Research Article, J Clin Exp Oncol Vol: 11 Issue: 1
A Single Centre Detailed Clinicopathological, Immunohistochemical and Follow Up Study of Male Breast Cancer Patients from Western India
Anjali Sharma1, Ajay Sharma2, Sanjeev Patni1, Ajay Bapna1, Nidhi Patni1, Anil Gupta1, Lalit Mohan Sharma3, Naresh Somani1, Naresh Ledwani1, Shashikant Saini1, Naresh Jakhotia1, Mudit Sharma1, Shubham Verma3 and Vandana Nunia4*
1Department of Pathology, Bhagwan Mahaveer Cancer Hospital and Research Centre, Jaipur, Rajasthan, India
2Department of Surgical Gastroenterology, Mahatma Gandhi Medical College, Jaipur, Rajasthan, India
3Department of Surgical Oncology, Bhagwan Mahaveer Cancer Hospital and Research Centre, Jaipur, Rajasthan
4Department of Zoology, University of Rajasthan, Jaipur, Rajasthan, India
*Corresponding author
Vandana Nunia, Department of Zoology, University of Rajasthan, Jaipur, Rajasthan, India
Tel: +91 7742462690;
E-mail: vandananunia@gmail.com
Received date: 31 December 2021, Manuscript No: JCEOG-22-51078;
Editor Assigned: 03 January 2022, PreQC No: JCEOG-22-51078 (PQ);
Reviewed Date: 13 January 2022, QC No: JCEOG-22-51078;
Revised Date: 20 January 2022, Revised Manuscript No: JCEOG-22-51078 (R);
Accepted Date: 28 January 2022, DOI: 10.4172/2324-9110.11.1.291
Abstract
Aim: The clinicopathological features, Immunohistochemical (IHC) characteristics, estimated recurrence, treatment and survival outcomes of Male Breast Cancer (MBC) patients were analyzed.
Methods: We have retrospectively evaluated the tumor registry data of 71 MBC (1.11% of total breast cases) patients from 2010 to 2018. Statistical analysis included the new Magee Equation 2 (nME2) for the calculation of Estimated Recurrence Score (ERS), Kaplan-Meier method to analyze survivals and cox survival model for multivariate prognostic analysis.
Results: Chief complaints, history, gross and microscopic characteristic of MBC patients were investigated. MBC molecular subtypes included luminal subtype A (57.74%), luminal subtype B (26.76%), HER-2 (12.67%) and TNBC (2.81%). Male breast cancer patients were more likely to be invasive carcinoma of No Special Type (NST) (95.77%), ER positive (84.50%) PR positive (77.46%) and Her 2/Neunegative (72.97%). Low, intermediate and high estimated recurrence scores were reported in 20, 37 cases and 14 cases respectively. In the follow up study metastasis was reported in 13 cases and recurrence in 5 cases and metachronous multiple primary tumor in 2 cases. Out of 71 cases 55 were effectively followed up, 5-year Overall Survival (OS) and Disease Free Survival (DFS) rates were 72.72% and 63.63% respectively. Multivariate analysis showed lymphovascular invasion, molecular subtypes, metastasis, age, tumor size, Ki-67 and intra-ductal components to be prognostic factors for survival of MBC.
Conclusion: Male breast cancer has a lower incidence rate and poor prognosis. MBC patients exhibited large tumor size, node positivity, metastasis, high percentage of hormonal receptor positivity, high Nottingham grade and estimated recurrence score. More emphasis should be placed on early diagnosis to
improve survival.
Abbreviations: MBC: Male Breast Cancer; IHC: Immunohistochemistry; ER: Estrogen Receptor; PR: Progesterone Receptor; HER2: Human Epidermal Growth
Factor Receptor 2; TNBC: Triple-Negative Breast Cancer; nME2: new Magee Equation 2; PET Scan: Positron Emission Tomography Scan; CT Scan: Computerized Tomography Scan; BRCA: Breast Cancer Gene; Invasive carcinoma (NST): Invasive carcinoma of No Special Type (NST); pTNM: Tumor, Nodes, and Metastases; ERS: Estimated Recurrence Score; OS: Overall Survival; DFS: Disease Free Survival.
Keywords: Male breast cancer; Molecular subtypes; Pathology; Immunohistochemistry; Recurrence acore; Followup
Introduction
Male breast cancer is having lifetime risk of approximately 1:1000, very low as compared to 1:8 in case of female breast cancer but recent data shows that the incidences of MBC are slowly rising [1-6]. It is usually a disease of elderly men and seen in 6th or 7th decade. MBC is an aggressive and uncommon disease and most of the patients diagnosed at advanced stage. Risk factors related to male breast cancer are old age, endocrine factors, genetic factors, exposure to radiation and hormones. It develops more commonly in male with some underlying medical conditions like high oestrogen/androgen ratio as seen in the cases of liver disease, obesity, testicular tumours and Klinefelter’s syndrome [7,8].
Although both male and female breast cancer diseases have similarities, there are notable differences in risk factors, prognosis, and survival. Male breast cancers are hereditary in up to 40% of cases, as compared to female breast cancer where it is only 5%-10% and germline mutations in BRCA2 gene are more common than BRCA1 [3]. MBC presents at older age, with low to intermediate grades, higher stage, higher rates of hormone receptor positivity, more lymph node involvement, increased secondary malignancies, distinct molecular profiles and overall poorer prognosis. Some researchers state that MBC imitate the features of post-menopausal female breast cancer. Mortality rate is high in MBC patients in comparison to female that may be due to late diagnosis [9]. These patients respond differently to treatment and biggest difference is in endocrine management [10].
MBC has a varying rate of incidence across different geographies and ethnic groups. There are also racial/ethnic differences observed in MBC treatment and outcomes, as black men are more likely to die from breast cancer than white men [9,11]. Therefore more clinicopathological studies from various geographical regions are required to improve the diagnosis and treatment outcome in MBC patients. These studies will help in better understanding of its biology and histogenesis so that gender specific treatment can be offered. The epidemiological data regarding MBC is little as compared to female counterpart and this is more in India where only a few clinical studies are available with limited samples [12-15]. Indian council of medical research data from population as well as hospital-based cancer registries’ statistics didn’t provide any separate data on breast cancer in male population. As our institute, being one of the leading super-speciality cancer institute, providing multi-modality/multi-disciplinary approach for investigation as well as treatment to cancer patients of north western part of India. We hope that our data regarding male breast cancer would help in better understanding of male breast cancer from pathological points of view.
Material and Methods
This was a retrospective study carried out at Bhagwan Mahavir cancer hospital and research centre Jaipur, Rajasthan, India, during the period of 2010 to 2018. Total 97 patients with male breast lesions were taken into consideration, of which 71 cases were malignant and 26 cases were benign breast lesions. Resection specimens of the breast received in the department of pathology were included in the study. Records were checked retrospectively for available data for clinical history and examination including age, chief complaints, side and duration of lesion at the time of presentation, lymph node status, habits of smoking, gutakha chewing, alcohol consumption and significant family history. Gross details were collected as side of breast involvement, size of tumours, quadrant of involvement, nipple areola status, cut surface of tumours, margin of tumour, base of the resection specimen, remaining breast tissue status and dissected lymph nodes. All this information was cross-checked and validated by available sources. Metastatic workup and treatment history were noted down as per the available records. Clinical, pathological, and biological features were assessed on all patients according to our routine practice. Microscopic details recorded from histopathology were histological diagnosis, differentiation, intraductal component, desmoplasia, margin of tumour, lymphocytic response, lympho-vascular invasion, perineural invasion, nipple areola, status of base and remaining breast tissue and lymph node status. Hematoxylin and Eosin (HE) stain was employed to stain the histological slides. Immunohistochemistry study was done on representative tumour blocks for ER, PR, Her-2/Neu and Ki-67. For ER and PR, H scoring system was employed to ascertain the proportion of stained cells and assess the intensity of the nuclear staining [16].
The HER2 test was scored from 0 to 3+ in which: Score 0 or 1 were reported as negative; 2+ as equivocal; and 3+ as positive. Specimens showing equivocal HER2 staining were outsourced (core diagnostic, SRL diagnostic, Medgenome, Gurgaon, Haryana) for further examination with the help of Fluorescent In Situ Hybridization (FISH) and their results were documented. The immunohistochemical stainings were used to classify the breast cancer cases into four subtypes: Luminal A (ER+ and/or PR+, HER2-, Ki-67<14%), luminal B (ER+ and/or PR+, HER2-, Ki-67 ≥ 14%), HER2-enriched (ER-, PR-, HER2+), and TNBC (ER-, PR-, HER2-).
Nottingham grade was calculated by combining nuclear grade, tubule formation, and mitotic rate. Each element was given a score of 1 to 3 and scores of all three components were added together to give the grade (range 3-9) [17,18]. New Magge’s Equation 2 (nME2) for estimated recurrence score was determined by using online tool based on clinical data (Nottingham grade, ER status, PR status, Her-2/Neu and tumour size). Magee ERS were compared with follow up of relapse and metastasis in the low vs. high recurrence score groups.
Statistical analysis
All the data received from hospital-based software and available files, was filled in the excel sheet. Further this numerical data of various parameters was analysed by calculating mean, mode, median, standard deviation, standard error of mean with the help of excel functions. The patients' follow-ups were carried out by telephone, electronic medical records and letters.
Disease Free Survival (DFS) was defined as the time from date of diagnosis to disease progression or death by any cause on the date of the last follow-up. Overall Survival (OS) was defined as the time from date of diagnosis until death by any cause. Disease Free Survival (DFS) was estimated by considering recurrence of breast cancer, multiple primary cancer and death as events. All patients were followed until December 31st 2019, or death from any cause. Kaplan-Meier methodology was employed to analyze survival and line plot was made using matplotlib module function of statistical programming language Python. Penalized cox model was used to investigate the effects of the clinical characteristics on patient survival. Coefficients of various variables were estimated using the penalized cox model in python. Model was trained using cox net survival analysis function from scikit-survival module.
Results
Total around 6375 breast tissues were received during 2010 to 2018 (up to 31st July) for reporting, out of 6375 breast tissues 97 cases (1.52% of total breast tissues) were reported as male breast lesions. Further out of 97 reported lesions 71 cases (73.19%) were malignant (1.11% of total breast tissues) and 26 cases (26.80%) were benign. MBC (n=71) cases included 68 cases for invasive carcinoma (NST) (68), 1 case each for mucinous carcinoma, invasive papillary carcinoma and squamous cell carcinoma.
Age ranged from 21 to 86, with median of 62 years, mean of 58.56 years, mode of 62 years. For the MBC cases (n=71) maximum 24 cases (33.80%) were reported in the 61-70 age group, 15 cases (21.12%) in 71-80, 14 cases (19.71%) in 51-60, 10 cases (14.08%) in 41-50 and 7 cases (9.85%) in 31-40 and 1 case (1.40%) reported in the 21-30 age group respectively. For MBC (n=71), most common side was left side in 35 cases (49.29% cases), right side in 32 cases (45.07% of cases) and not known in 4 cases (5.63% cases).
History and clinical characteristics
Out of total cases (n=71), lump/swelling was observed in 68 cases (95.77%), followed by pain in 2 cases (2.81%), nipple discharge in 1 case (1.40%) and no complain of ulcer was reported in any case. Duration of complaints was reported to be from 1 month to 120 months (mean 12.40 months, median 6 months). Lymphadenopathy (n=66) was reported in 21 cases (29.57%), not seen in 45 cases (63.38%) and not known in 5 cases (7.04%). Family history (n=51) of breast cancer was seen in 2 cases (3.92%). Habits of bidi/cigarette smoking/gutakha chewing or alcohol consumption were observed in 24 (33.80%) cases. History of other malignancy (n=52) was observed in 7 cases (13.46%) which included 5 cases (9.61%) of breast cancer, 1 case each (1.92% each) for Ewing’s sarcoma/primitive neuroectodermal tumor and carcinoma Pyriform fossa respectively (Table 1).
Table 1: Chief complaint and clinical characteristics of male breast cancer patients.
Gross and microscopic characteristic of tumor
Tumour size (n=67) ranged from 1 cm to 12 cm, with mean of 3.67 cm, standard deviation 2.18, standard error of mean 0.33. Tumour size of ≤ 2 cm was observed in 14.08% of cases (10 cases) 2 cm-5 cm in 69.01% of cases (49 cases), >5cm in 11.26% of cases (8 cases), and unknown in 5.63% of cases (4 cases). Gross status of nipple and areola (n=71), unremarkable in 52 cases (73.23%), retracted in 10 cases (14.08%), absent in 6 cases (8.45%) and eroded in 3 cases (4.22%). Tumour cut surface (n=71) was gray white in 68 cases (95.77%), cystic, yellowish and mucinous in 1 case (1.40%) each respectively. Margins of tumour were known in 71 cases, of which 64 cases (90.14%) were infiltrating and 7 cases were with expanding (pushing) margins (9.85%). Status of involvement of base was available for 71 cases, of which 69 cases (97.18%) were unremarkable and 2 cases (2.81%) were involved by tumour. Grossly, remaining breast (n=71) was unremarkable in 70 cases (98.59%) and fibrocystic in 1 case (1.40%) (Table 2).
Table 2: Gross characteristic of male breast cancer patients.
Intraductal component was observed in 8 cases (11.26%) and not seen in 63 cases (88.73%). Lymphocytic response was noted as mild in 63 cases (88.73%), moderate in 6 cases (8.45%) and severe in 2 cases (2.81%). Desmoplasia was noted as minimum in 16 cases (22.53%), moderate in 42 cases (59.15%) and marked in 13 cases (18.30%). Lympho-vascular invasion was observed in 32 cases (45.07%) and not seen in 39 cases (54.92%). Perineural invasion was seen in 5 cases (7.04%) and not seen in 66 cases (92.95%) (Table 3).
Table 3: Microscopic characteristics of male breast cancer patients (n=71).
pTNM staging
Most common tumour stage T2 was reported in 52 cases (73.23%), T1 in 10 cases (14.08%), T4 in 5 cases (7.04%) and T3 was seen in 4 cases (5.63%). Most common nodal status was seen as N0 (no nodal involvement) in 32 cases (45.07%), followed by N1 in 26 cases (36.61%), N2 in 8 cases (11.26%) and N3 in 5 cases (7.04%). As a part of metastatic work up, bone scan was available for 26 cases, of which 3 scans (4.22%) were abnormal whereas, 25 scans (32.39%) were found normal and no records available for 63.38% of cases (45 cases). PET CT scan (n=15) showed 13 out of 15 scans (86.66%) abnormal with metastasis, and 2 PET-CT scans (13.33%) were normal. Out of 13 abnormal scans single site of metastasis was observed in 6 cases (46.15% of abnormal scans), and multiple site metastasis in 7 cases (53.85% of abnormal scans). Most common site of metastasis observed was lymph nodes in 9 cases (69.23% of abnormal scans), followed by bone in 7 cases (53.85% of abnormal scans), lung in 6 cases (46.15% of abnormal scans), and 1 case each (7.69% of abnormal scans each) of brain, adrenal and head & neck region (Table 4).
Table 4: Patient clinically characterised by TNM staging.
Immunohistochemistry study
ER positivity was reported in 60 (84.50%) cases and negative in 11 (15.49%) cases. PR positivity was found in 55 (77.46%) cases and negative in 16 (22.53%) cases. For Her2Neu most common intensity pattern observed was 0 in 54 cases (72.97%), followed by 3+, 1+ and 2+ in 9 cases (12.16%), 8 cases (10.81%) and 3 cases (4.05%), respectively. After confirmation with FISH2 Her2Neu2+ equivocal cases were reported negative and 1 case was positive.
The vast majority of 41/71 cases were classified as luminal type A (57.74%), whereas 19/71 (26.76%) were luminal type B, 9/71 (12.67%) cases were classified as HER2 driven and 2/71(2.81%) TNBC cases were identified. All 41 luminal type A cases showed low Ki-67 values (<14%), the 19 cases were considered luminal type B because of high Ki-67 (≥ 14%).
Nottingham grade, recurrence score and follow up
Clinical follow-up data showed that 92.30% (48/52, data unknown in 19 cases) of patients received chemotherapy/hormonal therapy, 65.38% (34/52, data unknown in 19) received radiotherapy after initial surgery. Nottingham grade was interpreted and graded, grade 1 was reported in 23 cases (32.39%) 2 in 44 cases (61.97%) and grade 3 in 4 cases (5.63%). Estimated recurrence score nME2 (new Magee Equation 2) was calculated ranging from 10-46, with low ERS in 20 cases (28.16%), intermediate ERS in 37 cases (52.11%), high ERS in 14 cases (19.71%) (Table 5). In the actual follow up study distant metastasis was reported in 13 (23.63%) cases and tumor relapse in 5 (9.09%) cases and metachronous multiple primary tumor in 2 cases (3.63%). Out of 5 patients with tumor recurrence high and intermediate ERS were reported in 2 and 3 cases each respectively. In the metastasis study 3 cases reported high ERS, 8 intermediate and 2 cases reported low ERS values.
Table 5: Patient clinically characterised by Nottingham score and estimated recurrence score (n=71).
Out of 71 malignant cases follow up was available for 55 cases and 16 patients lost in follow up. The average follow-up time was five years. Out of 55 followed up cases, 15 (27.27%) patients died and overall 5-year OS and DFS of the remaining 40 patients was 72.72% and 63.63% respectively. Of the 15 patients who died, cause of death was unknown in two cases, 2 patients died of non-breast cancer causes and only 11 died of breast cancer (Figure 1).
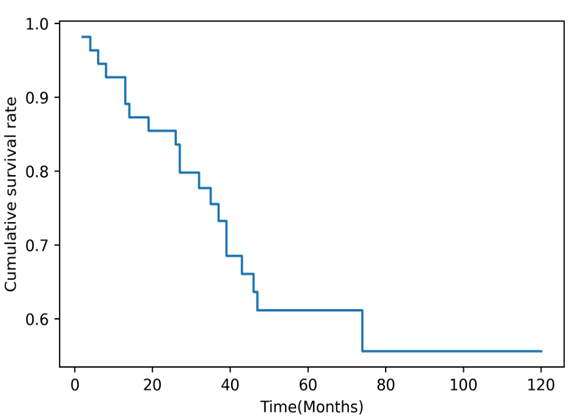
Figure 1: The Disease Free Survival (DFS) curve of patients with male breast cancer (n=55).
Multivariate analysis of factors affecting survival
Multivariate analysis showed that lymphovascular invasion (coefficient value=1.889), molecular subtypes (0.956), metastasis (0.421), age (0.333), tumor size (0.303), Ki-67 (0.0956) and intra-ductal components (0.0615) were associated with poorer survival of MBC patients.
Discussion
As occurrence of MBC is rare, clinical informations are generally available from small single institute based retrospective studies. In the present study we have retrospectively analysed 71 MBC patient samples. The incidence of MBC in our study was 1.11% which is consistent with the worldwide incidence. But, other studies of male breast cancer available from India have variably reported occurrence from 0.4% to 4.1%. In comparison to female breast cancer cases, male patients are slightly older at diagnosis. However, the data available from India have shown that disease is usually seen in younger age group. The median age at presentation in our study was 58 years which is almost a decade earlier than what is being reported in the west [12,13,19-21]. In our study we have invasive carcinoma (NST) (97.77%) as most common histological type; similar results were seen in several other male breast cancer studies also [22-25].
Male breast cancer is rarely diagnosed in asymptomatic phase [26]. MBC patients commonly present with a painless, retro-areolar mass followed by less commonly with skin ulceration, nipple retraction, bleeding from nipple and palpable axillary lymphadenopathy [1]. In our study we have found lump/swelling being the most common presentation in 95.77% of cases, nipple discharge in 1.40% cases, and pain in 2.81% cases. Other studies have also shown nipple involvement as an early event in a substantial number of patients [12,27-29]. We have observed intraductal component in 11.26% of cases and lymphovascular invasion in 45.07% of cases, similar findings were reported by Yu et al. [30]. Desmoplasia in our study was seen as moderate in 59.15% similar results were reported by Vermeulen et al. [31]. Breast cancer prognosis is worse in men as compared to women with higher tumour stage at presentation [32]. Most of our patients presented to us in advanced stage of disease and large tumor size. Most common pT observed in our study was pT2 in 73.23% cases, pT1 in 14.08% cases, pT3 in 5.63% cases and pT4 in 7.04% cases. MBC had a greater frequency of lymph node positivity at presentation. We have reported N1 in 36.61% cases, N2 and N3 in 11.26% and 7.04% cases respectively. Node positivity indicated advanced stage of disease. This is usually due to the poor awareness of early signs of the disease and lack of early detection by mammography. Male breast cancer is more likely to be node-positive with more frequent lymphovascular invasion as seen in various other studies [33,34].
MBC is believed to be a hormone-driven disease and has a high hormone (ER/PR) positive receptor status as compared to female counterpart [7,35]. In our study 84.50% cases showed ER positivity and 77.46% of cases were having PR positivity. Overexpression of HER2 also appears higher amongst male breast cancer cases and 12.67% were found to be HER2/Neu positive in our study. Similar results were seen in other studies done by Wan et al.; Silvestril et al.; Staruch et al. [36-38] also. Male breast cancers more often exhibited features associated with higher Nottingham grade and further high Magee equation score. We have reported Nottingham grade 2 in 44 cases (61.97%), followed by grade 1 in 23 cases (32.39%) and grade 3 in 4 cases (5.63%). Multiple studies have shown an independent prognostic significance of grade in breast cancer [39]. Another important breast cancer prognostic marker is Magee equations that have been developed as accurate tools for predicting recurrence in breast cancer patients using basic clinicopathological parameters. Magee equations provide a reasonable estimate of actual ODX recurrence score. In the present study new Magee equation 2 was used for estimated recurrence score calculated for risk stratification of patients and to predict outcomes. Low ERS (<18) was observed in 28.16% cases, intermediate score (≥ 18 to <30) in 52.11% cases and 19.71% cases had shown high score (≥ 30) by new Magee 2 equation. In the present study we have reported the average recurrence score in men 20.15 which is higher than the average recurrence score in women reported in the literature. Male breast cancer patients more commonly have intermediate recurrence score, while female breast cancer patients more commonly have low recurrence score [40]. In the actual follow up recurrence study we have observed single site of metastasis in 6 cases, and multiple site metastasis in 7 cases. MBC typically presents as an irregular, firm mass close to skin, nipple, and chest wall. Therefore, Invasion of these structures can occur early and later metastasize in other tissues as compared to females [41]. Findings of our study are similar to the study of Hou et al. [42] in which they studied 63 MBC patients and found increased risk for distant metastasis in MBCs with intermediate/high new Magee equation 2 ERS, but no increased risk for lymph node metastasis. Distant metastasis was significantly associated with positive lymph node, high Nottingham grade, and increased new Magee equation 2 ERS [36].
In contrary to female breast cancer only few studies have been conducted for molecular subtyping of male breast cancer that showed conflicting results, because of small groups and different immunohistochemical definitions. In the present study luminal subtype A was the most common in all patients (57.74%), followed by the luminal subtype B (26.76%), HER-2 subtype (12.67%) and TNBC (2.81%). In the similar study Kornegoor et al. [43] reported that luminal A and to a lesser extent luminal B types represent the vast majority of breast cancers in men. Molecular subtypes showed different features, recurrence patterns and survival. Therefore, molecular subtypes could provide clinically useful information of tumor biology and clinical behaviors, and could be used for determining treatment and surveillance strategies [44].
In our study, multi variant analysis showed age, tumor size, metastasis and lymphovascular invasion, molecular subtypes and Ki-67 to have a significant effect on survival. Age and large tumor size are associated with poor prognosis [45]. Lymphovascular invasion and metastasis, are associated with more aggressive biologic behavior of male breast cancer. Male breast cancer is more likely to be node-positive with more frequent lymphovascular invasion as seen in various other studies [33,34]. Male breast cancers showed higher proliferative activity, as measured by the Ki-67 proliferation index (mean labeling index of 33%). It may predict aggressive behavior of the tumor and higher histopathological grades [46]. Breast cancer prognosis is worse in men as compared to women with higher tumor stage at presentation [32].
Treatment for MBC largely follows management of postmenopausal breast cancer and depends on tumor size, location, stage, lymph node involvement and biologic characteristics such as estrogen receptor and HER2 expression. In our study 92.3% patients were given chemo/hormonal therapy and 65.38% radiotherapy after initial surgery. In the present study 5 year survival rate was 72.72% and same duration of follow-up was observed with Sanguinetti et al.; Yu et al.; Wan et al.; Hou et al.; Margaria et al.; Wick et al. [27,30,41,42,47,48]. The survival rate in males is low as compared to females as most cases of men are detected at very advanced stages. The less favorable results in male patients are due to the more advanced stage at presentation as well as a higher mean age at presentation leading to more co-morbidity [33,49].
The present study has the certain limitations of a retrospective study from a single institution conducted over a long time period with limited cases and incomplete clinical and follow-up informations. But it provides information of molecular subtype, immunohistochemical characterization in male breast cancer of Indian population, which to our knowledge, is rare in previous publications.
Conclusion
In the present study, we found invasive carcinoma of No Special Type (NST) as most common histopathological type. Multivariate analysis showed lymphovascular invasion, molecular subtypes, metastasis, age, tumor size, Ki-67 and intra-ductal components were associated with poor survival in MBC. We need to improve the public awareness of the disease for the early detection and better prognosis.
Authors Contribution
AS involved in study concept, design and clinical studies. SP, AG, LMS, NJ, NL, SS, NP, AB and NS performed clinical studies. MS performed experimental parameters, VN carried out data analysis and manuscript preparation. SK and SV helped in data analysis. AJS involved in critical evaluation of the manuscript. All authors approved the final version of the manuscript.
Ethics Approval
Study was conducted in accordance with declaration of Helsinki and approved by the ethics review committee of Bhagwan Mahavir cancer hospital, Jaipur.
References
- Giordano SH (2018) Breast cancer in men. N Engl J Med 378: 2311-20.
[Crossref], [Google Scholar], [Indexed] - Oto ED, Monti V, Cucchi MC, Masetti R, Varga Z, et al. (2015) X chromosome gain in male breast cancer. Human Pathology 46: 1908–1912.
[Crossref], [Google Scholar], [Indexed] - Turashvili G, Gonzalez-Loperena M, Brogi E, Dickler M, Norton L, et al. (2018) The 21-gene recurrence score in male breast cancer. Ann Surg Oncol 25: 1530.
[Crossref], [Google Scholar], [Indexed] - Collins LC, Goldblum JR, Lamps LW, McKenney JK, Myers JL (2018) Rosai and Ackerman’s surgical pathology (11th edtn). Philadelphia: Elsevier: 1434-1512
- Speirs V, Shaaban AM (2009) The rising incidence of male breast cancer. Breast Cancer Res Treat 115: 429–430.
[Crossref], [Google Scholar], [Indexed] - Hodgson NC, Button JH, Franceschi D, Moffat FL, Livingstone AS (2004) Male breast cancer: Is the incidence increasing? Ann Surg Oncol 11: 751–755.
[Crossref], [Google Scholar], [Indexed] - Di Lauro L, Maddalena B, Pizzuti L, Vici P, Sergi D, et al. (2015) Androgen receptor and antiandrogen therapy in male breast cancer. Cancer Letters 368: 20–25.
[Crossref], [Google Scholar], [Indexed] - Zabolotny BP, Zalai CV, Meterissian SH (2005) Successful Use of letrozole in male breast cancer: A case report and review of hormonal therapy for male breast cancer. Journal of Surgical Oncology 90: 26–30.
[Crossref], [Google Scholar], [Indexed] - Fentiman IS, Fourquet A, Hortobagyi GN (2006) Male breast cancer. Lancet 367: 595–604.
[Crossref], [Google Scholar], [Indexed] - Eggemann H, Bernreiter A, Reinisch M, Loibl S, Taran FA, et al. (2019) Tamoxifen treatment for male breast cancer and risk of thromboembolism: Prospective cohort analysis. Br J Cancer 120: 301–305.
[Crossref], [Google Scholar], [Indexed] - Weiss JR, Moysich KB, Swede H (2005) Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev 14: 20–26.
[Crossref], [Google Scholar], [Indexed] - Chikaraddi SB, Krishnappa R, Deshmane V (2012) Male breast cancer in Indian patients: is it the same? Indian J Cancer 49: 272-276.
[Crossref], [Google Scholar], [Indexed] - Rai B, Ghoshal S, Sharma SC (2005) Breast cancer in males: APGIMER experience. J Cancer Res Ther 1:31–33.
[Crossref], [Google Scholar], [Indexed] - Shah S, Bhattacharyya S, Gupta A, Ghosh A, Basak S (2012) Male breast cancer: A clinicopathologic study of 42 patients in Eastern India. Indian J Surg Oncol 3: 245–249.
[Crossref], [Google Scholar], [Indexed] - Andleeb A, Lone MM, Ahmad HI, Afroz F, Manzoor A, et al. (2016) Male breast cancer: A 10-year experience of a tertiary care center in North India. Clinical Cancer Investigation Journal 5: 521-526.
[Crossref], [Google Scholar] - Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11: 155-68.
[Indexed] - Bloom, HJ, Richardson WW (1957) Histological grading and prognosis in breast cancer: A study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 11: 359-377.
[Crossref], [Google Scholar], [Indexed] - Le-Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, et al. (1989) Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer 64: 1914-21.
[Crossref], [Google Scholar], [Indexed] - Shah P, Robbani I, Shah O (2009) Clinicopathological study of male breast carcinoma: 24 years of experience. Ann Saudi Med 29: 288–293.
[Crossref], [Google Scholar], [Indexed] - Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, et al. (2011) Incidence and outcome of male breast cancer: An international population-based study. J Clin Oncol 29: 4381–4386.
[Crossref], [Google Scholar], [Indexed] - Sundriyal D, Kotwal S, Dawar R, Parthasarathy KM (2015) Male breast cancer in India: Series from a cancer research centre. Indian J Surg Oncol 6: 384–386.
[Crossref], [Google Scholar], [Indexed] - Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, et al. (2007) Male breast cancer in the veterans affairs population: A comparative analysis. Cancer 109: 1471-7.
[Crossref], [Google Scholar], [Indexed] - Golshan M, Rusby J, Dominguez F, Smith BL (2007) Breast conservation for male breast carcinoma. Breast 16: 653-6.
[Crossref], [Google Scholar], [Indexed] - Culell P, Solernou L, Tarazona J, Roma J, Martí E, et al. (2007) Male breast cancer: A multicentric study. The Breast Journal 13: 213-215.
[Crossref], [Indexed] - Gentilini O, Chagas E, Zurrida S, Intra M, De Cicco C, et al. (2007) Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist 12: 512-5.
[Crossref], [Google Scholar], [Indexed] - Gennari R, Curigliano G, Jereczek-Fossa Ba, Zurrida S, Renne G, et al. (2004) Male breast cancer: A special therapeutic problem anything new? (Review). International Journal Of Oncology 24: 663-670.
[Crossref], [Google Scholar], [Indexed] - Sanguinetti A, Polistena A, Lucchini, R, Monacelli M, Galasse S, et al. (2016) Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our breast unit. International journal of surgery case reports 20: 8–11.
[Crossref], [Google Scholar], [Indexed] - Giordano SH (2005) A review of the diagnosis and management of male breast cancer. Oncologist 10: 471–479.
[Crossref], [Google Scholar], [Indexed] - Hill A, Yagmur Y, Tran KN, Bolton JS, Robson M, Borgen PI. Localized male breast carcinoma and family history. An analysis of 142 patients. Cancer. 1999;86:821–825.
[Crossref], [Google Scholar], [Indexed] - Yu XF, Yang HJ, Yu Y, Zou DH, Miao LL. A Prognostic Analysis of Male Breast Cancer (MBC) Compared with Post-Menopausal Female Breast Cancer (FBC). PLoS One. 2015;10(8):e0136670.
[Crossref], [Google Scholar], [Indexed] - Vermeulen MA, Slaets L, Cardoso F, Giordano SH, Tryfonidis K, van Diest PJ, et al. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer. 2017;82:219-27.
[Crossref], [Google Scholar], [Indexed] - Salvadori B, Saccozzi R, Manzari A, Andreola S, Conti RA, Cusumano F, et al. Prognosis of breast cancer in males: an analysis of 170 cases. Eur J Cancer. 1994; 30A(7): 930-935.
[Crossref], [Google Scholar], [Indexed] - Joshi MG, Lee AK, Loda M, Camus MG, Pedersen C, et al. (1996) Male breast carcinoma: An evaluation of prognostic factors contributing to a poorer outcome. Cancer 77: 490-498.
- McLachlan SA, Erlichman C, Liu FF, Miller N, Pintilie M. Male breast cancer: an 11 year review of 66 patients. Breast Cancer Res Treat. 1996;40 (3):225–230.
[Crossref], [Google Scholar], [Indexed] - Contractor KB, Kaur K, Rodrigues GS, Kulkarni DM, Singhal H. Male breast cancer: is the scenario changing. World Journal of Surgical Oncology2008; 6:58.
[Crossref], [Google Scholar], [Indexed] - Wan BA, Ganesh V, Zhang L, Sousa P, Drost L, Lorentz J, Vesprini D, Lee J, Rakovitch E, Lu FI, Eisen A, Yee C, Lam H, Chow E. Treatment Outcomes in Male Breast Cancer: A Retrospective Analysis of 161 Patients. Clin Oncol (R Coll Radiol). 2018 Jun;30(6):354-365.
[Crossref], [Google Scholar], [Indexed] - Silvestri1 V, Barrowdale D, Mulligan AM, Neuhausen SL, Fox S,Karlan BY et al. Male breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Research. 2016;18:15.
[Crossref], [Google Scholar], [Indexed] - Staruch RM, Rouhani MJ, Ellabban M. The surgical management of male breast cancer: Time for an easy access national reporting database? Ann Med Surg (Lond). 2016;9:41–49.
[Crossref], [Google Scholar], [Indexed] - Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, Blamey RW, Ellis IO. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008 Jul 1;26(19):3153-8.
[Crossref], [Google Scholar], [Indexed] - Liu J, Suresh A, Palettas M, Stephens J, Ganju, A et al., Outcomes, and Management Strategies of Male Breast Cancer: A Single Institution Comparison to Well-Matched Female Controls. Eur J Breast Health 2020; 16(3): 201-207.
[Crossref], [Google Scholar], [Indexed] - Wang-Rodriguez J, Cross K, Gallagher S, Djahanban M, Armstrong JM, Wiedner N, Shapiro DH Male Breast Carcinoma: Correlation of ER, PR, Ki-67, Her2-Neu, and p53 With Treatment and Survival, a Study of 65 Cases. Mod Pathol 2002; 15(8):85361.
[Crossref], [Google Scholar], [Indexed] - Hou Y, Moosavi HS, Wei L, Parwani AV, Li XB, Li Z. Magee Equation Recurrence Score Is Associated With Distal Metastatic Risk in Male Breast Carcinomas: Experience From Two Institutions. Am J ClinPathol. 2018;150(6):491-498.
[Crossref], [Google Scholar], [Indexed] - Kornegoor, R., Verschuur-Maes, A., Buerger, H. et al. Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol 25, 398–404 (2012).
[Crossref], [Google Scholar], [Indexed] - Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012 Feb;21(1):50-7.
[Crossref], [Google Scholar], [Indexed] - Ravi A, Bang H, Karsif K, Nori D. Breast cancer in men: prognostic factors, treatment patterns, and outcome. Am J Mens Health. 2012 Jan;6(1):51-8. Epub 2011 Aug 10.
[Crossref], [Google Scholar], [Indexed] - Mohammed AA Quantitative assessment of Ki67 expression in correlation with various breast cancer characteristics and survival rate; cross sectional study. Annals of Medicine and Surgery 2019 (48)129-134.
[Crossref], [Google Scholar], [Indexed] - Margaria E, Chiusa L, Ferrari LA, Canton OD, Pich A. Therapy and survival in male breast carcinoma: A retrospective analysis of 50 cases. Oncology Reports. 2000; 7: 1035-1039.
[Crossref], [Google Scholar], [Indexed] - Wick MR, Sayadi H, Ritter JH, Hill DA, Reddy VB, Gattuso P, Low-Stage Carcinoma of the Male Breast A Histologic, Immunohistochemical, and Flow Cytometric Comparison with Localized Female Breast Carcinoma. Am J ClinPathol 1999; 111:59-69.
[Crossref], [Google Scholar], [Indexed] - Ribeiro G. Male breast carcinoma A review of 301 cases from the Christie Hospital & Holt Radium Institute, Manchester. Br. J. Cancer. 1985;51:115-119.
[Crossref], [Google Scholar], [Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 



