Research Article, J Clin Exp Oncol Vol: 7 Issue: 1
Can FDG PET/CT and CT Exclude N2 and N3 Nodal Disease in Patients with Breast Cancer?
Muhammet Arslan1*, Bozkurt Gulek2, Aygul Polat Kelle3, Serkan Akbulut4, Erhan Ugurlu5, Isa Burak Guney6, Sinan Sozutok7 and Ozgur Kulahci8
1Department of Radiology, Pamukkale University, Denizli, Turkey
2Department of Radiology, Healty Science University, Adana, Turkey
3Department of Nuclear Medicine, Healty Science University, Adana, Turkey
4Department of General Surgery, Healty Science University, Adana, Turkey
5Department of Chest Diseases, Pamukkale University, Denizli, Turkey
6Department of Nuclear Medicine, Cukurova University, Adana, Turkey
7Department of Radiology, Healty Science University, Adana, Turkey
8Department of Pathology, Healty Science University, Adana, Turkey
*Corresponding Author : Muhammet Arslan
Department of Radiology, Pamukkale University, Camlaralti mah. 20100, Kinikli, Denizli, Turkey
Tel: +90 5057955960
E-mail: dr.marslan@hotmail.com
Received: October 17, 2017 Accepted: January 04, 2018 Published: January 10, 2018
Citation: Arslan M, Gulek B, Kelle AP, Akbulut S, Ugurlu E, et al. (2018) Can FDG PET/CT and CT Exclude N2 and N3 Nodal Disease in Patients with Breast Cancer?. J Clin Exp Oncol 7:1. doi: 10.4172/2324-9110.1000210
Abstract
Purpose: The purpose of this study was to investigate if PET-CT and CT were able to exclude N2 and N3 disease in patients with breast cancer.
Materials and Methods: In this study, the PET-CT reports of 211 patients who had been previously diagnosed with breast cancer and who had undergone PET-CT examinations and sentinel lymph node biopsies or axillary lymph node dissections, were reevaluated retrospectively. Whether PET-CT and CT were able to exclude N2 or N3 disease was the subject of evaluation of this study.
Results: It was found at the end of the study that PET-CT was able to exclude N2 and N3 involvement in 92,9 % of the cases, whereas CT alone could do the same exclusion in 93,6 %.
Conclusion: PET-CT and CT can well exclude N2 and N3 disease in patients with breast cancer. It may be suggested that PET-CT can replace sentinel lymph node biopsy in future preoperative evaluations.
Keywords: Breast cancer; PET/CT; Axillary nodal disease; Sentinel lymph node biopsy; Axillary lymph node dissections
Introduction
Axillary lymph node metastasis is an important issue in breast cancer patients, because it can alter the therapy and affect the prognosis [1,2]. Sentinel lymph node biopsy (SLNB) is still a popular procedure in the evaluation of the axilla. But the latest literature indicates that SLNB is not indeed as trustable as previously suggested [3]. In SLNB, the lymph node which is in the closest proximity to the primary tumor is excised and pathologically examined. If this lymph node is pathologically negative, then an axillary dissection is not performed. If the pathological findings are positive, an axillary dissection is out of question.
However, the American College of Surgeons Oncology Group (ACOSOG) Z0011 and Z0010 trials indicate that 1 or 2 axillary lymph node involvements do not necessarily indicate a grave prognosis [4]. In other words, patients with N1 nodal disease and positive SLNB may be undergoing unnecessary axillary lymph node dissections (ALND) [5]. If N2 and N3 disease can be excluded by means of PET-CT and CT, it may be possible to prevent patients from undergoing unnecessary ALND procedures. Besides, morbidities secondary to ALNB procedures may be prevented. Because PET-CT has the potential to disclose the whole axilla, distant pathological lymph nodes, too, may easily be demonstrated [6]. Having a stance on these findings, this study was built on the claim that PET-CT and CT could exclude N2 and N3 disease in patients with breast cancer.
Materials and Methods
At the beginning of the study, the PET-CT and CT reports of patients diagnosed with breast cancer between January 2015 and April 2017 were examined. The patients had not undergone any axillary operations. As an additional procedure, the CT components of these PET-CT examinations were reevaluated by a radiologist in order to look for any undiagnosed axillary involvement. There were a total of 253 patients at the beginning, but 42 patients were later excluded from the study because they had not been operated and did not have any pathological diagnosis.
The pathology and PET-CT and CT results of the remaining 211 patients were split into two groups, these being the N0-N1 and N2-N3 groups. Whether PET-CT and CT excluded N2 and N3 disease or not, was evaluated according to the negative predictive values. Ethical approval from an authorized ethical committee was also obtained.
Nodal disease was evaluated according to the number of pathologic lymph nodes, and these findings are presented in Table 1. Patients with no pathological lymph nodes are categorized as N0, whereas those with 1-3 nodes are labeled as N1, those with 4-9 nodes as N2, and those with 10 or more nodes as N3.
The FDG PET-CT examinations were performed in an integrated PET-CT scanner with a full ring high resolution LSO PET and a sixslice CT scanner (Siemens Biograph 6, Knoxville, USA). All patients fasted for 6 hours prior to the procedures. Serum glucose levels were measured prior to the examinations to make sure that the levels were under 200 mg/dl. Whole body images were acquired 60 minutes after the intravenous administrations of the FDG. The images were obtained from the vertex level to the proximal thigh region.
The CT phase of the study was performed in a 16-slice multidetector CT unit (GE Optima, Milwaukee, USA).
Lymph node assessments by CT were done based on certain malignant criteriae. These criteriae were as follows: short axis bigger than 1 cm, short to long axis ratio higher than 0.5, loss of fatty hilus, intranodal densities suggestive of necrosis, and contours of irregular shape.
Results
The average age of the 211 patients was 53,5 years. In 126 of these patients, there was a positive pathologic nodal disease (N1, N2, and N3). More than half of the patients (78) had N1 disease, whereas 48 patients had N2 and N3 disease. The pathological diagnoses of the breast masses came as invasive ductal cancer in 151, invasive lobular cancer in 38, and other invasive cancer types in 22, of the patients. The pathological results of those patients with nodal disease are shown in Table 1.
| Tumor Type | N0-N1 | N2-N3 |
|---|---|---|
| Invasive ductal | 81 | 3 |
| Invasive lobuler or mixed lobular | 22 | 4 |
| Other invasive | 16 | 0 |
Table 1: The pathological results of those patients with nodal disease.
Of the 79 patients with positive PET-CT findings, 53 (73,4 %) had their diagnoses verified with pathology results. In 21 patients (26,6 %), the pathology results were negative. On the other hand, 20 (15,2 %) of the 132 patients with negative PET-CT findings had positive pathological diagnoses, whereas the remaining 112 (84,8 %) had negative pathology results.
In 7 of the 132 patients with negative PET-CT findings, there were N2 and N3 diseases confirmed by pathological results. In N0 and N1 patients, the negative predictive values (NPV) for PET-CT and CT were found as 79,1 and 81,6, respectively (Table 2). There were no male patients in the study, as well as no patients with bilateral breast cancers.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| PET/CT | 62,7 | 88,6 | 77,6 | 79,1 | 78,6 |
| CT | 72,3 | 77,3 | 66,7 | 81,6 | 75,3 |
Table 2: In N0 and N1 patients, the negative predictive values (NPV) for PET-CT and CT were found as 79,1 and 81,6, respectively.
Discussion
Modern medicine has witnessed a transformation of both the diagnostic and therapeutic procedures into more noninvasive fashions. An example to this transformation has been the preference of SLNB to ALND in the axillary staging of breast cancer patients, during the last 20 years [7]. But even SLNB has its own share of morbidities, such as the development of seromas, paresthesia, and lymphedema; and this has led physicians to turn to even more noninvasive alternatives. Axillary ultrasonography (US), for example, is a widely used modality, because it is noninvasive and very easily available. But US, too, has its own drawbacks, such as its inability to visualize the whole axillary region and its user-dependency. The utilization of PET-CT in breast cancer patients is on a steady increase. Especially at the time of the initial diagnosis, most oncologists and oncologic surgeons demand routine PET-CT examinations in breast cancer patients. PET-CT provides the advantage of scanning the body as a whole for metastases, as well as depicting a very detailed examination of the axilla in terms of anatomy and activity.
The Z0011 study of the American College of Surgeons was aimed at determining if the SLNB-positive breast cancer patients needed a complementary axillary dissection or not. The Z0011 study was somewhat a limited study in some aspects, but the combined Z0010 and Z0011 studies have clearly demonstrated that a complimentary axillary dissection gives no benefit in SLNB-positive breast cancer patients [4,8].
ALND may not be beneficial for at least half of the patients with positive SLNB results. In fact, a sentinel lymph node is the sole positive node in nearly 40-60 % of these patients [9]. Therefore, some authors are now in doubt and thus question the profit of complementary axillary dissection in patients who present a low risk of metastasis beyond the sentinel loops. These authors have come to this point on the contentious issues of postoperative morbidity, the questionable profit of excising negative nodes, doubtful benefit to survival, and the rather rarity of axillary recurrence [8,9].
There are some studies on this issue in the literature. One is a meta-analysis done by Pennat et al. [10]. In their study, these authors analyzed 6 studies done on the subject, and they scrutinized these studies by splitting them into two groups, these being the PET / CTPET and CT groups [11-16]. Pennat et al found that PET-CT alone was more sensitive in the diagnosis, when compared to both CT and PET, separately. But the authors could not find significant differences in terms of specificity.
In a study done by Schmidt et al, no statistically significant differences could be found between PET-CT and MRI, in terms of sensitivity and specificity [15]. In our literature scanning, this was the only study which compared PET-CT and MRI. On the other hand, Hyun et al have commented that while MRI is not a perfect modality in terms of diagnostic performance, it still demonstrated a capacity to exclude nodal disease in 98,2 % of the cases [5]. Especially in patients who had not received neoadjuvant chemotherapy, this ratio was found to be even higher (99,6 %) [5].
There were a few limitations to our study. The first one was that the study was built on a retrospective basis and the PET-CT evaluations could only be made by examining the old reports of the studies. Another disadvantage was that the evaluation of the CT part of the PET-CT examinations which was performed by a radiologist could only be done by examining the axillary regions. Yet another important limitation was the fact that the majority of breast cancer patients had received neoadjuvant chemotherapy (NAC) prior to their operations. The administration of NAC may decrease the staging level and thus alter the axillary situation.
The efficacy of PET-CT may be more thoroughly investigated in bigger centers with higher numbers of patients, by splitting the patients into certain categories such as NAC-receiving and not-NACreceiving, and pre-NAC and post-NAC groups.
Another important advantage of PET-CT is its capability to scan the whole axilla, together with the whole body, thus enabling to comment on the general dissemination of the disease and the situation of the metastases. As can be seen in Figures 1-3, PET-CT can provide important diagnostic information about distant organ metastases, axillary conditions, and the situation of other lymph nodes.
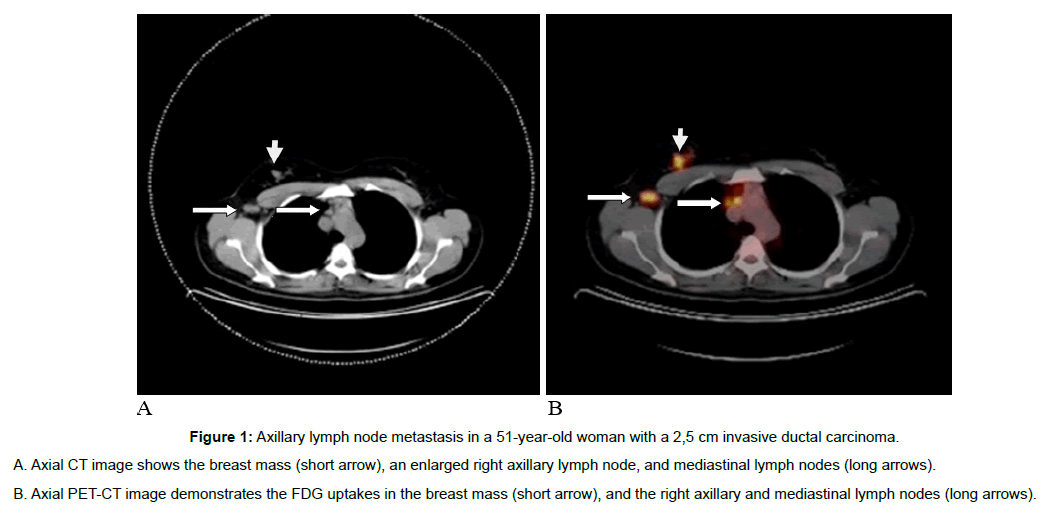
Figure 1: Axillary lymph node metastasis in a 51-year-old woman with a 2,5 cm invasive ductal carcinoma.
A. Axial CT image shows the breast mass (short arrow), an enlarged right axillary lymph node, and mediastinal lymph nodes (long arrows).
B. Axial PET-CT image demonstrates the FDG uptakes in the breast mass (short arrow), and the right axillary and mediastinal lymph nodes (long arrows).
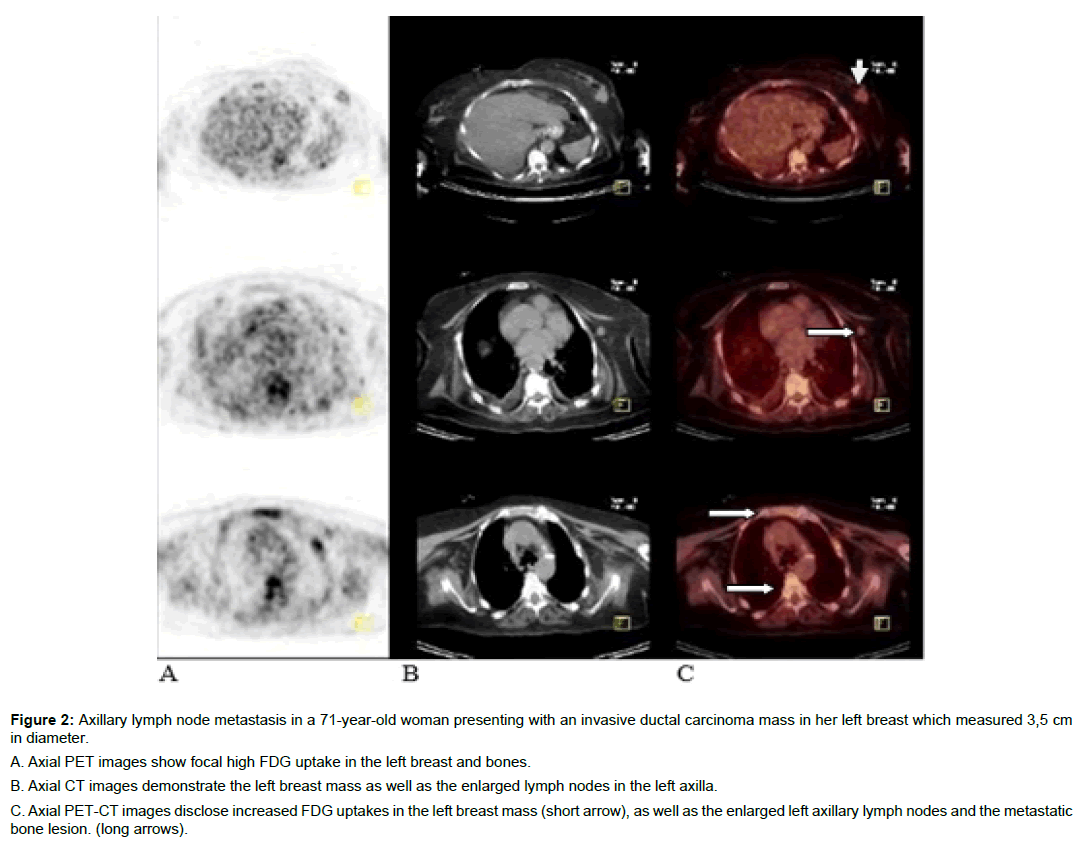
Figure 2: Axillary lymph node metastasis in a 71-year-old woman presenting with an invasive ductal carcinoma mass in her left breast which measured 3,5 cm in diameter.
A. Axial PET images show focal high FDG uptake in the left breast and bones.
B. Axial CT images demonstrate the left breast mass as well as the enlarged lymph nodes in the left axilla.
C. Axial PET-CT images disclose increased FDG uptakes in the left breast mass (short arrow), as well as the enlarged left axillary lymph nodes and the metastatic bone lesion. (long arrows).
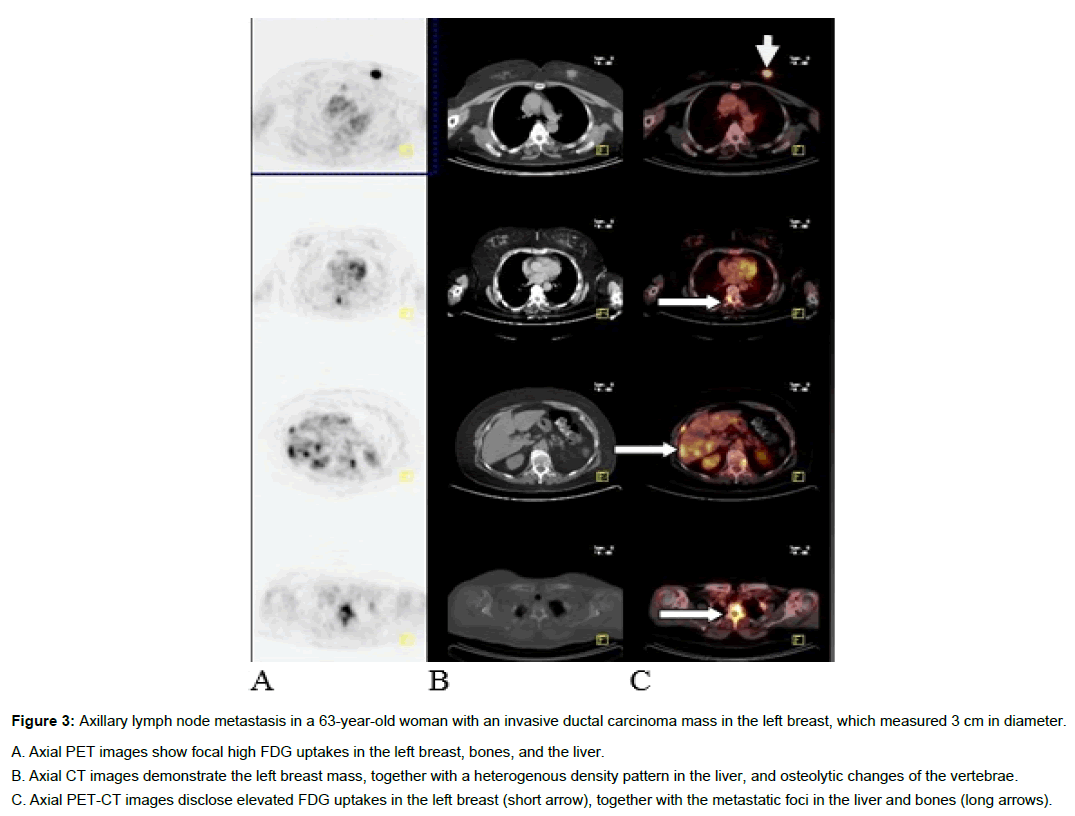
Figure 3: Axillary lymph node metastasis in a 63-year-old woman with an invasive ductal carcinoma mass in the left breast, which measured 3 cm in diameter.
A. Axial PET images show focal high FDG uptakes in the left breast, bones, and the liver.
B. Axial CT images demonstrate the left breast mass, together with a heterogenous density pattern in the liver, and osteolytic changes of the vertebrae.
C. Axial PET-CT images disclose elevated FDG uptakes in the left breast (short arrow), together with the metastatic foci in the liver and bones (long arrows).
Conclusion
As a conclusion, it was seen that FDG PET-CT and CT are able to exclude N2 and N3 axillary lymph node metastases (ALNM). No statistically significant differences could be found between FDG PETCT and CT, in terms of evaluating the axillary lymph nodes.
In patients with N2 and N3 nodal disease, those with a pathological diagnosis of invasive lobular cancer demonstrated a higher pseudo negative ratio, when compared to those patients with invasive ductal carcinoma.
It must be pointed that, in the future, PET-CT may well replace SLNB in the management of invasive ductal cancer patients.
References
- Banerjee M, George J, Song EY, Roy A, Hryniuk W (2004) Treebased model for breast cancer prognostication. J Clin Oncol 22: 2567-2575.
- Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS (1998) The sentinel node in breast cancer-a multicenter validation study. N Engl J Med 339: 941-946.
- Han A, Moon HG, Kim J, Ahn SK, Park I, et al. (2013) Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 16: 378-385.
- Caudle AS, Hunt KK, Tucker SL, Hoffman k, Gainer SM, et al. (2012) American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol 19: 3144-3151.
- Hyun SJ, Kim EK, Moon HJ, Yoon JH, Kim MJ (2016) Preoperative axillary lymph node evaluation in breast cancer patients by breast magnetic resonance imaging (MRI): Can breast MRI exclude advanced nodal disease?. European radiology 26: 3865-3873.
- Groheux D, Espié M, Giacchetti S, Hindié E (2013) Performance of FDG PET/CT in the clinical management of breast cancer. Radiology 266: 388-405
- Neal CH, Daly CP, Nees AV, Helvie MA (2010) Can Preoperative Axillary US Help Exclude N2 and N3 Metastatic Breast Cancer? Radiology 257: 335-341.
- Giuliano AE, Hunt KK, Ballman KV, Beitsch PD,Whitworth PW, et al (2011) Axillary dissection vs noaxillary dissection in women with invasive breast cancer and sentinel node metastasis:a randomized clinical trial. Jama 305: 569-575
- Bear HD (2008) Completion axillary lymph node dissection for breast cancer: immediate versus delayed versus none. J Clin Oncol 26: 3483-3484
- Pennant M, Takwoingi Y, Pennant L, Davenport C, Fry-Smith A, et al. (2010) A systematic review of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 14: 1-103.
- Fueger BJ, Weber WA, Quon A, Crawford TL, Allen-Auerbach MS, et al. (2005) Performance of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol 7: 369-376.
- Radan L, Ben-Haim S, Bar-Shalom R, Guralnik L, Israel O (2006) The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer 107: 2545-2551.
- Haug AR, Schmidt GP, Klingenstein A, et al. (2007) F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography in the follow-up of breast cancer with elevated levels of tumor markers. J Comput Assist Tomogr 31: 629-634.
- Veit-Haibach P, Antoch G, Beyer T, Stergar H, Schleucher R, et al. (2007) FDG-PET/CT in restaging of patients with recurrent breast cancer: possible impact on staging and therapy. Br J Radiol 80: 508-515.
- Schmidt GP, Baur-Melnyk A, Haug A, Heinemann V, Bauerfeind I, et al. (2008) Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PETCT. Eur J Radiol 65: 47-58.
- Dirisamer A, Halpern BS, Flöry D, Wolf F, Beheshti M, et al. (2010) Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol73: 294-299.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi