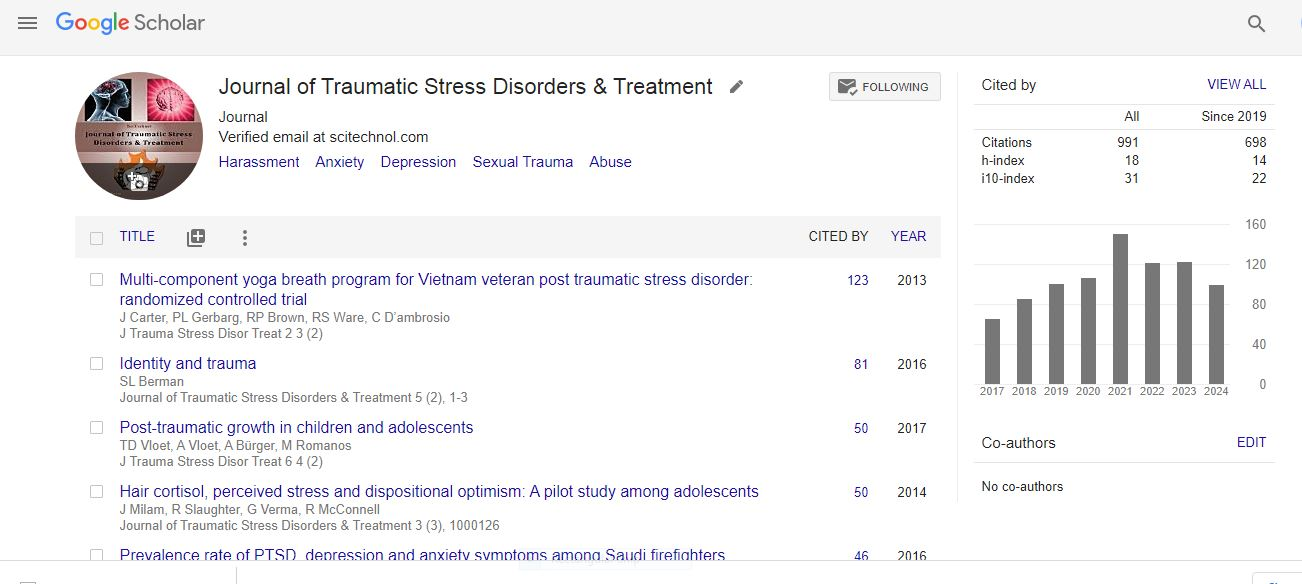
Research Article, J Trauma Stress Disor Treat Vol: 7 Issue: 1
A Double Blind, Placebo Controlled, Clinical Study to Evaluate the Efficacy and Safety of StressCare Capsules
Palaniyamma D1*, Debarati Bhar2 and Sushma C1
1Department of Clinical trial and Medical Services, The Himalaya Drug Company, India
2Kolkata Nursing Home, Kolkata, India
*Corresponding Author : Palaniyamma D, MBBS, MD
Medical Advisor, Department of Clinical trial and Medical Services, R&D, The Himalaya Drug Company, Makali, Bangalore – 562162, India
Tel: 91-80-67549920
E-mail: dr.palani@himalayawellness.com
Received: February 07, 2018 Accepted: February 24, 2018 Published: March 03, 2018
Citation: Palaniyamma D, Bhar D, Sushma C (2018) A Double Blind, Placebo Controlled, Clinical Study to Evaluate the Efficacy and Safety of StressCare Capsules. J Trauma Stress Disor Treat 7:1. doi: 10.4172/2324-8947.1000181
Abstract
Stress is a multifactorial stimulus that elicits responses at emotional, cognitive, behavioral and also at physiological levels. Thus the approach to manage stress should also be multifactorial. Among the herbal approaches, traditionally polyherbal formulations are used in Indian medicine. Present study on StressCare capsules was a Double Blind, Placebo Controlled, Clinical Study to evaluate its Efficacy and Safety in 100 volunteers presenting with clinical symptoms of stress and irritability. On entry, a detailed medical history was obtained to ascertain the presence of anxiousness followed by complete physical and psychological examination. All the people were randomly divided into StressCare and placebo groups, and people from both the groups received either StressCare or placebo for a period of 8 weeks, in a dose of 2 capsules twice daily, orally. Those involved in the study were evaluated clinically on entry at 4 weeks and at end of 8 weeks. All the subjects were clinically evaluated at regular intervals and statistical analysis was performed between the groups. Study results indicate that StressCare is safe and effective in the management of mild occasional stress and irritability as compared to placebo.
Keywords: Stress and Stress Related Condition; StressCare; Placebo
Introduction
Stress is a common buzz word in the modern lifestyle and related health concerns. When people reach out for help, they are often dealing with circumstances, situations, and stressors in their lives that leave them feeling emotionally and physically overwhelmed. This is because people feel that their resources or skills are not up to the mark to deal with the high levels of stress they are experiencing. Dr. Walter B. Cannon, a physiologist described the meaning of “stress” in terms of “fight or flight response” as a series of involuntary physiological and biochemical changes that prepare you to deal with threats of danger. This response was critical to the survival of primitive humankind when requiring quick bursts of energy to fight or flee predators such as the saber-toothed tiger [1].
Basically, stress is defined in at least three ways as per the context. First definition states that more the stress or stimulus, the bearer either human or other physical thing, will succumb to it. When the stimulus is too great, the collapse becomes inevitable. This is in line with Oxford dictionary’s definition of stress; “to subject (a material thing, a bodily organ, a mental faculty) to stress or strain; to overwork, fatigue.”
Selye (1956) gave a physiological response based definition of stress. It measured stress in terms of body’s stage wise response to stress from creating an internal alert till stage of exhaustion in health. This duration of stages gives the scope for handling the stress accordingly [2].
Third definition of stress proposes a comprehensive explanation including stimulus based and response based definitions and regarding stress as a dynamic process of both. Lazaraus and Folksman (1984) advocated this definition which emphasizes the involvement of cognitive, emotional and behavioral factors [3].
Thus, Definitions of stress provide only a snapshot of this dynamic process. In the Encyclopaedic Dictionary of Psychology this dynamic process is defined as “the way in which people realize and identify their problems, how they react to them and attempt to cope with them and the ‘cost’ of doing so” (Harre and Lamb) [4,5].
Although some stress is a natural and inevitable part of our lives, feeling burdened or unable to cope can be problematic and can affect the mental and physical well-being [6].
Constantly being exposed to stressful situations can be overstimulating and if we are constantly feeling stressed, we may begin to feel unable to manage the problems at hand. In order to avoid situations in which we feel “overloaded,” we must first identify what stresses us, what our threshold for stress is, and how we can most effectively manage stressful situations. No one event, regardless of how traumatic, can be detrimental to health [7]. Among various methods and techniques suggested for minimizing the effects of stress to individuals, some noteworthy are yoga, exercises, behavioral therapies, relaxation techniques, deep breathing, massages and others. These are non-drug therapies aimed to relieve stress. Alternative measures also include herbal preparations which are traditionally known to be helpful.
StressCare capsule is a polyherbal formulation that might help in coping to stressful conditions in healthy individuals.
Material and Methods
The study was aimed to evaluate the safety and efficacy of Stress Care Capsule in 100 individuals presenting with mild occasional Stress and irritability related parameters like loss of appetite, indigestion, weakness, occasional stress, fatigue, mood swings, occasional sleep disturbances, and occasional bowel disturbances in a prospective double blind placebo controlled study design. This study was conducted at Kolkata Nursing Home, Aatghara, Rajarhat Road, Rajarhat Gopalpur, Kolkata.
Study Procedure
Subjects who are above 18 years of age group were selected as per the subject selection criteria. Subjects with severe psychiatric disorder, pregnant and breastfeeding women, individuals with severe Hepatic/ cardiac/ mental disorder, subjects with history of alcohol or smoking abuse were excluded from the study. Individuals unwilling to provide informed consent or abide by the requirements of the study were also excluded from the study. On admission into the study, informed consent was obtained from subjects after explaining the nature of the study. The subjects were free to opt out from the study, if they desired so. The subjects were randomly assigned in two groups, A and B. Subjects in Group A received StressCare capsule in the dosage of Two capsule twice a day with meals for 8 weeks while the subjects in Group B received identical placebo in the same dosage. No other supplements were administered to these subjects.
Demographic data of subjects on entry is given in the Table 1. All the subjects were evaluated at the entry, end of Week 4 and end of Week 8. Detailed clinical information was collected from each subject about stress and related health concerns. General physical examination, systemic examination and laboratory investigations were recorded on a Case Report Form at all intervals. Clinical symptoms were assessed as per the scoring i.e., Nil (0), Mild(1), Moderate(2) and Severe(3) and overall clinical assessment was defined as cured, improved and unchanged. Hematological parameters like Hemoglobin, Total count, Differential count, ESR, Platelet count, Biochemical parameters like Random blood sugar, SGPT and Serum creatinine were carried out at entry and at the end of the study.
| Demographic Data | StressCare | Placebo |
|---|---|---|
| Number of Subject | 50 | 50 |
| Gender (Male: Female) | (30:20) | (30:20) |
| Age (Mean ± SD) in Years | 33.62 ± 10.64 | 34.78 ± 14.58 |
| Weights in Kgs | 54.88 ± 8.05 | 60.06 ± 10.06 |
| Diet (Veg: Non Veg) | (6:44) | (7:43) |
| Smoking (Yes: No) | (22:28) | (5:45) |
| Alcohol (Yes: No) | (5:45) | (5:45) |
Table 1: Demographic Data of Subjects on Entry (Mean ± SD).
Adverse events were to be reported or observed by the subjects with the information about the severity, date of onset, duration and the action taken. Relation of the adverse events to study interventions was predefined as certain, probable, possible and unlikely. For subjects withdrawing from the study, efforts were made to ascertain the reason for dropout. Non-compliance (defined as failure to take less than 80% of the intervention) was not regarded as intervention failure but the reason for non- compliance were noted.
Primary Endpoint
The primary endpoint was defined as overall clinical improvement in stress and irritability related condition like loss of appetite, indigestion, weakness, occasional stress, fatigue, mood swings, occasional sleep disturbances, occasional bowel disturbances, and sense of well being.
Statistical Analysis
Statistical analysis performed between the groups using Mann Whitney test for all parameters except for weight gain which was analysed using unpaired t-test. For blood pressure, hematological and biochemical parameters, analysis was performed using paired t test. Statistical analyses were performed using Graphpad Prism software Version 6.07, San Diego, California, USA.
Results
Effect of StressCare on scoring for general wellbeing is shown in the Table 2. Mild Indigestion at the baseline was 0.80 ± 1.01, and improved to 0.50 ± 0.79 at the end of Week 4 and further improved to 0.24 ± 0.43 at the end of Week 8 whereas in placebo group, the score at entry was 0.96 ± 1.28, 0.84 ± 1.22 at the end of Week 4 and 0.66 ± 1.04 at the end of Week 8. Between the group analysis has shown the significance at the end of the study with p<0.0334 in StressCare group.
| Parameter | Duration | StressCare | Placebo | Significance |
|---|---|---|---|---|
| Occasional Loss of Appetite | At Entry | 1.28 1.49 | 0.80±1.23 | ns |
| Week 4 | 0.76 ± 1.08 | 0.70 ± 1.15 | ns | |
| Week 8 | 0.48 ± 0.79 | 0.54 ± 0.99 | ns | |
| Mild Indigestion | At Entry | 0.80 ± 1.01 | 0.96 ± 1.28 | ns |
| Week 4 | 0.50 ± 0.79 | 0.84 ± 1.22 | ns | |
| Week 8 | 0.24 ± 0.43 | 0.66 ± 1.04 | P < 0.0334 | |
| Occasional Weakness | At Entry | 1.77 ± 1.27 | 1.32 ± 1.19 | ns |
| Week 4 | 1.02 ± 1.10 | 1.06 ± 1.02 | ns | |
| Week 8 | 0.58 ± 0.71 | 0.78 ± 0.82 | ns | |
| Occasional stress | At Entry | 1.20 ± 1.32 | 1.44 ±1.01 | ns |
| Week 4 | 0.57 ± 0.94 | 1.42 ± 1.11 | P < 0.0001 | |
| Week 8 | 0.45 ± 0.87 | 1.22 ± 1.09 | P < 0.0001 | |
| Mild Fatigue | At Entry | 2.33 ±1.38 | 1.85 ± 1.12 | ns |
| Week 4 | 1.68 ± 1.13 | 1.40 ± 1.07 | ns | |
| Week 8 | 1.10 ± 0.86 | 1.16 ± 0.96 | ns | |
| Mild Mood Swings | At Entry | 1.30 ± 1.55 | 1.77 ± 1.24 | ns |
| Week 4 | 0.92 ±1.29 | 1.78 ± 1.28 | P < 0.0001 | |
| Week 8 | 0.44 ± 0.70 | 1.52 ± 1.28 | P < 0.0001 | |
| Occasional Sleep disturbances | At Entry | 1.84 ±1.49 | 1.50 ± 1.30 | ns |
| Week 4 | 1.12 ± 1.30 | 1.28 ± 1.16 | ns | |
| Week 8 | 0.68 ± 0.98 | 0.94 ± 0.96 | ns | |
| Occasional Bowel Disturbances | At Entry | 0.58 ± 0.61 | 0.88 ± 0.87 | ns |
| Week4 | 0.52 ± 0.61 | 0.98 ±1.06 | P < 0.025 | |
| Week8 | 0.44 ± 0.61 | 0.84 ±1.06 | ns | |
| Sense of Wellbeing | At Entry | 0.24 ± 0.43 | 0.12 ± 0.48 | ns |
| Week4 | 0.22 ± 0.42 | 0.20 ± 0.53 | ns | |
| Week8 | 0.22 ± 0.42 | 0.24 ± 0.56 | ns |
Statistical analysis performed using Mann Whitney test for all parameters except for weight gain which was analyzed using unpaired t-test. Analysis for between the groups
Table 2: Effect of StressCare on Scoring for General Wellbeing.
Occasional stress was scored 1.20 ± 1.32 at the baseline that reduced to 0.57 ± 0.94 at the end of Week 4 and further reduced to 0.45 ± 0.87 at the end of Week 8 in the StressCare group. In the placebo group, initial score at the baseline was 1.44 ± 1.01, at the end of Week 4 was 1.42 ± 1.11, and 1.22 ± 1.09 at the end of Week 8. Between the group, analysis has shown statistical significance of p<0.0001 at the end of Week 4 and at the end of Week 8 in StressCare group as compared to placebo.
Mood Swings in the StressCare group at the baseline was 1.30 ± 1.55, at the end of Week 4 was 0.92 ± 1.29 and at the end of Week 8 was 0.44 ± 0.70. In placebo group, at baseline it was 1.77 ± 1.24, at the end of Week 4 was 1.78 ± 1.28 and at the end of Week 8 was 1.52 ± 1.28. Between the group analysis has shown a significance of p<0.0001 to StressCare Group over placebo group at the end of the study.
Occasional bowel disturbances was scored 0.58 ± 0.61, at the baseline, reduced to 0.52 ± 0.61 at the end of Week 4 and further reduced to 0.44 ± 0.61 at the end of Week 8 in the StressCare group. In the placebo group, initial score at the baseline was 0.88 ± 0.87, at the end of Week 4 was 0.98 ± 1.06, and 0.84 ± 1.06 at the end of Week 8. Between the group, analysis has shown statistical significance of p<0.025 at the end of Week 4 in StressCare group as compared to placebo. Other parameters like occasional loss of appetite, occasional weakness, mild fatigue, occasional sleep disturbances, and sense of well-being has been shown to reduce in the StressCare group as compared to placebo, but was not shown to be statistically significant.
Effect of StressCare on, hematology and clinical chemistry are given in Table 3. Hematological and biochemical parameters were carried out initially and at the end of the study to assess the safety profile of the StressCare capsule. Hematology and biochemical investigations carried out to evaluate safety of the product were within the normal limits to further establish the safety of the product.
| Parameters | Visit | Stresscare | Placebo |
|---|---|---|---|
| Total Leucocyte Count (/cu.mm.) | 7968 ± 1926 p<0.0001 |
8229 ± 2159 p<0.0001 |
|
| At entry | |||
| At end of Week 8 | 7208 ± 1511 | 7570 ± 1633 | |
| Neutrophils% | At entry | 68.74 ± 4.75 | 68.14 ± 4.71 |
| At end of Week 8 | 68.10 ± 2.56 | 67.04 ± 2.25 | |
| Lymphocytes % | At entry | 28.88 ± 4.68 | 29.56 ± 4.79 |
| At end of Week 8 | 29.24 ± 2.23 | 30.02 ± 2.00 | |
| Eosinophils % | At entry | 1.32 ± 0.62 | 1.24 ± 0.43 |
| At end of Week 8 | 1.58 ± 0.50 p<0.0079 |
1.58 ± 0.54 p<0.0001 |
|
| Monocytes % | At entry | 1.06 ± 0.31 | 1.08 ± 0.27 |
| At end of Week 8 | 1.08 ± 0.27 | 1.10 ± 0.30 | |
| Basophils % | At entry | 0.00 ± 0.00 | 0.00 ± 0.00 |
| At end of Week 8 | 0.06 ± 0.31 | 0.00 ± 0.00 | |
| Hemoglobin gm% | At entry | 12.64 ± 1.57 | 12.67 ± 1.72 |
| At end of Week 8 | 13.02 ± 1.33 p<0.0001 |
13.09 ± 1.36 p<0.0002 |
|
| ESR mm/hr | At entry | 13.94 ± 6.54 | 13.33 ± 6.03 |
| At end of Week 8 | 9.52 ± 3.64 p<0.0001 |
9.65 ± 3.30 p<0.0001 |
|
| Serum bilirubin mg/dl | At entry | 0.65 ± 0.18 | 0.62 ± 0.16 |
| At end of Week 8 | 0.56 ± 0.12 p<0.0001 |
0.55 ± 0.12 p<0.0007 |
|
| SGPT (U/L) | At entry | 24.82 ± 7.22 | 24.75 ± 6.85 |
| At end of Week 8 | 24.84 ± 6.77 | 24.92 ± 6.47 | |
| Serum Creatinine mg/dl | At entry | 0.81 ± 0.11 | 0.86 ± 0.11 |
| At end of Week 8 | 0.75 ± 0.08 p<0.0001 |
0.76 ± 0.08 p<0.0001 |
|
| RBS mg/dl | At entry | 98.58 ± 16.09 | 93.94 ± 13.81 |
| At end of Week 8 | 90.22 ± 10.87 p<0.0001 |
87.20 ± 9.62 p<0.0001 |
Table 3: Effect of StressCare on, Hematology and Clinical Chemistry.
Effect of StressCare on Quality of Life (WHOQOL) is given in Table 4. Analysis was carried out on physical, psychological, social relationships and environment domains at the time of entry end of Week 4 and at the end of Week 8. There was no statistical significant change in any of the domains except for environmental domain which showed a significance of p<0.0092 at the end of Week 4 in StressCare group compared to placebo. However, a trend of improvement was noticed for physical health and social relationship in both the groups.
| Domain | Visits | Placebo | Stresscare | Significance |
|---|---|---|---|---|
| Physical Health | At entry | 45.00 ± 10.54 | 45.08 ± 6.59 | ns |
| Week 4 | 50.71 ± 12.09 | 49.86 ± 8.17 | ns | |
| Week 8 | 51.96 ± 13.97 | 55.58 ± 7.08 | ns | |
| Psychological | At entry | 40.71 ± 7.03 | 39.52 ± 6.11 | ns |
| Week 4 | 40.71 ± 6.82 | 39.44 ± 5.65 | ns | |
| Week 8 | 40.43 ± 7.52 | 39.14 ± 6.62 | ns | |
| Social Relationships | At entry | 58.92 ± 16.76 | 56.52 ± 13.34 | ns |
| Week 4 | 59.02 ± 18.20 | 61.78 ± 12.83 | ns | |
| Week 8 | 59.14 ± 18.66 | 67.42 ± 9.42 | ns | |
| Environment | At entry | 29.76 ± 5.60 | 28.19 ± 4.03 | ns |
| Week 4 | 32.54 ± 6.29* | 29.51 ± 4.43 | p<0.0092 | |
| Week 8 | 33.65 ± 6.98 | 32.92 ± 5.54 | ns |
Table 4: Effect of StressCare on Quality of Life (WHOQOL).
Discussion
Stress can manifest itself in a variety of emotional, behavioral, and even physical symptoms, and the symptoms of stress vary enormously among different individuals. Common symptoms often reported by those experiencing excess stress include occasional sleep disturbances, muscle tension, bowel disturbances, and fatigue. Emotional and behavioral symptoms that can accompany excess stress include nervousness, anxiety, changes in eating habits including overeating, loss of enthusiasm or energy, and mood changes.
Among the various measures for minimizing the stress effects and improving the adaptation, traditional herbal preparations are widely used. These herbs with medical benefits help in relieving the physical impacts of stress and improve the sense of well-being. Though many herbs like Brahmi, Sankhapushpi, Aswagandha, and Satavari are well known traditionally for their stress relieving benefits, clinical studies to prove the beneficial effects of polyherbal formulations are not much.
The beneficial effects of StressCare Capsules could be due to the sum total effects of its valuable ingredients. Some of the ingredients have been discussed as below, Chyavanaprasha is a classical preparation and holds an important therapeutic application in Indian Medicine. It has potent restorative & tonic activity [8]. Various trials have shown rejuvenating and adaptogenic properties as well as in maintaining general well-being [9,10].
Capparis spinosa has rejuvenating properties, which correct and prevent the free radical-induced oxidative damage to various organs and systems [11]. It is known to protect liver, spleen, and kidney and thus help maintain general well-being [12]. Cichorium intybus contain escutetin and glycosides. The bio-active principles exhibit protective action on liver and kidney [13]. Trials also suggest that extract of Cichorium intybus has free radical scavenging activity thus helps maintain general well-being [14].
Solanum berries contain solasonine and sapogenins. They help improve the circulation in small vessels and also improve muscle function. Phenolic compounds present in Solanum nigrum extract are also helpful in maintenance of overall health [15].
Anthroquinone derived from Cassia occidentalis has action on the colon movements, and helps regular healthy bowel movements [16]. Cassia occidentalis is also used as a general tonic in weakness. It protects liver from various damaging agents [17]. Bark of Terminalia arjuna helps circulation and also helps lipid Metabolism [18]. It also possess nitric oxide suppressant, cardioprotective, antioxidant, membrane stabilizing activities; all these helps to maintain general health [19].
It has beneficial effects on gastrointestinal system and circulatory system. It is known to be beneficial in mild, spasmodic discomforts of gastrointestinal tract [20]. Selenium present in Achillea millefolium has antioxidant activity and helps in proper functioning of heart [21].
Tamarix gallica contain the bio-actives like polyphenols, gallic acids, ellagic acid, which has health promoting activities [22]. The extract also has protective action on liver which may be due to its antioxidant activity [23]. Safranal, isolated from Crocus sativus has shown activity in preventing oxidative damage in brain tissue. It is used as antioxidant in various health related conditions [24-25].
Curculigo orchioides contains actives like glycosides, curculigoside and alkaloid lycorine. It has beneficial effects on vigor, liver health and functions as a general tonic [26]. It also promotes overall immunity [27].
The extract of Caesalpinia digyna plant root has exhibited its protective action against free radicals in liver and kidneys due to its antioxidant activities [28].
Asparagus racemosus contains biochemically important minerals which play a role in the enhancement of its medicinal properties including rejuvenating, adaptogenic and strength promoting activities. Trials show that Asparagus racemosus root administration could also help in lipid metabolism [29].
Alkaloids in the Withania somnifera root are known to prevent cellular damage due to daily stress and delay the premature aging. It is known to support RBC levels and hemoglobin, and thus used as a health supplement [30].
Glycyrrhiza glabra helps maintain lipid metabolism [31]. It provides thick protective mucous for the linings of the stomach [32]. Centella asiatica is a known nervine tonic and also helps in supporting blood pressure already in the normal range and mild memory problems associated with aging, and improving absentmindedness. It also helps correct occasional sleep disturbances [33].
Shilajeet helps to control the carbohydrate metabolism. It increases appetite, and useful in occasional gastric discomfort [34]. Shilajeet also regulates excess lipids to restore within normal limits, and maintains well-being [35]. It is also found to provide positive influence on hormones and brain functions [36].
Terminalia chebula promotes health due to its antioxidant activities [37]. The extract is also known for its benefits in bowel cleansing [38]. The antioxidant activity of Mucuna pruriens was demonstrated by its ability to scavenge free radicals and also calming effects [39].
Myristica fragrans is used as stimulant, appetizer and to control flatulence. Studies also indicate its benefits on vigor, gastric mucosa and lipid metabolism [40]. Fruits of the plant Piper longum and its component piperine have immunomodulatory activity, which may help a person to maintain health [41]. It also enhances the bioavailability that potentiates action of other ingredients [42].
Syzygium aromaticum is used in common digestive symptoms [43]. The seed extract which contains the essential oils have potential antioxidant activities. It is also promoted as a general health supplement in expectant mothers [44].
Carum copticum seed extract has various health benefits on heart and liver and its use in maintaining health [45]. Curcuma longa have shown beneficial effects in conditions related to liver, heart, and immunity [46].
Adhatoda vasica extracts contain alkaloids such as vasicine, vasicinone and a quinazoline-alkaloid, and peganin. These improve the immunity of an individual and help in maintaining respiratory health [47]. Eclipta alba helps promote liver health and also known to enhance memory. Eclipta alba helps reduce free radicals due to its anti-oxidant properties [48]. Celastrus paniculatus seed enhances memory and reduces free radicals [49]. The plant extract reduces anxiety and keeps a person healthy [50]. Argyreia speciosa root helps improve the immunity of the person [51].
In this double blind, placebo-controlled study, the safety and efficacy of StressCare in mild occasional stress and related conditions were investigated. Improvement in stress and stress related condition following 8 weeks of StressCare treatment in comparison with placebo was noted. Since StressCare capsules consists of those herbal ingredients help to relieve stress, scavenge free radicals and toxins, improve immune responses and support cardiac and nervine functioning, it facilitates in protecting from further stress and possible tissue damage. Thereby StressCare helps to improve normal physiological immunity and tissue responses required for cellular regeneration and repair, thus reviving the physical capacity, enhanced ability to digest the ingested food and promote the sense of well-being.
Conclusion
This clinical study indicates good clinical efficacy of StressCare Capsules in promoting sense of well-being and improvement in mild occasional stress and irritability related clinical parameters like occasional loss of appetite, mild indigestion, occasional weakness, occasional stress, mild fatigue, mild mood swings, occasional sleep disturbances, occasional bowel disturbances, and sense of well-being. Maintenance of normal blood pressure and laboratory parameters in the normal range suggest that StressCare capsule is well tolerated and safe for human use. There were no adverse events either reported or observed in the clinical study. Thus it can be concluded that StressCare is safe and effective in the management of mild occasional stress and irritability.
References
- Stress & Stress Management (2010) Clinic Community Health Centre.
- Selye H (1956) The Stress of Life. New York, McGraw-Hill.
- Lazarus RS, Folkman S (1984) Stress: Appraisal and Coping. New York, Springer, USA.
- Harre R, Lamb R (1983) The Encyclopaedic Dictionary of Psychology. (Edtn), Oxford, Blackwell.
- Gillian Butler (1993) Definitions of stress. Occas Pap R Coll Gen Pract 61(1): 1–5.
- BL Seaward (2016) Managing Stress. (1st Edtn.) Jones & Bartlett, USA.
- Ballesteros D, Whitlock JL (2009) Coping: Stress management strategies. The Fact Sheet Series, Cornell Research Program on Self-Injury and Recovery. Cornell University, Ithaca, USA.
- The Ayurvedic Formulary of India: Anonymous (1978) Govt. of India. New Delhi; Part-1. (1st edtn) 30-31.
- Jagetia GC, Rao SK, Baliga MS, S Babu K (2004) The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: A preliminary study. Phytother Res 8(7): 561-565.
- Jagetia GC, Baliga MS (2004) The evaluation of the radio protective effect of Chyavanaprasha (an ayurvedic rasayana drug) in mice exposed to lethal dose of gamma-radiation: A preliminary study. Phytotherpy Res 18(1): 14-18.
- Bonina F, Puglia C, Ventura D, Aquino R, Tortora S, et.al. (2002) In vitro antioxidant and in vivo photoprotective effects of a lyophilized extract of Capparis spinosa L buds. J Cosmet Sci 53(6): 321-335.
- Khare CP (2004a) Encyclopedia of Indian Medicinal Plants. Springer, Germany.
- Khare CP (2004b) Encyclopedia of Indian Medicinal Plants. Springer. Germany.
- Schaffer S, Schmitt S, Muller WE, Eckert GP (2005) Antioxidant properties of mediterranean food plant extracts: Geographical differences. J. Physiology and Pharmacology 56(1): 115-124.
- Uma SA, Bharti O (2008) In vitro 5-Lipoxygenase inhibition of polyphenolic antioxidants from undomesticated plants of South Africa. J. Medicinal Plants Research 2(9): 207-212.
- Asolkar LV, Kakkar KK, Chakre OJ (1992) Second Supplement to Glossary of Indian Medicinal Plants with active principles: A-k (1965-1981). New Delhi, India.
- Usha K, Mary GK, Hemalatha P (2007) Hepatoprotective effect of Hygrophilia spinosa and Cassia occidentalis on carbon tetrachloride induced liver damage in experimental rats. Indian J Clinical Biochemistry 22(2): 132-135.
- Dwivedi S (2007) Terminalia arjuna Wight & Arn.: A useful drug for cardiovascular disorders. J.
- Ethnopharmacol 114(2): 114-129.
- Chander R, Singh K, Khanna AK, Kaul SM, Puri A, et al. (2004) Antidyslipidemic and antioxidant activities of different fractions of Terminalia arjuna stem bark. Indian J Clinical Biochemistry 19(2): 141-148.
- Khare CP (2007a) Indian Medicinal Plants: An illustrative dictionary. Springer. New Delhi, India.
- Krishaiah D, Sarbatly R, Bono A (2007) Phytochemical antioxidants for health and medicine-A move towards nature. Biotechnology and Molecular Biology Review. 1(4): 97-104.
- Khare CP (2004d) Encyclopdia of Indian Medicinal Plants. Springer. Germany.
- Sehrawat A, Sultana S (2006) Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated, and 2-AAF promoted hepatocarcinogenesis in male wistar rats. Life Sciences 79(15): 1456-1465.
- Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, et al. (2009) Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak J Pharma Sci 22(1): 102-106.
- Hosseinzadeh H, Sadeghnia HR (2005) Safranol, a constituent of Crocus sativus (Saffron), attenuated cerebral ischemia induced oxidative stress damage in rat hypothalamus. J Pharm Pharmaceut Sci 8(3): 394-399.
- Khare CP (2004e) Encyclopdia of Indian Medicinal Plants. Springer. Germany.
- Bafna AR, Mishra SH (2006) Immunostimulatory effect of methanol extract of Curculigo orchioides on immunosuppressed mice. J Ethnopharmacol 104(1-2): 1-4.
- Srinivasan R, Chandrasekar MJ, Nanjan MJ, Suresh B (2007) Antioxidant activity of Caesalpinia digyna root. J Ethnopharmacol 113: 284-291.
- Nishant P, Visavadiya, Narasimhacharya AVRL (2007) Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesteremic rats. eCAM 6(2): 219-226.
- Khare CP (2004f) Encyclopdia of Indian Medicinal Plants. Springer. Germany; 480-481.
- Asgary S, Dinani JN, Madani H, Mahzoni P, Naderi GH (2007) Effect of Glycyrrhiza glabra extract on aorta wall atherosclerotic lesion in hypercholesterolemic rabbits. Pak J Nutrition 6(4): 313-317.
- Khare CP (2004g) Encyclopdia of Indian Medicinal Plants. Springer. Germany.
- Sushma T, Shinjini S, Kishor P, Sangeeta G, Gambhir IS (2008) Effect of Centella asiatica on mild cognitive impairment (MCI) and other common age-related clinical problems. Digest J. Nanomat Biostruct 3(4): 215-220.
- Chopra RN, Chopra IC, Handa KL, Kapur LD (1958) Indigenous Drugs of India. (2nd Edtn) U N Dhur & Sons Private Ltd. Kolkata, India.
- Sankhla A, Mathur PN, Sankhla AK, Dashora PK (1992) Comparative efficacy of Shilajeet and gum guggulu (Commiphora mukul) in preventing diet induced hypercholesterolemia in wistar rats. Indian J Clinical Biochemistry 7(1): 45-48.
- Shibnath Ghosal (1990) Chemistry of Shilajit, an immunomodulatory Ayurvedic Rasayana. Pure & Appl Chem 62(7): 1285-1288.
- Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC (2003) Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull 26(9): 1331-1335.
- Lee HS, Won NH, Kim KH, Lee H, Jun W, et al. (2005) Antioxidant effects of aqueous extract of Terminalia chebula in vivo and in vitro. Biol Pharm Bull 28(9): 1639-1644.
- Dhanasekaran M, Tharakan B, Manyam BV (2008) Antiparkinson drug: Mucuna pruriens shows antioxidant and metal chelating activity. Phytother Res 22(1): 6-11.
- Somani R, Karve S, Jain D, Jain K, Singhai AK (2008) Phytochemical and pharmacologiocal potential of Myristica fragrans Houtt. A Compressive view. Pharmacognosy Review 2(4): 68-76.
- Sunila ES, Kuttan G (2004) Immunomodulatory and antitumour activity of Piper longum Linn. and piperine. J Ethnopharmacol 90(2-3): 339-346.
- Abdel Moneim ES, Imam MO, Amin A. El Khalifa (2007) Nutritive Value of Clove (Syzygium aromaticum) and Detection of Antimicrobial Effect of its Bud Oil. Res. J. Microbiol 2(3): 266-271.
- Dhulap S, Anita M, Hirwani RR (2008) Phytopharmacology of Elettaria cardamomum. Pharmacognosy Review 2(4): 27-35.
- Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akthar MS (2005) Studies on the antihypertensive, antispasmodic, bronchodilator, and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol 98(1-2): 127-135.
- Jain S, Shrivastava S, Nayak S, Sumbhate S (2007) Recent trends in Curcuma longa Linn. Pharmacognosy Review 1(1): 119-128.
- Kumar M, Samarth R, Madhu K, Selvan SR, Saharan B, et al. Protective effect of Adhatoda vasica Nees against radiation-induced damage at cellular, biochemical, and chromosomal levels in Swiss albino mice. eCAM 2007; 4(3):343-350.
- Majumdar AS, Saraf MN, Andrades NR, Kamble RY (2008) Preliminary studies on the antioxidant activity of Tribulus terrestris and Eclipta alba. Pharmacognosy Review 4(3): 102-107.
- Kumar MHV, Gupta YK (2002) Antioxidant property of Celastrus paniculatus wild. A possible mechanism in enhancing cognition. Phytomedicine 9(4): 302-311.
- Rajkumar R, Kumar EP, Sudha S, Suresh B (2007) Evaluation of anxiolytic potential of Celastrus oil in rat models of behavior. Fitoterapia 78(2): 120-124.
- Gokhale AB, Damre AS, Saraf MN (2003) Investigations into the immunomodulatory activity of Argyreia speciosa. J Ethnopharmacol; 84(1): 109-114.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi