Research Article, J Clin Exp Oncol Vol: 6 Issue: 4
Comparing Gefitinib and Erlotinib With Regard To Brain Metastases Recurrence in EGFR-Mutant Non-Small Cell Lung Cancer Patients
Kenji Nakahama1*, Akihiro Tamiya1, Yoshihiko Taniguchi1, Yoko Naoki1, Masaki Kanazu2 and Shinji Atagi3
1Department of Internal Medicine, National Hospital Organization Kinki-chuo Chest Medical Center, Nagasone-cho 1180, Kita-ku, Sakai City, Osaka 591- 8555, Japan
2Department of Respiratory Medicine, National Hospital Organization, National Toneyama Hospital, 5-1-1 Toneyama, Toyonaka, Osaka 560-8552, Japan
3Clinical Research Center, National Hospital Organization Kinki-chuo Chest Medical Center, Nagasone-cho 1180, Kita-ku, Sakai City, Osaka 591-8555, Japan
*Corresponding Author : Kenji Nakahama
Department of Internal Medicine, National Hospital Organization Kinki-chuo Chest Medical Center, Nagasone-cho 1180, Kita-ku, Sakai City, Osaka 591-8555, Japan
Tel: +81-72-252-3021
Fax: +81-72-252-3041
E-mail: nakahama_kenji@outlook.jp
Received: May 19, 2017 Accepted: May 30, 2017 Published: June 02, 2017
Citation: Nakahama K, Tamiya A, Taniguchi Y, Naoki Y, Kanazu M, et al. (2017) Comparing Gefitinib and Erlotinib With Regard To Brain Metastases Recurrence in EGFR-Mutant Non-Small Cell Lung Cancer Patients. J Clin Exp Oncol 6:4. doi: 10.4172/2324-9110.1000190
Abstract
Brain metastases of lung cancer are associated with a poor prognosis. Little research has been conducted to directly compare erlotinib with gefitinib regarding the frequency of central nerve system recurrence. This is the first study to directly compare erlotinib with gefitinib in terms of brain metastases recurrence rates in patients with EGFR-mutant NSCLC who had no brain metastasis at the time of starting TKI treatment. This was a single-center retrospective study. Advanced or recurrent non-small cell lung cancer patients with no brain metastases at the time of starting initial tyrosine kinase inhibitor treatment who received either gefitinib or erlotinib monotherapy were selected. The primary endpoint was the incidence of brain metastases, and secondary endpoints included the objective response rate, progression-free survival, overall survival, and Post-Progression Survival in subgroups based on tyrosine kinase inhibitor treatment and the occurrence of brain metastases. There were 119 patients in the gefitinib group and 13 patients in the erlotinib group. Brain metastases at disease progression were observed in 16 patients in the gefitinib group, and in no patients in the erlotinib group (13.5% vs. 0%, p=0.37). The median overall survival was 29.2 months in the gefitinib group and was not reached in the erlotinib group (p=0.14). The median PPS was 15.5 months in the gefitinib group and 23.7 months in the erlotinib group (p=0.11). Based on the occurrence of brain metastases, Post-Progression Survival was significantly longer in the no brain metastases group (8.0 months vs. 17.9 months, p=0.01). These data showed the possibility of a lower central-nerve-system recurrence rate with erlotinib compared with gefitinib. Post-Progression Survival in patients with brain metastases was significantly shorter than that of patients without brain metastases.
Keywords: Brain metastases; EGFR-mutant lung cancer; Erlotinib; Gefitinib; Non-small cell lung cancer
Introduction
In the field of lung cancer, brain metastases correlate with a poor prognosis and deterioration in the patient’s quality of life [1].
More than 25% of patients with lung cancer develop brain metastases during their disease course [2,3]. Previous studies have shown that the incidence of brain metastases is higher with adenocarcinoma than with other subtypes of non-small cell lung cancer (NSCLC) [4,5]. In particular, it was reported that the incidence rate of brain metastases is higher in epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma than in EGFR-wild type lung adenocarcinoma [6].
Gefitinib and erlotinib are oral EGFR tyrosine kinase inhibitors (TKIs) that have demonstrated superior progression-free survival (PFS), objective responses, and more favorable safety profiles than standard first-line platinum-based doublet chemotherapy in patients with EGFR-mutant NSCLC. In these patients, gefitinib and erlotinib achieved response rates of 56% to 74%, and a median PFS of 10 to 14 months [7-11]. Although EGFR-TKIs have improved the outcome of EGFR mutation positive NSCLC, central nerve system (CNS) recurrence after a good response to the first TKI treatment remains a serious problem. Given the adverse effects of brain metastases on survival, prevention of such metastases during chemotherapy, including EGFR-TKI therapy, is important.
It is reported that TKIs reduced brain metastasis recurrence compared with chemotherapy in first-line treatment of EGFR mutation positive NSCLC [12]. However, it is unclear whether there is a difference between TKIs in their ability to prevent brain metastasis occurrence.
CNS recurrence after an initial response to gefitinib was observed in 25-33% of patients with EGFR-mutant NSCLC [13]. In contrast to gefitinib, the CNS recurrence rate of erlotinib was 1-8% [12,14-16]. Although several reports indicate erlotinib superiority over gefitinib in the treatment of brain metastases, little research has been conducted to directly compare erlotinib with gefitinib in terms of the frequency of CNS recurrence during treatment with each agent.
The present study was undertaken in order to investigate whether there was a difference between the effects of gefitinib and erlotinib on the CNS recurrence rate when we treated NSCLC patients without brain metastases.
Materials and Methods
Patients
The study was a single-institution retrospective study of advanced or recurrent NSCLC patients harboring EGFR mutations. Patients were selected based on the following inclusion criteria: diagnosed with EGFR mutation-positive advanced or recurrent non-squamous NSCLC that was confirmed histologically or cytologically; received either gefitinib or erlotinib monotherapy as the initial TKI treatment between December 2009 and December 2014 at Kinki-chuo Chest Medical Center; and had no brain metastases based on computed tomography (CT) or magnetic resonance imaging (MRI) at the time of starting initial TKI treatment. Patients who had received chemotherapy before TKI treatment were eligible for the study. Patients who received combination therapy of TKI with other antitumor drugs, such as bevacizumab or cytotoxic chemotherapy, were excluded.
Patient characteristics at baseline, including sex, age; smoking history, histology, staging, EGFR mutation status, and the administration line (e.g. first-line) of initial TKI treatment were investigated. All participants provided written informed consent. This study was approved by the Ethics Review Board and Institutional Review Board at our hospital.
Outcomes
The primary endpoint was the incidence rate of brain metastases during initial TKI treatment. The secondary endpoints included the objective response rate (ORR), PFS, overall survival (OS), Post-Progression Survival (PPS) in subgroups based on TKI treatment, and PPS in subgroups based on the occurrence of brain metastases.
PFS was calculated from the date of initiation of TKI administration to the date of detection of disease progression or of death from any cause. The PFS was censored at the date of the last visit for patients who were alive without any documented disease progression. The OS was calculated from the date of initiation of TKI administration to the date of death. The OS was censored at the date of the last visit for patients whose deaths could not be confirmed.
The presence of brain metastases was estimated at the time of disease progression. Brain imaging, such as CT and MRI, was not necessarily needed if patients did not show any neurologic findings. Tumor responses were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
Statistical analysis
The incidence rates of brain metastases and ORR were compared using Fisher’s exact test. PFS, OS, and PPS were analyzed using a log-rank test. Hazard ratios (HR) and confidence intervals (CI) were estimated using a Cox proportional hazards model. To analyze the PFS and OS, survival curves were drawn using the Kaplan-Meier method. P values below 0.05 were considered significant. All statistical analyses were performed using JMP version 13.0 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
One hundred and nineteen NSCLC patients in the gefitinib group and 13 patients in the erlotinib group fulfilled all of the eligibility criteria. Clinical characteristics of the patients at baseline are summarized according to treatment group in Table 1. The participants in the gefitinib group had a median age of 72 years, while this was 64 years in the erlotinib group. Sixty-six percent of patients in the gefitinib group and 69% of patients in the erlotinib group were female. In both groups, more than half of the patients were never smokers. Most patients in both groups had stage IV disease and adenocarcinoma histology. In the erlotinib group, only 1 patient had an uncommon EGFR mutation that was not the deletion of exon 19 (Del 19) or L858R. In the gefitinib group, about 8% of patients had uncommon mutations. In the gefitinib group, 93 patients received the drug as first-line therapy, 25 patients received it as second-line therapy, and only 1 patient received it as third-line. In the erlotinib group, 8 patients received the treatment as first-line therapy, and 5 received it as second-line therapy. We did not analyze performance status (PS) as we could not collect sufficient data.
| Gefitinib (n=119) | Erlotinib (n=13) | |
|---|---|---|
| Age (Median) | 72 | 64 |
| Sex Male Female |
40 79 |
4 9 |
| Smoking status Smoker Non-smoker Unknown |
55 60 4 |
6 7 0 |
| Histology Adenocarcinoma Squamous cell carcinoma NOS |
108 1 10 |
12 1 0 |
| Stage IIIB IV post-operative recurrence post-radiation therapy recurrence |
7 78 29 5 |
0 10 3 0 |
| EGFR Mutation status del19 L858R other |
57 52 10 |
8 4 1 |
| TKI administration line 1st 2nd 3rd |
93 25 1 |
8 5 0 |
Table 1: Baseline Characteristics.
Treatment outcomes
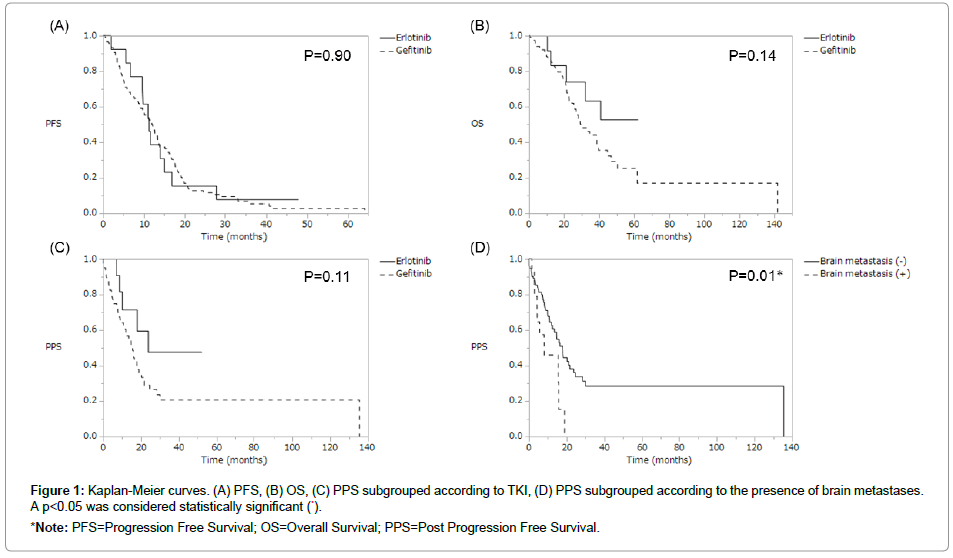
Table 2 shows outcomes according to the TKI treatments. The median follow-up was 20.3 months, and was significantly longer in erlotinib group (19.1 vs. 31.9 months, p=0.02). Disease progression was observed in 102 patients in the gefitinib group, and in 12 in the erlotinib group. The median PFS was 12.0 (95% CI: 9.1-13.4) months in the gefitinib group and 11.3 (95% CI: 6.6-15.1) months in the erlotinib group (p=0.90). The ORR was 56.3% in the gefitinib group and 76.9% in the erlotinib group (p=0.24). The median OS was 29.2 (95% CI: 24.4-39.7) months in the gefitinib group and was not reached in the erlotinib group (p=0.14). The median PPS was 15.5 (95% CI: 10.9-18.9) months in the gefitinib group and 23.7 (95% CI: 8.3-NR) months in the erlotinib group (p=0.11).
| Gefitinib (n=119) | Erlotinib (n=13) | P value | |
|---|---|---|---|
| Brain metastasis occurrence No. of patients % of patients |
16 13 |
0 0 |
0.35 |
| Objective response rate No. of patients % of patients |
67 56 |
10 77 |
0.24 |
| Median PFS (95%CI) | 12.0 (9.1-13.4) | 11.3 (6.6-15.1) | 0.90 |
| Median OS (95%CI) | 29.2 (24.4- 39.7) | Not reach (12.3- ) | 0.14 |
| Median PPS (95%CI) | 15.5 (10.9-18.9) | 23.7 (8.3- ) | 0.11 |
Table 2: Outcomes according to TKI treatments.
Brain metastases at disease progression were observed in 16 of 119 patients in the gefitinib group, while no patients in the erlotinib group developed brain metastases (0% vs. 13.5%, p=0.37). There was no significant difference between the 2 groups in the brain metastases rate, PFS, OS, ORR, or PPS; however, there were favorable trends for OS and PPS in the erlotinib group (Figure 1). The HRs of erlotinib to gefitinib for PFS, OS, and PPS were 0.96 (95% CI: 0.50-1.67, p=0.89), 0.52 (95% CI: 0.18-1.19, p=0.13), and 0.48 (95% CI: 0.17-1.10, p=0.09), respectively.
Figure 1: Kaplan-Meier curves. (A) PFS, (B) OS, (C) PPS subgrouped according to TKI, (D) PPS subgrouped according to the presence of brain metastases. A p<0.05 was considered statistically significant (*).
*Note: PFS=Progression Free Survival; OS=Overall Survival; PPS=Post Progression Free Survival.
When we assessed PPS in subgroups based on the occurrence of brain metastases at disease progression, PPS was significantly longer in the no brain metastases group compared with the brain metastasis group (8.0 vs. 17.9 months, p=0.01), and the HR of brain metastases was 2.36 (95% CI: 1.10-4.63, p=0.03) (Table 3).
| Brain metastasis (+) (n=16) | Brain metastasis (-) (n=97) | P value | |
|---|---|---|---|
| Median PPS (95% CI) | 8.0 (2.9-15.8) | 17.9 (12.5-23.7) | P=0.01* |
| Hazard Ratio (95%CI) | 2.36 (1.10-4 63) | P=0.03* | |
*P<0.05
Table 3: Post-progression free survival sub grouped according to brain metastases recurrence.
In the brain metastases group, only 1 patient had an uncommon EGFR mutation; this patient also had the del19 mutation. All other patients who experienced brain metastases had a common mutation that indicates Del 19 or L858R.
Discussion
To the best of our knowledge, this is the first study to directly compare erlotinib with gefitinib in terms of brain metastases recurrence rates in patients with EGFR-mutant NSCLC who had no brain metastasis at the time of starting TKI treatment. There was no significant difference in the incidence rate of brain metastases between erlotinib and gefitinib treatment in patients with NSCLC. However, there was no brain metastasis recurrence at the time of disease progression during erlotinib treatment.
The high incidence of CNS recurrence in lung cancer during systemic chemotherapy has been attributed to limited penetration of certain drugs into the cerebrospinal fluid (CSF) [13,17]. The bloodbrain barrier (BBB) prevents drugs from reaching a brain tumor; therefore, it can be presumed that the BBB permeability of agents into the CNS is very important in the treatment of patients with brain metastases. The penetration rate of TKI from the plasma to the CSF was better for erlotinib (2.8%) than it was for gefitinib (1.1%). In addition, it was reported that in 25 leptomeningeal carcinomatosis patients, erlotinib achieved better cytological clearance of the CSF than gefitinib [18].
When we analyzed PPS in subgroups according to brain metastases recurrence at the time of disease progression, the no brain metastases group exhibited longer PPS. Similar to previous studies, our study shows poor prognosis with brain metastasis in this group of patients [1]. It was reported that symptomatic CNS relapse was a common progression in NSCLC patients; some of these patients could not receive further cytotoxic chemotherapy because of the CNS relapse, suggesting the potential risk of missing subsequent therapy [19].
In this study, although OS and PPS were not significantly different between the 2 TKI groups, there were better tendencies for both OS and PPS in the erlotinib group compared with the gefitinib group. One of the reasons for these tendencies may be the difference in the brain metastasis recurrence rate.
The first limitation of our study is that it was a single-center retrospective study and there were only a small number of patients in the erlotinib group. Because of the small sample size, statistically significant after there were no differences between erlotinib and gefitinib, including in the incidence of brain metastases. A second limitation is that not all patients were examined by brain imaging at the time of disease progression if the patient did not show any neurological symptoms. As a result, we might have missed asymptomatic brain metastases, resulting in the low brain metastases rate.
In conclusion, our data showed the possibility of a lower recurrence rate with erlotinib than with gefitinib in NSCLC patients with mutant EGFR. This is the first study to directly compare erlotinib with gefitinib in terms of brain metastases recurrence rates in patients with EGFR-mutant NSCLC with no brain metastasis at the time of starting TKI treatment. Furthermore, PPS in patients with brain metastases was significantly shorter than in patients without brain metastases. Most comparative studies to evaluate the efficacy of TKIs have been designed to detect differences in the ORR, PFS, and OS. However, considering the negative prognostic effect of brain metastasis, a prospective study or a large observational study focused on the difference in brain metastases recurrence between patients receiving erlotinib and gefitinib is warranted.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
References
- Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, et al. (2010) The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96: 17-32.
- Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F (2013) EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol 111: 1-10.
- Langer CJ, Mehta MP (2005) Current management of brain metastases, with a focus on systemic options. J Clin Oncol 23: 6207-6219.
- Cox JD, Scott CB, Byhardt RW, Emami B, Russell AH, et al. (1999) Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys 43: 505-509.
- Gore E, Choy H (2004) Non-small cell lung cancer and central nervous system metastases: should we be using prophylactic cranial irradiation? Semin Radiat Oncol 14: 292-297.
- Han G, Bi J, Tan W, Wei X, Wang X, et al. (2016) A retrospective analysis in patients with EGFR-mutant lung adenocarcinoma: is EGFR mutation associated with a higher incidence of brain metastasis? Oncotarget 7: 56998-57010.
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947-957.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, et al. (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380-2388.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239-246.
- Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735-742.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11: 121-128.
- Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, et al. (2012) The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res 18: 4406-4414.
- Omuro AM, Kris MG, Miller VA, Franceschi E, Shah N, et al. (2005) High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 103: 2344-2348.
- Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, et al. (2017) Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol 22: 70-78.
- Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong V, et al. (2016) First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA oncol 2: 305-312.
- Atagi S, Goto K, Seto T, Yamamoto N, Tamura T, et al. (2016) Erlotinib for Japanese patients with activating EGFR mutation-positive non-small-cell lung cancer: combined analyses from two Phase II studies. Future oncol 12: 2117-2126.
- Lee YJ, Choi HJ, Kim SK, Chang J, Moon JW, et al. (2010) Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 116: 1336-1343.
- Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, et al. (2012) Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 70: 399-405.
- Kato Y, Hotta K, Takigawa N, Nogami N, Kozuki T, et al. (2014) Factor associated with failure to administer subsequent treatment after progression in the first-line chemotherapy in EGFR-mutant non-small cell lung cancer: Okayama Lung Cancer Study Group experience. Cancer Chemother Pharmacol 73: 943-950.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi