodes of tuberculosis.70
This is similar to our study where mean FVC among new and recurrent cases was 80.03 and 53.58 %, FEV1 was 72.74 and 51.33 %,
FEV1/FVC ratio was 73.89 and 69.25, MEF 25-75% was 48.97 and 33% respectively.
In a study by Chushkin et al., values for FEV1 and FEV1/FVC ratio were compellingly lower in patients with history of two or more
treatment episodes for PTB compared to patients who had only one episode. FEV1, FVC and FEV1/FVC percentage predicted decreased by 16.83%, 12.28% and 7.70 respectively.18
Patients who were diagnosed with recurrent pulmonary tuberculosis had a 2.8 fold higher risk of having lower lung function parameters post treatment in comparison to new cases16
Studies conducted in other regions of the world also show that recurrent tuberculosis is associated with worse spirometry outcomes. A population wide study done in Korea by Lee and colleagues showed that patients with previous TB had a 3 fold chance of having obstructive airway disease71
CONCLUSION
PTB causes lung function impairment even after treatment. Lag time, Duration after Treatment and Number of PTB episodes play a significant role in the degree of decline in lung function.
Spirometry is a simple, and easily reproducible, subjective test which should be utilized for follow up in post PTB patients. Significant lung function decline has been observed in patients after the first decade of treatment completion. Spirometry should be performed in all treated PTB patients as a screening tool to monitor the decline in lung function.
Recurrent PTB will contribute to significant decline in lung function especially on the severity of restrictive lung disease based on FVC and DLCO. Early identification and treatment of PTB patients goes a long way in preventing development of recurrent TB and MDR-TB.
On conclusion, “The completion of Tuberculosis therapy is only the beginning of TB illness.”
References
- Rosenblatt MB. Pulmonary tuberculosis: evolution of modern therapy. Bulletin of the New York Academy of Medicine. 1973 Mar;49(3):163.
- Daniel TM, Bates JH, Downes KA. History of tuberculosis. Tuberculosis: pathogenesis, protection, and control. 1994 May 16:13-24.
- Adigun R, Singh R. Tuberculosis.
- Realini L, De Ridder K, Palomino JC, Hirschel B, Portaels F. Microaerophilic Conditions Promote Growth of Mycobacterium genavense. Journal of clinical microbiology. 1998 Sep 1;36(9):2565-70.
- World Health Organization. Global tuberculosis report 2020. World Health Organization; 2020.
- Singh B, Chaudhary O. Trends of pulmonary impairment in persons with treated pulmonary tuberculosis. Int J Med Res Prof. 2015;1(1):8-11.
- Guliani A, Bhalotra B, Parakh U, Jain N. Even asymptomatic patients may have serious lung function impairment after successful Tb treatment. InB52. TUBERCULOSIS: TREATMENT 2014 May (pp. A3203-A3203). American Thoracic Society
- Chung KP, Chen JY, Lee CH, Wu HD, Wang JY, Lee LN, Yu CJ, Yang PC. Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics. 2011;66(4):549-56.
- Ranu H, Wilde M, Madden B. Pulmonary function tests. The Ulster medical journal. 2011 May;80(2):84
- Menon B, Nima G, Dogra V, Jha S. Evaluation of the radiological sequelae after treatment completion in new cases of pulmonary, pleural, and mediastinal tuberculosis. Lung India: Official Organ of Indian Chest Society. 2015 May;32(3):241
- Parveen Bobby K. Functional evaluation in Pulmonary Tuberculosis Sequelae patients in a tertiary care centre, Chengalpattu (Doctoral dissertation, Chengalpattu Medical College, Chengalpattu)
- Ramos LM, Sulmonett N, Ferreira CS, Henriques JF, Miranda SS. Functional profile of patients with tuberculosis sequelae in a university hospital. Jornal
- Banu Rekha VV, Ramachandran R, Kuppurao KV, Rahman F, Adhilakshmi AR, Kalaiselvi D, Murugesan P, Sundaram V, Narayanan PR. Assessment of long term status of sputum positive pulmonary TB patients successfully treated with short course chemotherapy. Indian Journal of Tuberculosis. 2009;56(3):132-4
- Singla N, Singla R, Fernandes S, Behera D. Post treatment sequelae of multi- drug resistant tuberculosis patients. Indian J Tuberc. 2009 Oct;56(4):206-12
- Verma SK, Kumar S, Narayan KV, Sodhi R. Post tubercular obstructive airway impairment. Indian J Allergy Asthma Immunol. 2009 Jul;23(2):95-
- Brasileiro de Pneumologia. 2006 Feb;32(1):43-7.
- Manji M, Shayo G, Mamuya S, Mpembeni R, Jusabani A, Mugusi F. Lung functions among patients with pulmonary tuberculosis in Dar es Salaam–a cross-sectional study. BMC pulmonary medicine. 2016 Dec;16(1):58.
- Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000 Jan 1;55(1):32-8.
- Chushkin MI, Ots ON. Impaired pulmonary function after treatment for tuberculosis: the end of the disease?. Jornal Brasileiro de Pneumologia. 2017 Feb;43(1):38-43.
- Jung KH, Kim SJ, Shin C, Kim JH. The considerable, often neglected, impact of pulmonary tuberculosis on the prevalence of COPD. American journal of respiratory and critical care medicine. 2008 Aug 15;178(4):431.
- Kolb M, Collard HR. Staging of idiopathic pulmonary fibrosis: past, present and future. European Respiratory Review. 2014 Jun 1;23(132):220-4.
- Sailaja K, Rao HN. Study of pulmonary function impairment by spirometry in post pulmonary tuberculosis. Tuberculosis. 2015 May 25;4(42):25
- Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. American Review of Respiratory Disease. 1971 May;103(5):625-40.
- Filley EA, Bull HA, Dowd PM, Rook GA. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumour necrosis factor. Immunology. 1992 Dec;77(4):505
- Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. European respiratory journal. 2011 Jan 1:erj00154-2011.
- Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. The Journal of infectious diseases. 2005 Oct 1;192(7):1201-10.
- Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, Feldman C. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. European Respiratory Journal. 1998 Aug 1;12(2):351-6.
- Muniyandi M, Rajeswari R, Balasubramanian R, Nirupa C, Gopi PG, Jaggarajamma K, Sheela F, Narayanan PR. Evaluation of post-treatment health-related quality of life (HRQoL) among tuberculosis patients. The International Journal of Tuberculosis and Lung Disease. 2007 Aug 1;11(8):887-92
- Global Tuberculosis report 2014 – www.who.int/tb/publications/globalreport/ en
- Dr.Jyotsna joshi .Spirometry in practice . A real life approach. Mumbai. cipla ltd
- Al-Hajjaj MS. Predictive factors of poor lung function in cured tuberculosis patients. Bahrain Med Bull. 2002 Mar;24(1):19-22.
- Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006 Mar 1;61(3):259-66.
- Pasipanodya JG, Miller et al. Pulmonary Impairment after Tuberculosis. Chest 2007 jun ; 131 (6): 1817- 24
- www.who.int/tb/publications/globalreport/
- Agarwala A, Maikap MK, Panchadhyayee P, Mandal P, Roy PP. Chronic airway obstruction in post tubercular fibrosis cases: A serious lung function changes. Int J Res Med Sci 2016;4:5294-6
- Brashier B, Gangavane S, Valsa S, Gaikwad SN, Ghorpade SV, Mandrekar S, et al. Almost Half the Patients Treated for Pulmonary Tuberculosis (TB) Show Evidence of Obstructive Airways Disease (OAD). In: European Respiratory Society Annual Congress, Stockholm, Sweden; 15-19 September, 2007. [Abstr. E2585]
- Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013; 17: 67–75
- Eun Joo Lee, Sang Yeub Lee et al. Routine Pulmonary Function test can estimate the extent of tuberculous destroyed lung. Scientific World Journal 2012: 835031
- Venkateshiah SB et al. The utility of Spirometry in diagnosing Pulmonary restriction. Lung 2008; 186(1) : 19-25
- R. Long, B. Maycher, A. Dhar, J. Mafreda, E. Hershfield, N. Anthonisen Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function Chest, 113 (April (4)) (1998), pp. 933-943
- Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax 2000; 55: 198–204
- Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb) 2011; 91: 497–509
- Hatipoglu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 1996; 51: 397–402.
- Murata K, Itoh H, Todo G, et al. Centrilobular lesions of the lung: demonstration by high-resolution CT and pathologic correlation. Radiology 1986; 161: 641– 645
- Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings – early active disease and sequential change with antituberculous therapy. Radiology 1993; 186: 653–660
- Ehrlich RI, Adams S, Baatjies R, et al. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis 2011; 15: 886–891
- D’Aquino LC, Rodrigues SC, De Barros JA, Rubin AS, Rosário Filho NA, de Castro Pereira CA. Predicting reduced TLC in patients with low FVC and a normal or elevated FEV. J Bras Pneumol. 2010;36(4):460-7.
- Seiscento M, Vargas FS, Antonangelo L, et al. Transforming growth factor β-1 as a predictor of fibrosis in tuberculous pleurisy. Respirology 2007; 12: 660–663
- R. Long, B. Maycher, A. Dhar, J. Mafreda, E. Hershfield, N. Anthonisen Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function Chest, 113 (April (4)) (1998), pp. 933-943
- Pefura-Yone EW, Kengne AP, Tagne-Kamdem PE, Afane-Ze E. Clinical significance of low forced expiratory flow between 25% and 75% of vital capacity following treated pulmonary tuberculosis: a cross-sectional study. BMJ open. 2014 Jul 1;4(7).
- Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiological reviews.
- Hamutcu R, Rowland JM, Horn MV, Kaminsky C, MacLaughlin EF, Starnes VA, Woo MS. Clinical findings and lung pathology in children with cystic fibrosis. American journal of respiratory and critical care medicine. 2002 Apr 15;165(8):1172-5.
- Marcus H, Yoo OH, Akyol T, Williams Jr MH. A randomized study of the effects of corticosteroid therapy on healing of pulmonary tuberculosis as judged by clinical, roentgenographic, and physiologic measurements. American Review of Respiratory Disease. 1963 Jul;88(1):55-64.
- Kim MA, Kim SH, Zo JH, et al. Right heart dysfunction in post-tuberculosis emphysema. Int J Tuberc Lung Dis 2004; 8: 1120–1126
- Tegegn A, Yazachew M. Delays in tuberculosis treatment and associated factors in Jimma Zone, Southwest Ethiopia. Ethiopian Journal of Health Sciences. 2009;19(1).diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC infectious diseases. 2009 Dec 1;9(1):91.
- Schneider D, McNabb SJ, Safaryan M, Davidyants V, Niazyan L, Orbelyan S. Reasons for delay in seeking care for tuberculosis, Republic of Armenia, 2006–2007. Interdisciplinary perspectives on infectious diseases. 2010 Jan 1;2010.
- Lambert ML, Van Der Stuyft P. Delays to tuberculosis treatment: shall we continue to blame the victim?
- World Health Organisation (WHO), Global Tuberculosis Control, World Health Organisation, Geneva, Switzerland, 2011
- Ngahane BH, Nouyep J, Motto MN, Njankouo YM, Wandji A, Endale M, Ze EA. Post-tuberculous lung function impairment in a tuberculosis reference clinic in Cameroon. Respiratory medicine. 2016 May 1;114:67-71.
- Madegedara D, Nandadeva D, Dhanasekera S, Kumara H. Lag period for diagnosing and starting treatment for pulmonary tuberculosis patients. European Respiratory Journal. 2014 Sep 1;44(Suppl 58).
- Bello S, Afolabi RF, Ajayi DT, Sharma T, Owoeye DO, Oduyoye O, Jasanya J. Empirical evidence of delays in diagnosis and treatment of pulmonary tuberculosis: systematic review and meta-regression analysis. BMC public health. 2019 Dec;19(1):820.
- Baez-Saldana R., Lopez-Arteaga Y., Bizarron-Muro A., Ferreira-Guerrero E., Ferreyra-Reyes L., Delgado-Sanchez G. et al. A novel scoring system to measure radiographic abnormalities and related spirometric values in cured pulmonary tuberculosis. PLoS One. 2013; 8: e78926
- World Health Organization. Diagnostic and treatment delay in tuberculosis. 2006.
- Adenager GS, Alemseged F, Asefa H, Gebremedhin AT. Factors associated with treatment delay among pulmonary tuberculosis patients in public and private health facilities in Addis Ababa, Ethiopia. Tuberculosis research and treatment. 2017 Feb 27;2017.
- Mahendradhata Y, Lestari T, Probandari A, Indriarini LE, Burhan E, Mustikawati D, Utarini A. How do private general practitioners manage tuberculosis cases? A survey in eight cities in Indonesia. BMC research notes. 2015 Dec 1;8(1):564.
- Vargha.G et al. Fifteen year follow up of lung function in obstructive and nonobstructive pulmonary tuberculosis. Acta med hung 1983; 40 (4) :271-6
- Meghji J, Lesosky M, Joekes E, Banda P, Rylance J, Gordon S, Jacob J, Zonderland H, MacPherson P, Corbett EL, Mortimer K. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax. 2020 Mar 1;75(3):269-78.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi