Research Article, J Regen Med Vol: 9 Issue: 3
Adipose Derived Stem Cell Engraftment Improves Erectile Function in a Rat Model of Cavernosal Nerve Injury
Reza Izadpanah1*, Zahra Brabadi1, Fatemeh Daneshimehr1, Eckhard U. Alt1, Amit G. Reddy2, Nora M. Haney2, Kenneth J. DeLay2, Laith Alzweri2, Wayne J.G. Hellstrom2, Bryant Song3, Travis Chen3, Kevin Swan3, Philip Kadowitz3, James E. Anaissie4 and Bysani Chandrasekar5
1Heart and Vascular Institute, Tulane University School of Medicine, New Orleans, USA
2Department of Urology, Tulane University School of Medicine, New Orleans, USA
3Department of Pharmacology, Tulane University School of Medicine, New Orleans, USA
4Department of Urology, Baylor College of Medicine, Houston, TX USA
5Harry S. Truman Veterans Memorial Hospital, Department of Medicine, University of Missouri School of Medicine, Columbia, USA
*Corresponding Author: Reza Izadpanah
Applied Stem Cell Laboratory Heart and Vascular Institute, Department of Medicine Tulane University School of Medicine, USA
Tel: 19857050938
E-mail: rizadpan@tulane.edu
Received: July 17, 2020 Accepted: August 24, 2020 Published: August 31, 2020
Citation: Izadpanah R, Brabadi Z, Daneshimehr F, Alt EU, Reddy AG, et al. (2020) Adipose Derived Stem Cell Engraftment Improves Erectile Function in a Rat Model of Cavernosal Nerve Injury. J Regen Med 9:3. doi: 10.37532/jrgm.2020.9(3).167
Abstract
Adipose-derived-stem-cells (ADSC) have shown promise in treating
erectile dysfunction (ED). Here we investigated the effect of ADSC
engraftment in restoring erectile function (EF) following nerve injury
during radical prostatectomy. Sprague-Dawley rats (4 groups; n=8/
group) underwent: 1) laparotomy (Lap) and immediate closure
(Sham); 2) Lap with bilateral cavernosal nerve injury (BCNI) (Crush);
3) Lap with BCNI and intracavernosal injection (ICI) of GFP+-ADSC
at surgery (INJ-1); and 4) Lap with BCNI and ICI of GFP+-ADSC
twice (at surgery and after three weeks) (INJ-2). Six weeks postBCNI, EF was measured via intracorporal pressure (ICP) response
following cavernosal nerve stimulation at 2.5V, 5V, and 7.5V. Penile
and major pelvic ganglion (MPG) tissue were analyzed to detect
GFP+-ADSC by immunohistochemistry. Data showed a significant
decrease in EF in the Crush group compared to Sham at 5V and
7.5V (P<0.01). While EF was significantly improved in both INJ-1
and INJ-2 groups compared to the Crush group (5V and 7.5V;
P<0.01), it was comparable between INJ-1 and INJ-2 groups at
higher voltages. Interestingly, no GFP+-ADSCs were identified in
both penile and MPG tissues in all four groups 6 weeks post-BCNI.
These data indicate that a single intracavernosal administration of
ADSCs is sufficient to improve EF following nerve injury during
radical prostatectomy.
Keywords: Adipose-Derived stem cells, Erectile dysfunction, Bilateral cavernosal nerve injury, Prostatectomy, Nerve regeneration
Abbreviations
Adipose-derived-stem-cells (ADSC) Erectile dysfunction (ED), Erectile function (EF), Post-radical prostatectomy ED (post-RP ED), Laparotomy (Lap), Bilateral cavernosal nerve injury (BCNI), Intracavernosal injection (ICI), Major pelvic ganglion (MPG), Prostate cancer (PCa), Stem cell therapy (SCT), Minimum Essential Medium Eagle (MEM), Fetal bovine serum (FBS), Green fluorescent protein (GFP), Multiplicity of infection (MOI), Dulbecco’s Modified Eagle’s medium (DMEM)
Introduction
Erectile dysfunction (ED) is defined as the inability to obtain or maintain an erection satisfactory for intercourse, and affects up to 50% in men over 70 years of age [1]. A common iatrogenic cause of ED is radical prostatectomy (RP), the treatment of choice for organconfined prostate cancer (PCa); the most common solid malignancy in men. Unfortunately, the most common complication of RP is ED caused by surgical injury to the cavernous nerves, often causing a clinically significant reduction in erectile function (EF) and quality of life [2].
Although ED is a common complication after RP, it has proven difficult to treat. Providers have a plethora of options to treat post- RP ED, known as penile rehabilitation. While this can be helpful in increasing the quality of erection, the results are often difficult to sustain without surgery or dependence on medications [3-6].
Stem cell therapy (SCT) has recently gained momentum as a novel approach to treat post-RP ED [7]. Promising results are obtained with SCT in several pre-clinical animal models, including the rat model of bilateral cavernosal nerve injury (BCNI), summarized in a recent comprehensive systemic review and meta-analysis (7), and starting to show success in human clinical trials as well [8]. Adipose tissuederived stem cells (ADSC), because of their abundance and ease of collection, have become the cells of choice in SCT [9]. ADSCs have been reported to exert regenerative effects on cavernous nerve and smooth muscle via a paracrine mechanism [10].
Although SCT has proven effective in early clinical trials, the mechanisms by which they improve post-RP ED are not fully understood. Interestingly, the injected labeled stem cells that migrate to the site of nerve injury have been shown to rapidly disappear after administration, regardless of the site of injection (e.g., intracavernous injection or ICI, tail vein), indicating that the secreted products of these engrafted stem cells likely operate in improving ED via a paracrine mechanism [7]. In fact, injection of an ADSC-derived cell lysate can restore EF almost as effectively as ADSCs, suggesting that most benefit is derived from the biomolecules released from the stem cells [11]. Moreover, the therapeutic benefit was similar at both one and three months after injection [7], suggesting that the major benefits of SCT occur early after administration. Therefore, we hypothesized that early post-injury injection of ADSCs is maximally effective compared to repeat ADSC engraftments. We further believe that ADSC engraftment enhances a reparative process via a paracrine mechanism, and further improve the therapeutic potential of SCT in post- RP ED.
Materials and Methods
Stem cell culture and characterization
Rat ADSCs at passage 1 were obtained from iXCells Biotechnologies (San Diego, CA) and cultured at 37°C and 5% CO2 in MEM (Minimum Essential Medium Eagle) growth medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/ streptomycin. Cells of passage 2 were labeled with green fluorescent protein (GFP) using a lentiviral vector (multiplicity of infection [MOI] of 5) and polybrene®. Forty-eight hours after transduction, GFP expressing (GFP+) ADSCs were collected by FACS. GFP+ADSCs were characterized by analyzing the expression of surface markers like CD105, CD90, CD44, CD34, CD45, CD4, CD11b, and CD68 (Invitrogen Corp., Carlsbad, CA) using a Beckman-Coulter Epics FC500 flow cytometer.
In addition, GFP+ADSCs were assessed for their multilineage differentiation capacity into adipocytes and osteoblasts according to a previously described protocol [12]. Briefly, GFP+ADSCs at passage 3 were seeded at a density of 10000 and 5000 cells/cm2 for adipogenic and osteogenic differentiation, respectively, in 6-well plates containing complete medium. Upon attachment, the cells were cultured in their relative induction medium. Adipogenic induction medium consisted of Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% FBS, 1%penicillin/streptomycin, 2 mM L-glutamine (Biochrom AG), 1 mM ascorbat-2-phosphate (Sigma Aldrich Co. LLC), 0.5 μM dexamethasone, 0.5 mM isobutyl methylxanthine, 50 μM indomethacin and 10 μg/ml insulin (Biochrome AG) [12]. The osteogenic differentiation medium consisted of 10 nM dexamethasone (Sigma-Aldrich, St Louis, MO, USA), 10 mM β-glycerol phosphate (Sigma-Aldrich) and 50 μg ml−1 ascorbic acid-2-phosphate (Sigma- Aldrich) [13].
Undifferentiated GFP-transduced ADSCs served as a control. The cells were cultured under standard conditions in a humidified atmosphere with 5% CO2 at 37°C for 14 days. The medium was changed every three days. The cells then were fixed in 4% paraformaldehyde for 30 min and stained with Oil Red O and Alizarin Red to detect adipogenic or osteogenic differentiation, respectively. The stained cells were observed under an optical microscope. The expanded GFP+ ADSCs in passage 4 were injected into the corpora of each animal (1x106 cells in 100 μl PBS).
Animal and experimental design
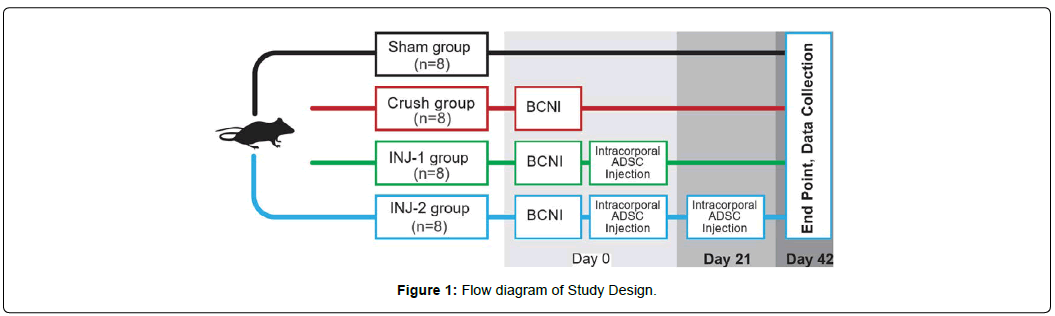
This investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (DRR/National Institutes of Health, 1996). All protocols were approved by the Institutional Animal Care and Use Committee at Tulane University School of Medicine, New Orleans, LA. Eight to ten-week-old male Sprague- Dawley rats (n=32; Charles River, Wilmington, MA) weighing 292-428 grams (10-12 weeks at time of initial operation) were used. Rats were kept at two rats per cage pre- operatively, and then one rat per cage post-operatively. Their water and diet were provided ad libitum. The animals were randomly assigned to four groups of eight each: Group 1 (Sham group) underwent sham surgery, in which a midline laparotomy was performed under anaesthesia and then closed; Group 2 (Crush group) underwent surgery to induce BCNI as detailed below; Group 3 (EXP- 1) underwent BCNI + a single injection of ADSCs, and Group 4 (EXP- 2) underwent BCNI + two injections of ADSCs (administered at the time of surgery and after three weeks). After six weeks, all animals were evaluated for EF. Six weeks was chosen as the final timepoint based on similar pre-existing studies. On a systematic review and meta-analysis of over 20 studies examining the effects of stem cells in a similar model, the median follow-up time was 4 weeks (in 14 of the studies), and was found that ICI stem cells disappeared from the penis rapidly, putting the efficiency of prolonged follow-up into question [7]. The study design is illustrated in (Figure 1). A power analysis was not performed for this proof of concept study. The number of rats per group was instead based on precedent established in preexisting literature on the injection of stem cells in BCNI animal models, most of which used between 5-10 rats per group [7, 11, 14-18].
Cavernous nerve crush surgery
Nerve crush surgery was performed as described in our previous studies [19, 20]. Briefly, rats were anesthetized using intraperitoneal injection of ketamine (100mg/kg; Vet One, 13985-704-10) and xylazine (10mg/kg; et One, 13985-584-10). The prostate was exposed via a midline laparotomy. The major pelvic ganglion (MPG) and cavernous nerve (CN) were identified bilaterally. In the sham group, no further manipulation was performed. In the BCNI groups, both cavernous nerves underwent crush injury by applying a #5 Dumont forceps two times for 30 seconds, 3-5mm distal to the MPG. Adequate crush was confirmed by an observable change in color to gray, with the neurolemma remaining intact. The abdomen was then closed in two layers using absorbable suture and staples.
ADSC administration
After anesthesia, approximately one centimeter of the corpora cavernosa of the penis was exposed using a clamp on the glans penis. A rubber tourniquet was applied to the base of the penis, and injected with 1x106 GFP+ ADSCs in 100 μL of PBS at the “3 o’clock” position of the corpus cavernosum at a 90-degree angle to the skin, directly above the tourniquet, using a 30-gauge Hamilton syringe and under the guidance of a microscope. This injection technique, including size of the needle and the concentration of stem cells, is among the most commonly used in the existing literature for intracavernosal stem cell transplantation [7, 21-23]. The suspension was carefully delivered over three minutes to avoid shearing of cells. The needle was left in place for one additional minute to allow for sufficient diffusion. The tourniquet was then released and the needle removed. No significant penile damage or hematoma formation was observed in any of the animals.
Erectile response
At six weeks after crush injury, rats were anesthetized using Inactin hydrate 98% (Sigma- Aldrich, Inc., St. Louis, MO) at 100 mg/kg. The right crura was cannulated with a 25G needle connected to a Namic Perceptor DT pressure transducer (Navilyst Medical, Marlborough, MA) and a data acquisition system (Biopac MP 100ACE, Santa Barbara, CA) to measure intracorporeal pressure (ICP). The right carotid artery was cannulated for continuous measurement of mean arterial pressure (MAP). The CN distal to the crush injury was stimulated with a square pulse stimulator (Grass Instruments SD9 stimulator, West Warwick, RI) at a frequency of 20 Hz, duration 0.5ms, and pulse width of 0.2 ms at increasing voltages (2.5V, 5V, and 7.5V) for one minute, with 3-5 minute intervals between stimulations. The response in ICP was measured using the software, and the ratio between ICP and MAP (ICP/MAP) obtained at the peak of erectile response was calculated to normalize for variations in systemic blood pressure. MPG and corporal tissue were harvested and embedded in paraffin for further immunohistochemical (IHC) analysis.
Immunohistochemistry
Both MPG and penile tissues were harvested following EF measuring six weeks post- surgery. Both tissues were fixed in 4% paraformaldehyde for 24 hours followed by 70% ethanol, embeded in paraffin, and then sectioned (5μm-thick). After antigen retrieval using Rodent declooker10x (# RD913, 40 min), endogenous peroxidase activity was quenched with 3% H202 (5 min at RT). Biotin/avidin blocking was performed with Avidin-Biotin (# AB972, 10 mins each), and protein block was done using Rodent Block.m (# RBm961, 30 mins). Following incubation with anti-GFP primary antibody (1:300 for 1h) and a secondary antibody (Mouse on Mouse, # Rp-polymer # mm 420, for 30 min), the sections were counterstained and blued with CaT Hemotoxylin (# CATHE 45s) and Tacha’s Bluing Solution 10x (# HTBLu,45s), respectively. Finally, the samples rinsed with deionized H2O were dried, submerged in xylene, and mounted with cover slips using Acrymount (#SL80).
Fluorescence microscopy
In vivo homing of GFP+ ADSCs to the site of injury was tracked using confocal microscope (NIS-ELEMENTS AR 4.60). ADSC were excited with a 561 nm CW laser and detected through a 593 nm/640 nm filter.
Statistical analysis
Data are shown as the mean ± standard error of mean or foldchange difference. Statistical difference between multiple groups was determined using a two-sided t-test in Microsoft Excel, as the data did not require a more complex level of statistical analysis. P < 0.05 were considered significant.
Results
Characterization of ADSCs
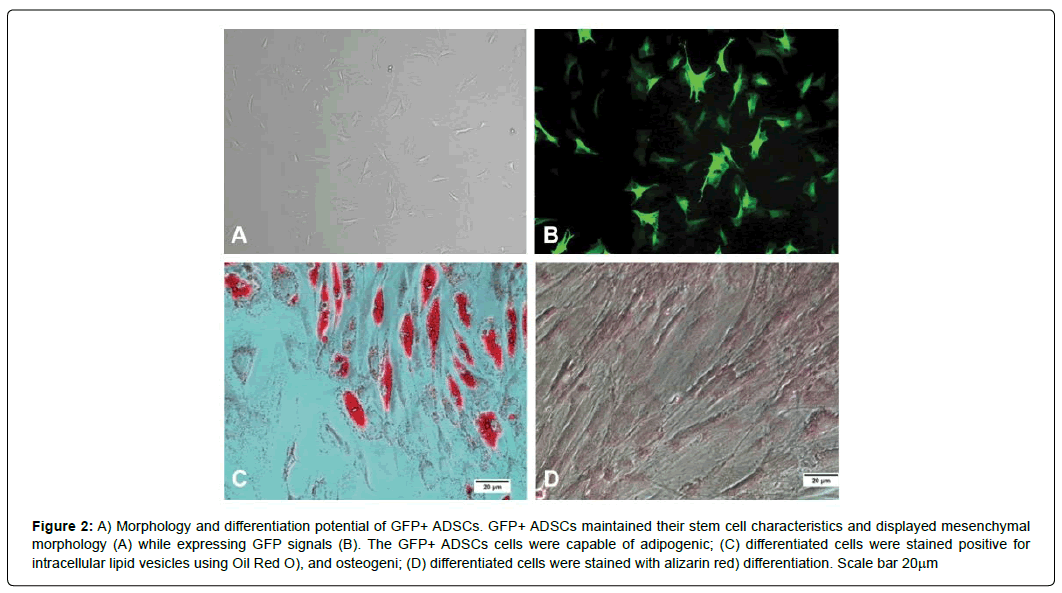
The ADSCs were transduced with a lentiviral vector expressing GFP. The surface expression of CD105, CD90, CD44, CD34, CD45, CD4, CD11b, and CD68 on ADSCs was analyzed by flow cytometry. The results show that GFP+ ADSCs expressed the mesenchymal markers such as CD44, CD90, and CD105, but lacked the expression of hematopoietic markers such as CD11b, CD34, and CD45. The GFP+ ADSCs showed fibroblastic morphology (Figure 2A) while maintaining positive GFP expression (Figure 2B). The forced expression of GFP in ADSCs did not affect self-renewal rates and differentiation potential when compared to non-transduced ASCs. Further, the data in (Figure 2C and 2D) show the osteogenic and adipogenic differentiation potential of GFP+ ADSCs, respectively. We used the GFP+ ADSCs in all engraftment studies.
Figure 2: A) Morphology and differentiation potential of GFP+ ADSCs. GFP+ ADSCs maintained their stem cell characteristics and displayed mesenchymal morphology (A) while expressing GFP signals (B). The GFP+ADSCs cells were capable of adipogenic; (C) differentiated cells were stained positive for intracellular lipid vesicles using Oil Red O), and osteogeni; (D) differentiated cells were stained with alizarin red) differentiation. Scale bar 20μm
Assessment of erectile function
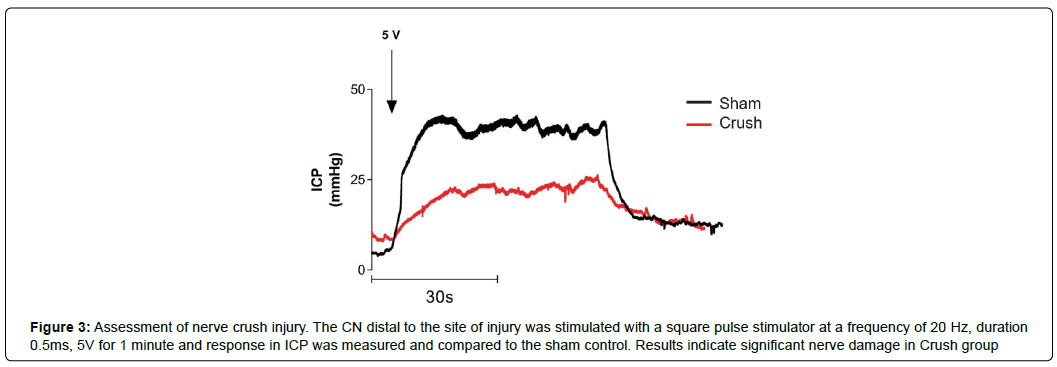
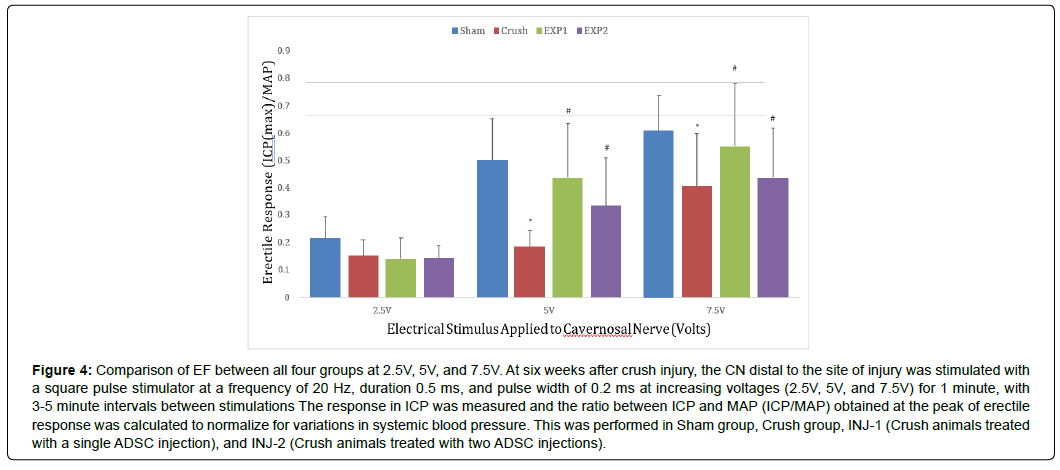
As illustrated in (Figure 1), four groups of Sprague-Dawley rats (n=8/group) underwent: 1) laparotomy and immediate closure (Sham group); 2) Lap with BCNI (Crush group); 3) Lap with BCNI and ICI injection of GFP+ ADSCs at surgery (EXP-1 group); and 4) Lap with BCNI and ICI of GFP+ ADSCs twice (at surgery and after three weeks) (EXP-2 group). To evaluate EF, the maximum ICP to MAP ratio (ICP: MAP) was measured six weeks after surgery at 2.5V, 5V, and 7.5V stimulation (Figure 3). P-values for comparison between groups at different voltages are presented in (Table 1). Rats in the Crush group demonstrated a significant decrease in EF when compared to sham at 5V (0.172 vs. 0.501, P<0.001) and 7.5V (0.276 vs. 0.610, P<0.001), as well as at 2.5V, albeit with no significance (0.156 vs 0.217, P=0.09). The Crush group also had significantly lower EF when compared to rats in the EXP-1 group at 5V (0.172 vs. 0.489, P=0.002) and 7.5V (0.276 vs. 0.601, P=0.002), as well as when compared to the EXP-2 group at 5V (0.172 vs. 0.387, P<0.05 at 5V) and 7.5V (0.059 vs. 0.118, P=0.004), although these relationships were not statistically significant for either group at 2.5V. When compared to the Sham group, both the EXP-1 and EXP-2 groups showed restored EF at all voltages. Moreover, the EF was similar between the EXP- 1 and EXP- 2 groups at all voltages. A graphical comparison of the four groups at each voltage is shown in (Figure 4).
Figure 3: Assessment of nerve crush injury. The CN distal to the site of injury was stimulated with a square pulse stimulator at a frequency of 20 Hz, duration 0.5ms, 5V for 1 minute and response in ICP was measured and compared to the sham control. Results indicate significant nerve damage in Crush group
Figure 4: Comparison of EF between all four groups at 2.5V, 5V, and 7.5V. At six weeks after crush injury, the CN distal to the site of injury was stimulated with a square pulse stimulator at a frequency of 20 Hz, duration 0.5 ms, and pulse width of 0.2 ms at increasing voltages (2.5V, 5V, and 7.5V) for 1 minute, with 3-5 minute intervals between stimulations The response in ICP was measured and the ratio between ICP and MAP (ICP/MAP) obtained at the peak of erectile response was calculated to normalize for variations in systemic blood pressure. This was performed in Sham group, Crush group, INJ-1 (Crush animals treated with a single ADSC injection), and INJ-2 (Crush animals treated with two ADSC injections).
| 2.5V | 5V | 7.5V | |
|---|---|---|---|
| Sham vs Crush | 0.089 | 0.001 | 0.001 |
| Sham vs EXP-1 | 0.073 | 0.892 | 0.909 |
| Sham vs EXP-2 | 0.051 | 0.236 | 0.422 |
| Crush vs EXP-1 | 0.681 | 0.002 | 0.002 |
| Crush vs EXP-2 | 0.626 | 0.027 | 0.004 |
| EXP-1 v EXP-2 | 0.976 | 0.317 | 0.578 |
Table 1: P-value comparison of maximum ICP/MAP between each group at all voltages.
Staining analysis
While all of the GFP+ ADSCs demonstrated high transduction (Figure 2B), no GFP+ stained cells were detected in histology. Neither immunohistochemistry nor florescent confocal imaging showed a positive GFP signal in intracorporally engrafted ADSCs 6 weeks after the first injection in penile and MPG tissues. A comparison of GFPstained groups for MPG and penile tissue is shown in Figure 5A (IIV) and 5B (I-IV), respectively
Discussion
Current penile rehabilitation therapies for post-RP ED have shown limited long- term efficacy, leaving much to be investigated. While stem cell-based ED therapies have shown encouraging outcomes in both animal and human studies, not much is known about the mechanism of action, the proper dosage, and the treatment strategy that will exert maximal therapeutic benefit. In this study, we compared the effect of a single or a two-time ICI administration of ADSCs on improving EF post-RP ED. Our data show that while a single ICI administration of ADSCs significantly improved EF in a rat model of post-RP ED, a second administration did not further improve EF. Moreover, histologic analysis revealed that the injected labelled cells were not detected after 6 weeks post-administration, suggesting that the injected cells do not embed in the tissue, and suggest that the soluble mediators secreted by these cells might have contributed to improvement in EF in a paracrine manner.
Although the mechanisms involved in stem cell-mediated tissue regeneration are not fully understood, it is hypothesized that stem cells affect surrounding tissue via a paracrine signalling system [24]. For example, ADSCs have been shown to contribute to cytoprotecting, cell survival, and immunomodulation of surrounding cells by induction and secretion of a variety of growth factors and cytokines, such as TNF-γ, vascular endothelial growth factor, and multiple neurotrophins that increase repair of nNOS (neuronal nitric oxide synthase)-expressing neurons in vivo and in vitro [25]. In support of this notion, administration of ADSC-derived cell lysates has been shown to restore EF similar to that of ADSCs [11]. Modifying growth factor expression in stem cells has been shown to have therapeutic potential [26]. Regardless of the location of stem cell injection (intracavernosal peri-prostatic, MPG, tail-vein, and more), studies have shown that stem cells migrate to the bone marrow and then to the site of BCNI within days of administration [27]. It was originally thought that injected stem cells embed into the targeted tissue and transdifferentiate into resident cells, thus “regenerating” the damaged tissue. However, permanent engraftment and trans-differentiation of labelled stem cells were not detected [28]. With respect to cavernosal nerve regeneration studies, no labelled stem cells were detected 2-12 weeks after injection, which was also observed in this study, arguing against the incorporation and long-term presence of stem cells in injected tissues [7]. This also implies that the beneficial effects may be during the early period after administration via a paracrine mechanism.
Considering the paracrine mode of action and the observed beneficial effects soon after injection, we hypothesized that a second administration of stem cells might further improve the therapeutic benefit due to another iteration of paracrine signalling. To the best of our knowledge, this is the first study to examine the effects of repeated ADSC administration in any animal model simulating ED. Interestingly, although administration of stem cells leads to improved EF after BCNI, there was no demonstrable improvement in EF with the second injection as compared to a single injection. This suggests that a majority of benefit from ADSCs occurs during early periods of ADSC administration at the site of nerve injury, and that repeated administration may not have any added benefit. It is possible that the inflammatory environment at the site of injury that is conducive to the recruitment of stem cells from the bone marrow is no longer present, or not strong enough over time. It may also be possible that injection into the corpora may cause trauma leading to fibrosis and inflammation, and with repeated injections this may be attenuated and counteract the benefit derived from additional stem cell treatments. Future studies may benefit from a mechanistic evaluation of the corpora after injection to better answer this question.
Although this study has a similar design to previously published reports, it is not without limitations. Firstly, the Dumont forceps- induced BCNI model used in this study can be highly variable between surgeons and groups, questioning consistency with the technique. That being said, a study comparing this model with other models of BCNI (i.e., bulldog clamp induced, neurotomy, etc.) showed that all approaches significantly decrease EF compared to sham controls, and did not vary statiscially from each other, indicating that our BCNI approach has acceptable levels of reproducibility [29]. Moreover, in the current study, all surgical procedures were performed by a single investigator, minimizing variability. Secondly, due to a limited study duration of six weeks, we were unable to examine whether ICI of ADSCs has long-term or late-appearing adverse effects in our model. The largest study duration in this field of research is three months, demonstrating the need for longer trial durations [7]. Additionally, histologic analysis was a secondary outcome of interest and thus no PCRs, WBs or IHCs were performed to identify fibroblast markers (such as Vimentin, FSP-1), limiting the histologic implications of this study. Lastly, some may argue that injecting stem cells at the time of injury does not allow for an injury to develop. However, studies have shown that the same beneficial effect when injected at the time of injury or four weeks afterwards [30].
Conclusion
Our study confirms the potential benefit of ICI of ADSCs as an effective treatment for ED. Our results also show that a single dose of ADSCs results in maximum improvement in ED, compared to twotime administration. However, further studies are needed to better characterize this phenomenon and elucidate the effects of repeat stem cell treatments.
Acknowledgments
This work was supported by a grant from the Alliance of Cardiovascular Researchers (to EA). RI is supported in part by the Elsa U. Pardee Foundation. BC is a recipient of the Department of Veterans Affairs Research Career Scientist award (#IK6BX004016), and is supported by the U.S. Department of Veterans Affairs, Office of Research and Development-Biomedical Laboratory Research and Devel- opment (ORD-BLRD) Service Award VA-I01-BX004220. In addition, we would like to thank the Department of Pathology and Cancer Center at Tulane University School of Medicine for flow cytometry
References
- Araujo AB, Travison TG, Ganz P, Chiu GR, Kupelian V, et al (2012) Erectile dysfunction and mortality. The journal of sexual medicine.6:2445-2454.
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, et al (2016) Patient- Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med.375:1425-1437.
- Hong EK, Lepor H, McCullough AR (1999) Time dependent patient satisfaction with sildenafil for erectile dysfunction (ED) after nerve-sparing radical retropubic prostatectomy (RRP). Int J Impot Res.11:S15-22.
- Liu C, Lopez DS, Chen M, Wang R (2017) Penile Rehabilitation Therapy Following Radical Prostatectomy: A Meta-Analysis. J Sex Med.14:1496-1503.
- Gur S, Sikka SC, Kadowitz PJ, Silberstein J, Hellstrom WJ (2015) update of erectile dysfunction management following radical prostatectomy: from basic research to clinical management. Curr Pharm Des.11:1440-1454.
- Kim TH, Ha YS, Choi SH, Yoo ES, Kim BW, et al (2016) Factors predicting outcomes of penile rehabilitation with udenafil 50 mg following radical prostatectomy. Int J Impot Res.28:80.
- Shan H, Chen F, Zhang T, He S, Xu L (2015) Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS One.10: e0121428.
- Yiou R, Hamidou L, Birebent B, Bitari D, Lecorvoisier P, et al (2016) Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. European urology.69:988-991.
- Alwaal A, Zaid UB, Lin C-S, Lue TF (2015) Stem cell treatment of erectile dysfunction. Advanced drug delivery reviews.82:137-144.
- Qiu X, Villalta J, Ferretti L, Fandel TM, Albersen M, et al (2012) Effects of intravenous injection of adipose‐derived stem cells in a rat model of radiation therapy‐ induced erectile dysfunction. The J Sex Med.9:1834-1841.
- Albersen M, Fandel TM, Lin G, Wang G, Banie L, et al (2010) Injections ofadipose tissue‐derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med.7:3331-3340.
- Gierloff M, Petersen L, Oberg H-H, Quabius E, Wiltfang J (2014) Adipogenic differentiation potential of rat adipose tissue-derived subpopulations of stromal cells. Journal of Plastic, Reconstructive & Aesthetic Surgery.67:1427-1435.
- Kang KS, Hong JM, Kang JA, Rhie J-W, Jeong YH (2013) Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med.45:6.
- Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ (2010) Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol.184:1560-1566.
- Jeong HH, Piao S, Ha JN, Kim IG, Oh SH (2013) Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/brain-derived neurotrophic factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology.8:1108
- Kim IG, Piao S, Lee JY, Hong SH, Hwang TK, et al (2013) Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A.19:14-23.
- Miyamoto K, Inoue S, Kobayashi K, Kajiwara M, Teishima J (2014) Rat cavernous nerve reconstruction with CD133+ cells derived from human bone marrow. J Sex Med.11:1148-1158.
- Choi WY, Jeon HG, Chung Y, Lim JJ, Shin DH, et al (2013) Isolation and characterization of novel, highly proliferative human CD34/CD73-double-positive testis- derived stem cells for cell therapy. Stem Cells Dev.22:2158-2173.
- Albersen M, Kendirci M, Van der Aa F, Hellstrom WJ, Lue TF (2012) Multipotent stromal cell therapy for cavernous nerve injury‐induced erectile dysfunction. J Sex Me.9:385-403.
- Katz EG, Moustafa AA, Heidenberg D, Haney N, Peak T, et al (2016) Pioglitazone enhances survival and regeneration of pelvic ganglion neurons after cavernosal nerve injury. Urology.89:76-82.
- You D, Jang MJ, Lee J, Suh N, Jeong IG, et al (2013) Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue- derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate.73:278-286.
- You D, Jang MJ, Lee J, Jeong IG, Kim HS, et al (2013) Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology.81:104-110.
- Ying C, Yang M, Zheng X, Hu W, Wang X (2013) Effects of intracavernous injection of adipose-derived stem cells on cavernous nerve regeneration in a rat model. Cell Mol Neurobiol.33:233-240.
- Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regenerative medicine.5:121-143.
- Salgado A, Reis R, Sousa N, Gimble J (2010) Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Current stem cell research & therapy.5:103-110.
- Kim SJ, Choi SW, Hur KJ, Park SH, Sung YC, et al (2012) Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury. Korean J Urol.53:726-32.
- Fandel TM, Albersen M, Lin G, Qiu X, Ning H, et al (2012) Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol.61:201-210.
- Gnecchi M, Zhang Z, Ni A, Dzau VJ (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res.103:1204-1219
- Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP (2006) Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med.3:77-83.
- Qiu X, Fandel TM, Ferretti L, Albersen M, Orabi H, et al (2012) Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol.62:720-727
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi