Research Article, Jrgm Vol: 13 Issue: 1
A Prospective Single-Donor Cellular Analysis of Two Different Intradiscal Autologous Platelet-Rich Plasma Systems
Jennifer Solomon1*, Mohammed Mostafa Sharaf2, Christopher Kyriakides3, Richard Abiuso4, Nicholas Beatty5, Christopher Lutz1, Meredith Prysak1, Gregory Lutz1
1Regenerative Sportscare Institute, United States of America
2New York University, Regenerative Sportscare Institute, United States of America
3NYU Langone, Regenerative Sportscare Institute, United States of America
4Tufts University, Regenerative Sportscare Institute, United States of America
5Hospital for Special Surgery, New York, USA
*Corresponding Author: Jennifer Solomon
Regenerative Sportscare Institute, United States of America
E-mail: solomonj@regensportscare
Received: 28-Dec-2023, Manuscript No. JRGM-23-123883;
Editor assigned: 30-Dec-2023, PreQC No. JRGM-23-123883 (PQ);
Reviewed: 12-Jan-2024, QC No. JRGM-23-123883;
Revised: 15-Jan-2024, Manuscript No. JRGM-23-123883 (R);
Published: 22-Jan-2024, DOI:10.4172/2325-9620.1000286
Citation: Solomon J, Sharaf MM, Kyriakides C, Abiuso R, Beatty N, et al. (2024) A Prospective Single-Donor Cellular Analysis Of Two Different Intradiscal Autologous Platelet-Rich Plasma Systems. J Regen Med 13:1.
Copyright: © 2024 Solomon J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Intradiscal autologous platelet rich plasma (PRP) is a therapy for patients with chronic discogenic lower back pain. Recent clinical studies suggest that a PRP preparation with a higher platelet concentration may improve clinical outcomes. The purpose of this study was to compare a newly designed PRP system (DiscCath™, LLC) with a traditional PRP system (Emcyte Corporation) to determine if higher platelet concentrations were attainable from the same donor.
Methods: Prospective single-donor cohort study at an outpatient interventional orthobiologics clinic involving 31 participants (17 male and 14 female) who supplied 64 distinct PRP samples (one patient’s PRP was analyzed on two separate treatment appointments). Baseline peripheral blood cell count data, and PRP cell count data that included absolute platelet count (ABS PLT), platelet fold increase (PLT Fold), white blood cell counts and differential (WBC; LYM, MON, GRA), and hematocrit percentage (HCT).
Results: DiscCath™ produced a PRP product with a statistically higher ABS PLT (6.95 vs 5.65 billion platelets) and higher PLT Fold (15.92X vs 12.48X) when compared to EmCyte. DiscCath™ also produced a PRP with a higher ABS WBC (44.63 vs 41.86 million) and a lower HCT (10.30 vs 11.54 percent) when compared to EmCyte, which did not achieve statistical significance.
Conclusion: The DiscCath™ PRP System concentrated platelets to higher levels than the Emcyte PRP System. This may have clinical implications in optimizing the intradiscal treatment of patients with degenerative disc disease. Clinical studies are needed to evaluate if the DiscCath™ PRP System improves clinical outcomes in intradiscal PRP therapy.
Keywords: Platelet-rich plasma, Intradiscal, Platelets, Systems, Single-Donor
Keywords: Platelet-rich plasma, Intradiscal, Platelets, Systems, Single-Donor
Introduction
Intradiscal Platelet Rich Plasma (PRP) is an emerging novel regenerative therapy for patients with Chronic Low Back Pain (CLBP) from Degenerative Disc Disease (DDD). There are now multiple clinical studies, including three randomized controlled trials, that have demonstrated different clinical outcomes despite studying similar patient populations [1-3]. One potential explanation for this phenomenon may be the type of PRP used, as well as the dose of platelets administered. There are a wide variety of PRP systems with varying degrees of cellular content. The optimal platelet dosing and the ideal type of PRP for intradiscal use has not yet been established.
If we analyze the clinical outcomes studies heretofore that have been published we can ascertain some trends that may lead us to developing a potentially improved PRP system for intradiscal use [4]. Additionally, one of our first goals with intradiscal orthobiologics is patient safety. With the increased awareness of the role that bacteria play in disc degeneration, an orthobiologic that takes this into consideration may have a beneficial therapeutic effect [5]. A recent review has suggested that a high platelet concentration leukocyte rich PRP may be the safest and lead to improvement in clinical outcomes in patients suffering from degenerative disc disease [6-8]. The concept behind using this method is that we are potentially killing two birds with one stone: healing painful annular fissures while at the same time correcting the dysbiosis inside the disc that may contribute to chronic pain and degeneration.
The annulus fibrosus has been shown to respond to PRP-derived growth factors, which has limited volume capacity for injection [9]. Additionally, laboratory studies of PRP have demonstrated a linear relationship between growth factor content and platelet concentration [10]. Recent studies have suggested that higher platelet concentrations may lead to improved clinical outcomes [11,12]. However, there are limitations of current commercially available point-of-care PRP systems to concentrate platelets to higher cellular levels [13]. Most systems concentrate platelets to three to five times baseline platelet counts, however, some systems can achieve ten times baseline [14-16]. Previous comprehensive reviews of commercial kits provide fold increases above baseline [17] which both DiscCath™ and Emcyte exceed by a significant margin, with DiscCath™ exceeding baseline by 15.92 fold and Emcyte returning 12.48 fold above baseline [18,19].
The DiscCath™ PRP system was specifically designed with an industry partner (Ranfac Corporation, Avon, MA) to potentially increase platelet concentrating abilities beyond what current commercial point-of-care systems are able to achieve. The aim is that higher concentrations of platelets would yield higher growth factor loads which may potentially improve clinical outcomes [20] with intradiscal PRP procedures. The intent was to design a PRP point-of-care system that could consistently achieve a greater than fifteen times platelet fold increase that we could then take into new clinical trials to assess. Additionally, because of the risk of infection and the potential role that bacteria may play in disc degeneration, the intent was to design a high concentration leukocyte-rich PRP (LR-PRP) system.
Thus, the purpose of this study was to compare the cellular content of DiscCath™ PRP system to the PRP system (Emcyte Corporation) we used in our previous clinical study to assess whether or not this newer system could concentrate platelets and white blood cells to higher levels in a single-donor model.
Materials and Methods
Patient Selection
This study involved the prospective analysis of 64 distinct PRP preparations from 31 consecutive patients, between the dates of December 9, 2021 and February 17, 2022. It was concluded that the scope of this study qualified for exempt review and retroactive patient consent was not required. All research and analyses were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its amendments. In terms of limitations, patient data was included for analysis given the following conditions: weight is greater than 110 lbs, non-pregnant, self-reported healthy, free of COVID-19, cold, and flu symptoms, and reports no history of prior infection within two weeks. For both pre- and post-injection, three-part differential CBCs were collected. The values from the performed CBCs were then stored and tabulated for data analysis.
Within the collection of these samples, the samples were then grouped based on which manufacturer’s kit was used, with one group being designated towards the EmCyte system, and the other to the DiscCath™ kit.
Ethics Approval and Consent to Participate: This observational study was determined to be exempt by the IntegReview IRB (OBX-1005).
Study Design
Primary Hypothesis
There will be a statistically significant difference in concentrations of the cellular components between the two PRP systems.
Participant Inclusion
Participants who received leukocyte poor PRP were excluded, and those who received PRP injections with leukocyte rich PRP were included from this trial. Additionally, patients who exhibited a low hemoglobin count (<10) and a lower platelet count (<100) were excluded from receiving the treatment (Table 1).
| Patient Demographics | |
| Number of Distinct Samples | 64 |
| Number of Patients | 31 |
| Age Range Distribution | <29: 1 30-49: 5 50-69: 17 >70: 8 |
| Average Age | 58 |
| Gender Distribution | M: 17 F: 14 |
Table 1: Analysis of Patient Demographics
Study Protocol
Each kit used follows a standard protocol for preparation of injection, with this injection being prepared in accordance with manufacturer guidelines. For the EmCyte kit(PurePRP® SP), 60 mL of peripheral blood is drawn from the patient and added to the apparatus. The first spin is performed at 3800 rpm for 2 minutes (on an Eppendorf AG 08/15 , 5702/R, A-4-38) followed by decanting and transferring the upper layer along with its buffy coat to a new tube to be spun for another 3800 rpm spin for 6 minutes. Following this, the platelets are then collected and injected into the targeted tissue.
For DiscCath kit, 80 ml of peripheral blood is drawn from the patient and added to the apparatus. The first spin is performed at 3,800 rpm for 2 minutes (same centrifuge, or list model and rotor cm), and the second spin is performed at 4,200 rpm for six minutes.
Before analyzing the samples in the hemacytometer, each sample was diluted at a 1:1 ratio with platelet poor plasma in order to circumvent upper measurement limitations associated with the hemacytometer. The differences in numbers were then remedied by doubling the received result prior to tabulation.
Three samples are then collected, labeled “PRE”, “POST”, and “PPP”. PRE corresponds to the initial concentrations of platelets and white blood cells in the first sample of whole blood collected. POST refers to the samples after concentration using either DiscCath™ or EmCyte technique prior to injection. PPP refers to a sample of the “Platelet Poor Plasma” that is a byproduct of the PRP preparation.
The samples were then analyzed with a HORIBA ABX Micros ES60 hemacytometer, with a complete blood count being performed in order to tabulate the necessary parameters needed for comparison and analysis between the two systems.
The following parameters were isolated and recorded from the PRE and POST CBC results: PLT and WBC, with the WBC data being further broken down into Lymphocytes (LYM), Monocytes (MON), and Granulocytes (GRA). The data then underwent statistical analysis to draw conclusions about differences between the two kits.
Results
As evidenced in (Table 2), the study was allowed to proceed with the 34 selected patients as their platelet concentration and white blood cell concentration numbers were within normal and healthy ranges.
| Average Whole Blood Concentrations | |
| Mean | |
| Platelet concentration (x 106/ml) | 176.94 ± 48.23 |
| White blood cell concentration (x 106/ml) | 4.87 ± 1.69 |
| Lymphocyte concentration (x 106/ml) | 1.52 ± 0.61 |
| Monocyte concentration (x 106/ml) | 0.32 ± 0.16 |
| Granulocyte concentration (x 106/ml) | 3.03 ± 1.30 |
| Pre injection hematocrit (%) | 37.95 ± 10.79 |
Table 2: Mean whole blood characteristics pre injection from 34 patients
DiscCath™ PRP System vs. EmCyte PurePRP II Evaluation
For the comparison of the two systems, several parameters were derived from the accumulated data. Outside of the aforementioned raw data consisting of PRE and POST samples, calculations were then performed to determine the percentage increase in both white blood cells and platelet counts, this data was then labeled as “[Parameter] Fold Increase”. The equation to calculate the fold increase using WBC Fold Increase as an example is as follows:

Additionally, in order to compare the total number of platelets injected between the two systems, Absolute Platelet Count was calculated. The calculation was performed with the sample equation as follows, with the constant 2.5 representing the amount of solution injected, as 3 mL injectates were prepared, with 0.5 mL being saved for analysis:

Following the evaluation of these values, the primary focus then became comparison, so in order to compare the two systems, a question was posed regarding the most appropriate statistical test to perform. As two different populations with unequal variances are being compared, a two tailed T-test assuming unequal variances was performed in order to delineate any significant statistical differences.
Furthermore, additional breakdowns for leukocyte data were collected, and then divided into Lymphocytes (LYM), Monocytes (MON), and Granulocytes (GRA), with the same calculations and evaluations being performed. Post injection Hematocrit percentages were also measured to ensure that both systems’ values stayed within the manufacturer’s indicated range.
Statistical Analyses Performed
T-tests for the analysis parameters were performed assuming unequal variances because the two systems are inherently different and from different manufacturers, so assuming equal variances in this case would be unfeasible. For this series of tests, a standard confidence interval of 5% was used.
Based on the observed P values, the parameters in which P<α are “Post injection platelet concentration”. “Platelet fold increase”, “Absolute platelet count”, and “Lymphocyte fold increase”. For the other parameters, no statistically significant difference was observed. In a previous paper on the topic of intradiscal biologics, it was observed that there is a direct correlation between granulocyte count and C acne recovery, which then illustrates the importance of maximizing granulocyte levels within procedure (Table 3).
| PRP Preparation System | |||
| DiscCath™ Platelet Separator 80mL | EmCyte Pure PRP II 60mL | P-value (α=0.05) | |
| Post injection platelet concentration (x 106/ml) | 2784.44 ± 1041.44 | 2261.25 ± 483.10 | 0.0139 * |
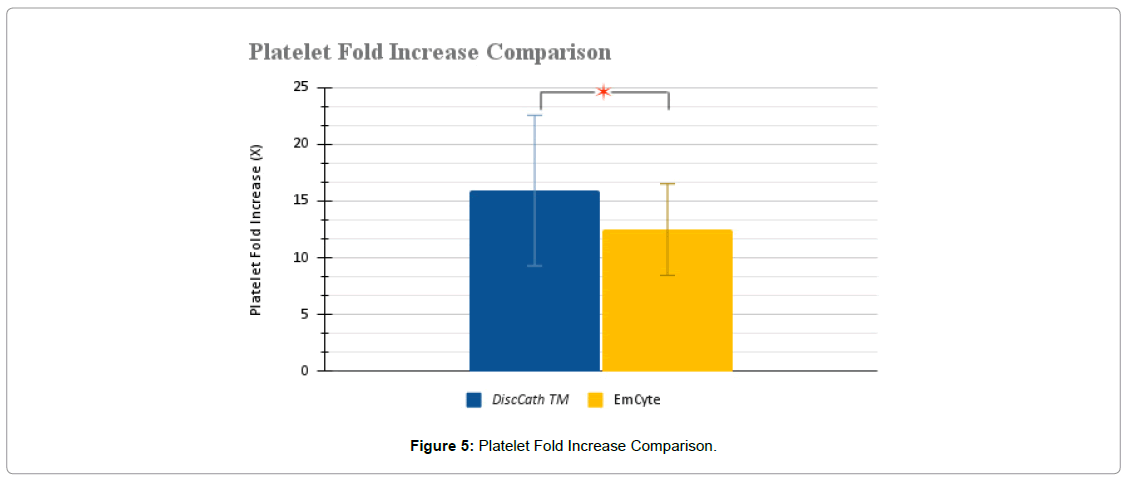
| Platelet fold increase (X) | 15.92 ± 6.63 | 12.48 ± 4.04 | 0.0152 * |
| Post injection white blood cell concentration x 106/ml) | 44.63 ± 19.07 | 41.86 ± 14.93 | 0.5254 |
| White blood cell fold increase (X) | 8.67 ± 3.68 | 8.22 ± 3.78 | 0.6335 |
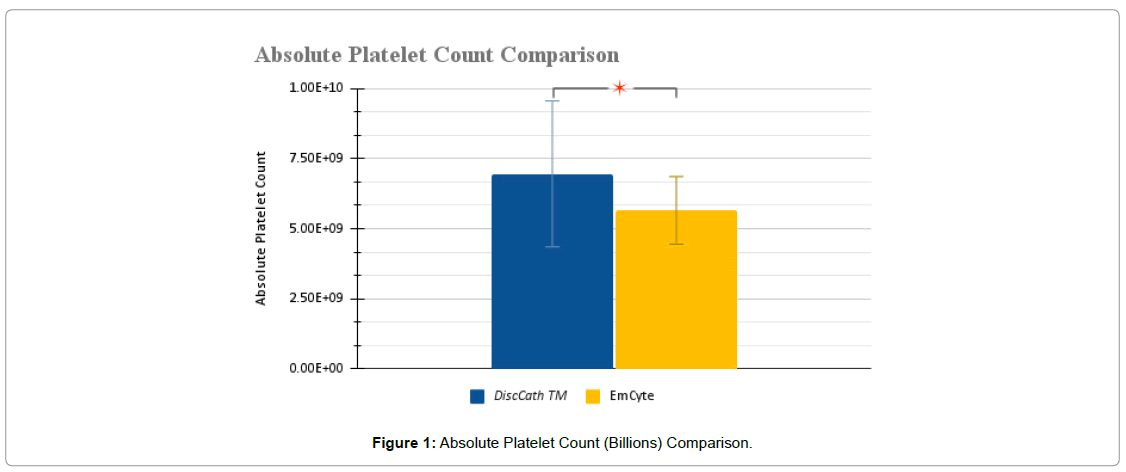
| Absolute platelet count (x 106/ml) | 6.95 ± 2.60 | 5.65 ± 1.21 | 0.0139 * |
| Post injection lymphocyte concentration (x 106/ml) | 27.11 ± 11.30 | 22.47 ± 7.46 | 0.0589 |
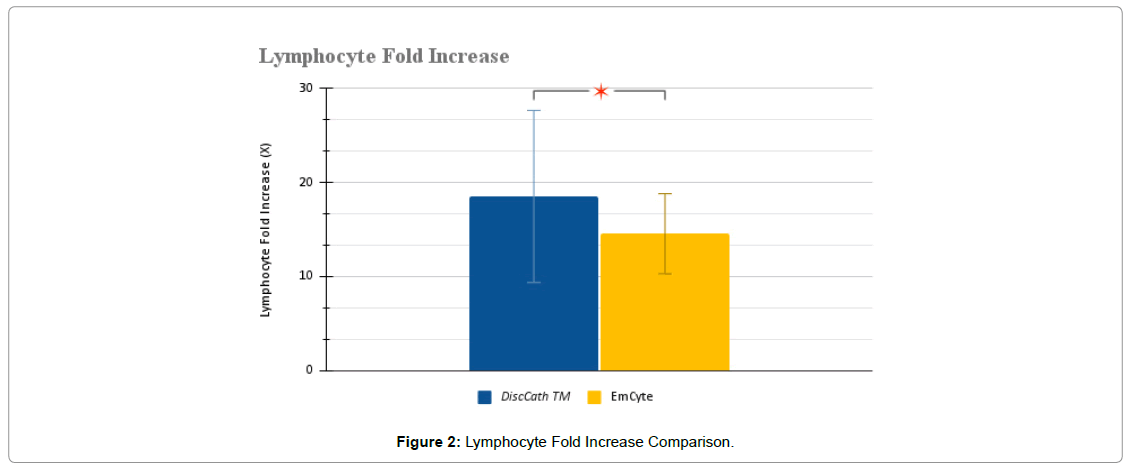
| Lymphocyte fold increase (X) | 18.5 ± 9.15 | 14.54 ± 4.25 | 0.0314 * |
| Post injection monocyte concentration (x 106/ml) | 5.39 ± 3.01 | 4.57 ± 1.81 | 0.1984 |
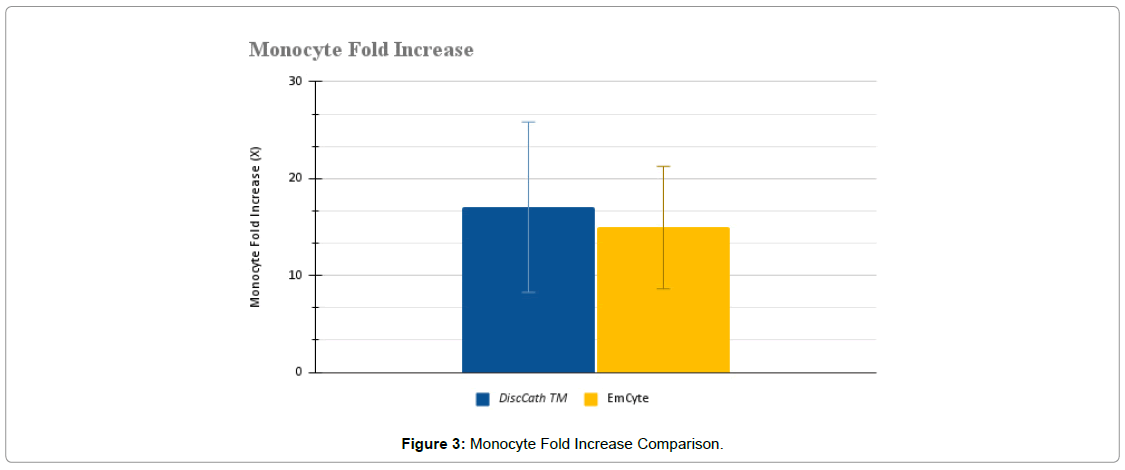
| Monocyte fold increase (X) | 17.06 ± 8.79 | 14.95 ± 6.33 | 0.2778 |
| Post injection granulocyte concentration (x 106/ml) | 12.48 ± 7.45 | 15.37 ± 7.44 | 0.1292 |
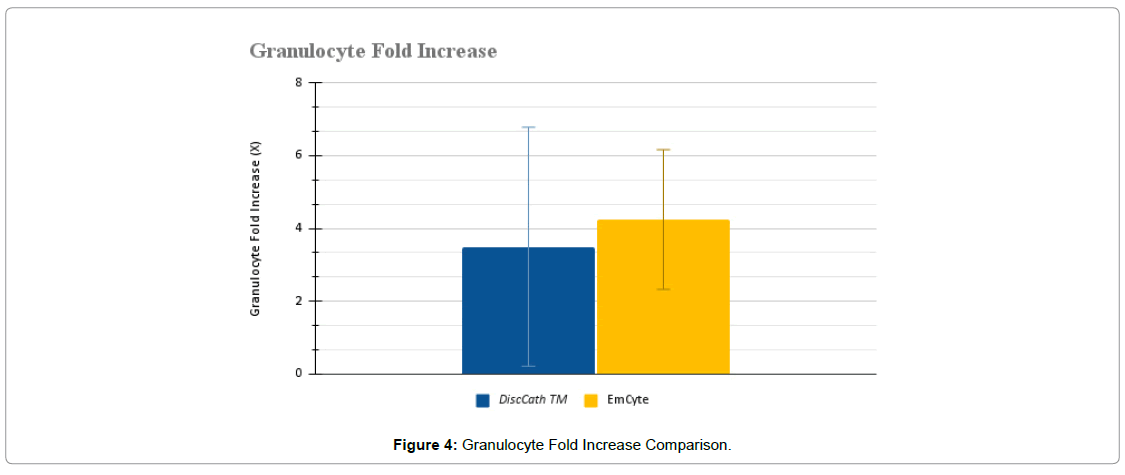
| Granulocyte fold increase (X) | 3.50 ± 3.29 | 4.24 ± 1.92 | 0.2744 |
| Post injection hematocrit (%) | 10.30 ± 4.88 | 11.54 ± 3.53 | 0.2499 |
Table 3: Statistical summary of average PRP characteristics obtained from analysis
Platelet Concentrations
Initially, both analyzed systems delivered platelets to the targeted region while maintaining a positive fold increase, indicating that platelet levels above the baseline were injected. Comparing the two systems together, it is evident that based on T-test analysis that DiscCath™ maintains statistical significance to the EmCyte system with a reported P-value of 0.0139 in terms of absolute platelet counts (Figure 1-5), and a P-value of 0.0152 in terms of the fold increase. Additionally, on average, DiscCath™ (2784.44 ± 1041.44 x 106/ml) Delivered approximately 23% more platelets than EmCyte (2261.25 ± 483.10 x 106/ml) into the injection site.
White Blood Cell Concentrations
There were no statistically significant differences between DiscCath™ and EmCyte in terms of white blood cell changes. While the P-value was not below the alpha value and it was therefore impossible to codify the difference between the two systems, it was observed that DiscCath™ (44.63 ± 19.07 x 106/ml) delivered approximately 6% more WBCs than EmCyte (41.86 ± 14.93 x 106/ml) into the injection site.
Leukocyte Characterization and Composition Comparison
The collected WBC data was then broken down further into specific lymphocytes, monocytes, and granulocytes (Figure 2-4).
From these numerical observations, statistical significance was found in the lymphocyte fold increase associated with DiscCath™ (P=0.0314), and was also close to statistical significance in the absolute number of lymphocytes delivered (P=0.0589). DiscCath™ (27.11 ± 11.30 x 106/ ml) delivered approximately 20% more lymphocytes than EmCyte (22.47 ± 7.46 x 106/ml) into the injection site.
There were also no statistically significant differences between the two systems in terms of monocytes (P=0.1984) and granulocytes (P=0.1292). DiscCath™ (5.39 ± 3.01 x 106/ml), on average, delivered approximately 17% more monocytes than EmCyte (4.57 ± 1.81 x 106/ml). Alternatively, EmCyte (15.37 ± 7.44 x 106/ml) produced approximately 23% more post injection granulocytes than DiscCath™ (12.48 ± 7.45 x 106/ml).
Hematocrit
DiscCath™ (10.30 ± 4.88 %) maintained a post injection hematocrit that was approximately 10% lower than that of EmCyte (11.54 ± 3.53 %), with a lower hematocrit being advantageous due to it being an indicator of more platelets, white blood cells, and growth factors within the sample.
Graphs and Visualizations of Aforementioned Findings
*Assuming α=0.05
Safety
There were no reported complications associated with both DiscCath™ and EmCyte
Discussion
Physician confidence in the choice of a PRP kit over other comparable kits is based on each manufacturer’s reported contents, particularly because CBC analysis on PRP preparations at point-of-care is uncommon in clinical practice. The characterization of PRP kits during clinical use is critical to confirm the content of the injectate, which may coincide with a kit’s efficacy in potential for tissue healing. What gets measured can be better managed to improve the quality control of the PRP product. This will hopefully lead to improvement in clinical outcomes of intradiscal biologic therapies.
The current study highlights several important points about the comparative performance of the DiscCath™ Platelet Separator 80ml kit versus the EmCyte PurePRP II 60-mL kit during routine medical use outside of a controlled experimental setting. Our data provide a clear view of the cellular makeup of both kits using the leukocyte-rich preparation method in a non-experimental setting, with statistically significant increases in platelet concentrations. The DiscCath™ system produced a mean platelet fold increase of 15.92-x over baseline, compared to the Emcyte System which produced a mean platelet fold increase of 12.48-x over baseline. Of the leukocytes which were noticeably enriched, lymphocytes had an increase of 19-x over the baseline, monocytes 17-x over the baseline, and granulocytes 3-x over the baseline. Both kits consistently produced on average a >8-fold increase in PLT concentration during routine clinical use, confirming both manufacturer’s reported concentration levels. Of note, the original determinations by the manufacturer Emcyte were performed on a Coulter Ac-T diff 2 Hematology Analyzer and for DiscCath™ on a Horiba ABX Micros ES60. Thus the performance conformed to expected values for both PLT and GRA enrichment parameters under conditions of routine clinical use.
The %HCT levels reported by EmCyte for the device are <1% for Protocol A and < 20% for Protocol B. Our data confirms that Protocol B reproducibly returned a %HCT of <20% during routine clinical use. The %HCT levels reported by DiscCath™ for the device are HCT < 20% for Leukocyte Rich Protocol. Our data also confirmed that Protocol B returned a %HCT of <20%. These patients also had a higher-than-expected GRA enrichment of 3-fold. The findings also revealed a significant increase in mean absolute counts of PLTs, LYMs, and GRAs, but differences in monocytes were not significant between the two volumes. The reason for this is unknown.
Widespread characterization of the injectate at point-of-care is the crucial first step toward fully elucidating the mechanism of action for PRP in regenerative medicine applications. Further evaluation of the therapeutic window of PRP may allow for a simple qualitative assessment for clinicians to use prior to injection with the intention of significantly improving patient outcomes. The need for specific data on cell count content is further discussed below.
The data derived from this study elucidates statistically significant differences in platelet concentration efficacy between two commercially available PRP systems. In the two systems studied, DiscCath™ proved superior in providing consistently higher PLT concentrations. The Emcyte system had less variability, yet lower mean platelet concentrations per milliliter of injectate. In clinical practice, this study indicates that when physicians are making modifications in injectate volumes for smaller anatomical sites of treatment, they may do so with confidence in substantial increases in PLT concentration and absolute PLT counts. This alone is a significant numerical advantage in terms of platelets delivered and potential improvement in growth factor delivery in small volume sites of injection, such as within the intradiscal space. Defining the absolute platelet level of an injectate in relation to patient improvement is difficult, however ensuring substantial enrichment is necessary to the viability of these procedures. Further characterization is needed to bridge this gap of knowledge. Studying patient outcomes compared to the absolute platelets and leukocytes received per injection may lead to greater understanding in platelet levels associated with efficacy.
References
- Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, et al (2016) Lumbar Intradiskal Platelet-Rich Plasma (Prp) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM R;8(1):1-10
- Zielinski MA, Evans NE, Bae H, Kamrava E, Calodney A, et al. (2022) Safety And Efficacy Of Platelet Rich Plasma For Treatment Of Lumbar Discogenic Pain: A Prospective, Multicenter, Randomized, Double-Blind Study. Pain Physician; 25(1): 29.
- Schepers M, Schepers MO, Groot D, Kleinjan EM, Pol MM, Mylenbusch H, et al.(2022). Effectiveness Of Intradiscal Platelet Rich Plasma For Discogenic Low Back Pain Without Modic Changes: A Randomized Controlled Trial. Intervent Pain Med; 1(1): 100011.
- Muthu S, Jeyaraman M, Chellamuthu G, Jeyaraman N, Jain R, et al. (2022) does The Intradiscal Injection Of Platelet Rich Plasma Have Any Beneficial Role In The Management Of Lumbar Disc Disease?. Global Spine J; 12(3): 503-514.
- Gilligan CJ, Gilligan CJ, Cohen SP, Fischetti VA, Hirsch JA, Czaplewski LG (2021). Chronic Low Back Pain, Bacterial Infection And Treatment With Antibiotics. Spine J; 21(6): 903-914.
- Lutz GE (2023) Intradiscal Leukocyte Rich Platelet Rich Plasma for Degenerative Disc Disease. Phys Med Rehabil Clin N Am; 34(1):117-133.
- Prysak MH, Lutz CG, Zukofsky TA, Katz JM, Everts PA, et al. (2019) Optimizing The Safety Of Intradiscal Platelet-Rich Plasma: An In Vitro Study With Cutibacterium Acnes. Regen Med; 14(10): 955-967.
- Jerome MA, Lutz C, Lutz GE (2021) Risks Of Intradiscal Orthobiologic Injections: A Review Of The Literature And Case Series Presentation. Int J Spine Surg; 15(s1): 26-39.
- Akeda K, An HS, Pichika R, Attawia M, Eugene JM, et al.(2006) Platelet-Rich Plasma (Prp) Stimulates The Extracellular Matrix Metabolism Of Porcine Nucleus Pulposus And Anulus Fibrosus Cells Cultured In Alginate Beads. Spine; 31(9): 959-966.
- Sundman EA, Cole BJ, Fortier LA (2011) Growth Factor And Catabolic Cytokine Concentrations Are Influenced By The Cellular Composition Of Platelet-Rich Plasma. Am J Sports Med; 39(10): 2135-2140.
- Lutz C, Cheng J, Prysak M, Zukofsky T, Rothman R, et al. (2022) Clinical Outcomes Following Intradiscal Injections Of Higher-Concentration Platelet-Rich Plasma In Patients With Chronic Lumbar Discogenic Pain. Int Orthop; 46(6): 1381-1385.
- Jain D, Goyal T, Verma N, Paswan AK, Dubey RK (2020) Intradiscal Platelet-Rich Plasma Injection For Discogenic Low Back Pain And Correlation With Platelet Concentration: A Prospective Clinical Trial. Pain Med; 21(11): 2719-2725.
- Dhurat R, Sukesh M (2014) Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg; 7(4): 189.
- Oudelaar BW, Peerbooms JC, Huis in ‘t Veld R, Vochteloo AJ(2019) Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med; 47(2): 479-487.
- Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH (2017) Analysis of platelet-rich plasma extraction: variations in platelet and blood components between 4 common commer cial kits. Orthop J Sport Med; 5(1):1-8.
- Prysak MH, Kyriakides CP, Zukofsky TA, Reutter SE, Cheng J, et al. (2021) A retrospective analysis of a commercially available platelet-rich plasma kit during clinical use. PM R; 13(12):1410-1417.
- Samadi P, Sheykhhasan M, Khoshinani HM (2019) The use of platelet-rich plasma in aesthetic and regenerative medicine: a comprehensive review. Aesth Plast Surg; 43: 803-814.
- EmCyte Corporation, (2015) One System Two Protocols: PurePRP II. https://emcyte.com/about-us/mission-quality-policy/8- pure-category/23-one-system-two-protocols.html.
- Cervos Corporation. KeyPRP Platelet Separator: KeyPRP Resources https://www.cervos.com/keyprp-resources0
- Kuffler DP (2019) Variables affecting the potential efficacy of PRP in providing chronic pain relief. J Pain Res; 12:109-116.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi