Short Communication, J Regen Med Vol: 9 Issue: 3
Comparison of Vitronectin and Fibronectin as Substrates during the Culture of Human Embryonic Stem Cells
Goabaone Gaobotse*
Department of Biological Sciences and Biotechnology, Botswana International University of Science and Technology, Palapye, Botswana
*Corresponding Author: Goabaone Gaobotse
Department of Biological Sciences and Biotechnology, Botswana International University of Science and Technology, Private Bag 16, Palapye, Botswana
E-mail: gaobotseg@biust.ac.bw
Received: July 27, 2020 Accepted: October 07, 2020 Published: October 14, 2020
Citation: Gaobotse G (2020) Comparison of Vitronectin and Fibronectin as Substrates during the Culture of Human Embryonic Stem Cells. J Regen Med 9:3. doi: 10.37532/jrgm.2020.9(3).169
Abstract
The feeder free culturing of pluripotent stem cells aims to eliminate the use of mouse embryonic fibroblasts (MEFs) as substrate during in vitro culturing. Here, the potential of fibronectin and vitronectin to keep stem cells undifferentiated was compared using two different stem cell lines (HUES1 and HUES7). Cells were cultured on vitronectin and fibronectin for 3 weeks. Results showed that cells cultured in fibronectin appear to adhere slightly well initially while those in vitronectin detached and bulked up in culture. However, cells cultured on either substrate were well attached and expressed Oct4 and Nanog at the end of the 3-week protocols. This signals the capacity of both vitronectin and fibronectin to keep stem cells undifferentiated after a few passages. Such comparisons of stem cell culture substrates allow the maximization of stem cell culture conditions that are specific to the cell line in use.
Keywords: Vitronectin, HUES1, HUES7, Pluripotency, Fibronectin, Oct4, Nanog
Introduction
HUES are 17 cell lines (HUES1 –HUES17) that were derived and characterized by Melton and colleagues at Harvard University in Boston, USA [1]. These cell lines were derived from IVF excess embryos that had been obtained with consent. These cell lines were shown to proliferate on mouse embryonic fibroblasts (MEFs) and expressed markers of undifferentiated stem cells such as Oct4, SSEA- 4, TRA-1-60 and TRA-1-81. They were also reported to display compact colonies and prominent nucleoli which are typical attributes of pluripotent stem cells.
Early methods of culturing hESCs were similar to methods used to culture mouse embryonic stem cells (mESCs) [2]. However, there is need for the development of feeder-free culture methods to improve the clinical application of hESC derived cultures. The feeder free culturing system of hESCs was first developed in the early 2000s [3]. In this system, hESCs are cultured on matrigel or laminin in MEF conditioned medium. Recently, matrigel has been commonly used as an alternative substrate not only for the growth of hESCs but also for hESC studies [4]. Laminin has been shown to be an efficient hESC culture substrate [5]. Other substrates such as hydrogels [6] have also been studied.
Extracellular matrix (ECM) molecules are said to regulate hESC self-renewal by interacting with integrin cell-surface receptors [7]. Vitronectin is an ECM glycoprotein that is most abundant in bone and serum. It can support the long-term self-renewal of hESCs [8]. Fibronectin is a ubiquitously expressed ECM glycoprotein that is important in vertebrate development and plays a role in the longterm self-renewal of hESCs [9]. It has been shown previously that MEFs secrete fibronectin [10]. In fact, fibronectin has been shown to support the propagation of hESCs even in semi-defined culture conditions [11].
The study of stem cell adhesion and survival molecules is still insufficient. One of the greatest challenges of using proteins from natural products is batch-to-batch variability. The major aim of this study was to compare the potential of vitronectin and fibronectin maintaining stem cells undifferentiated after a few passages on two different stem cell lines. This was determined by both morphological observation and pluripotency gene expression analysis. This study is important because knowing the most appropriate substrate for a specific cell line enables improved and optimal propagation of that cell line.
Materials and Methods
Cell culture of hUES1 stem cells on vitronectin and fibronectin
Cells used in this study were cells that have been cultured on MEF feeder layers prior to their transfer into fibronectin and vitronectin matrices. HUES1 cells were at passage number 14 (p14) while HUES7 cells were at passage number 9 (p9). Vitronectin and fibronectin solutions (1-part fibronectin, Millipore, Billerica, MA, USA: 19 parts PBS w/o Ca2+ and Mg2+, PAA Laboratories Inc.) were applied to 6-well plates and the plates were incubated overnight at 4°C. Following incubation, hUES1 and HUES7 cells were collected into 15 ml tube which was then centrifuged (Sigma 3-16K centrifuge, SciQuip, Shropshire, UK) for 3 minutes at 700 xg. The supernatant was discarded and the tube was gently tapped to break up the cell pellet. The cells were then resuspended in hESC feeder-free medium (Table 1). Vitronectin and fibronectin solutions were then removed from the plates that had been incubated overnight. Cells were plated onto the vitronectin and fibronectin coated wells in 3 ml hESC feederfree media per well. They were fed with pre-warmed feeder-free media 48 hours after plating and every 24 hours thereafter. The hESCs were passaged (as described above) at a 1:3 ratio using TrypLETM Express until they reached at least 90% confluency. Cells were cultured over a three week period and morphology was recorded and captured at day 0 and immediately before each of the 3 passages on days 6, 14 and 21. Before, during and after the culture protocol of HUES1 and HUES7 stem cells, cell characterization and authentication was performed by real-time PCR to check the cells for their undifferentiated state through the expression of Oct4 and Nanog (Tables 2 and 3). Cells were also tested for culture contaminants such as mycoplasma. The data of various analyses were expressed as mean ± standard deviation. All experiments were carried out in triplicate to improve accuracy.
| Medium | Constituents | Final Concentration | Supplying Company |
|---|---|---|---|
| Feeder-Free Base medium (FF medium) | Advanced DMEM F12 | 90% | PAA |
| L-Glutamine 2mM | 1% | PAA | |
| BSA (0.5g BSA + 50ml PBS) | 0.10% | Sigma/PAA | |
| β-Mercaptoethanol 0.09 mM | 0.20% | Gibco | |
| Non-Essential Amino Acids | 1% | PAA | |
| Lipid Supplement x100 | 1% | Gibco | |
| N2 Supplement x100 | 1% | Gibco | |
| B27 Supplement x50 | 2% | Gibco |
Table 1: Stem cell feeder-free medium used for culturing HUES1 and HUES7 stem cells.
| Primary Antibody Target | Species& Isotype | Concentration | Dilution | Supplying Company |
|---|---|---|---|---|
| Oct-04 | Mouse IgG | 250µg/ml | 0.111111111 | BD Biosciences |
| Nanog | Goat IgG | 100µg/ml | 0.111111111 | R&D Systems Inc. |
Table 2: Primary antibodies used for immunofluorescence staining. Their species, isotypes, concentrations and suppliers are also shown.
| Secondary Antibody Target | Species | Concentration | Dilution | Supplying Company | Conjugation |
|---|---|---|---|---|---|
| Anti-Mouse IgG | Donkey | 2mg/ml | 0.180555556 | Invitrogen | Alexa Fluor 488 |
| Anti-Goat IgG | Donkey | 2mg/ml | 0.180555556 | Invitrogen | Alexa Fluor 594 |
Table 3: Secondary antibodies used for immunofluorescence staining. Their species, isotypes, concentrations, suppliers and conjugations are also shown.
Determination of pluripotency of HUES1 and HUES7 stem cells
Immunofluorescence staining
All cells for immunofluorescent staining analysis were cultured in 24-well plates. Staining was performed for intracellular markers Oct4 and Nanog which are 2 of the 3 pluripotency regulatory genes. In brief, cells were fixed with 4% paraformaldehyde (PFA) for 15 minutes at room temperature. After fixation, PFA was discarded and the cells were washed 3 times with PBS solution (PBS, 0.05% Tween, 0.1% BSA; PAA Laboratories). Seventy-five μl of blocking/permeabilization solution (PBS, 10% serum, 0.1% Triton X) was then applied for 30 minutes at room temperature if appropriate. The serum used for the blocking solution was from the species that the secondary antibody was raised in. The blocking solution was then discarded. Seventy-five μl of primary antibody solution at specified dilutions in 1% serum, 0.1% Triton X for intracellular antigens and PBS solution were applied overnight at 4°C. Primary antibody dilutions were determined by dose response curves. Following overnight incubation, cells were then washed 3 times with PBS solution. Then, 75 μl of secondary antibody solution at specified dilutions in 1% serum, +/-0.1% Triton X and PBS were applied for 1hour at room temperature. Cells were then washed 3 times with PBS solution. 1:50000 dilute DAPI solution (Invitrogen, Oregon, USA) was applied for 5 minutes in the dark. Cells were then washed 3 times and immediately visualized with microscopy after the 3rd wash.
Results
HUES1 and HUES7 cell culture on vitronectin and fibronectin
HUES1 and HUES7 cells were cultured in a feeder-free system on either vitronectin or fibronectin. The morphology of the hESCs was regularly assessed by phase contrast microscopy to check that they are healthy and to assess their propagation pattern. Generally, assessment of cell morphology showed that the cells formed compact layers (Figures 1 and 2) as cell propagation continued with days. The cells appeared to form consistent mono-layers. However, cells that were cultured on fibronectin (for both HUES1 and HUES7) showed a lot more adherence to the culture dish when compared to those on vitronectin especially at passages 2 and 3.
Pluripotency marker expression in hUES1 and HUES7 stem cells cultured on vitronectin and fibronectin
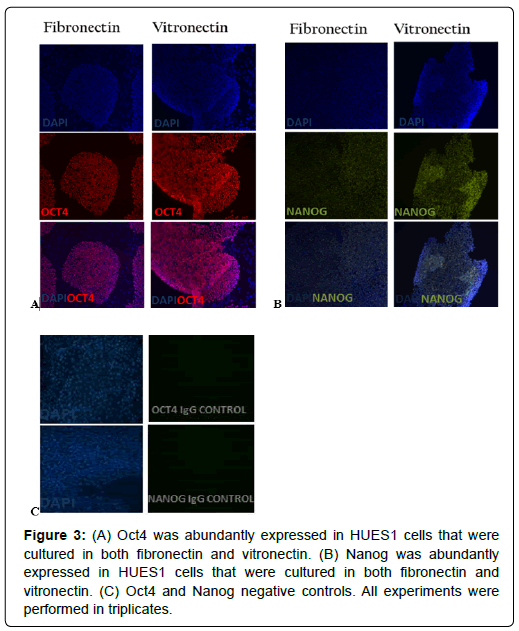
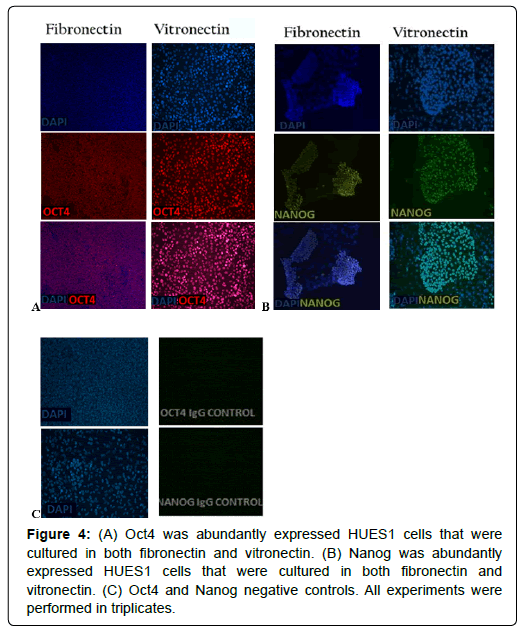
The pluripotency of the HUES1 and HUES7 cells was tested by staining the cells for intra-cellular markers of pluripotency; Oct4 and Sox2. Cells on both vitronectin and fibronectin adequately expressed the nuclear localised Oct4 (Figures 3A and 4A) and Nanog (Figures 3B and 4B). Negative controls showed negligible secondary antibody cross-reactivity (Figures 3C and 4C).
Figure 3: (A) Oct4 was abundantly expressed in HUES1 cells that were cultured in both fibronectin and vitronectin. (B) Nanog was abundantly expressed in HUES1 cells that were cultured in both fibronectin and vitronectin. (C) Oct4 and Nanog negative controls. All experiments were performed in triplicates.
Discussion
The efficiency and clinical application of pluripotent stem cells relies, to a large extent, on prudent culture techniques that maximize the number of cultured colonies as well as the clinical applicability of those colonies. This is what regenerative medicine as a discipline is dependent on. The stem cell niche is central to the attachment and proliferation of cells in culture. It is for this reason that research should be done on the different components on the ECM, which is an important component of the stem cell niche.
In this study, both fibronectin and vitronectin were shown to support the propagation of HUES1 and HUES7 cells as well as the maintenance of pluripotency in these cells for 3 weeks. In fact, recombinant vitronectin have been shown to promote self-renewal and pluripotency in multiple stem cell lines through αVβ5 Integrin [8]. Another study has reported a novel substrate that comprises of both vitronectin and fibronectin in combination with other matrix components such as collagen IV and laminin. This is something that could be performed in parallel in different cell lines to test the efficacy of such a recombinant substrate.
This study has shown that the expression of stem cell pluripotency markers was retained over a few passages of HUES1 and HUES7 cells in both vitronectin and fibronectin. These substrates, therefore, can serve equally as viable replacements for Matrigel in a cell culture system involving a chemically defined medium. As [12] explains, culturing embryonic stem cells in a chemically defined system is much more beneficial to the clinical applications of the cells. HUES1 cells have been previously shown to express integrin receptors for a number of ECM components, among them fibronectin and vitronectin [8]. These receptors are responsible for facilitating cell adhesion during the in vitro culturing of these cells. FGF2 signalling in hESCs [13] and integrin signalling in mESCs [14] have been shown regulate stem cell self-renewal. It would be of great interest to find out which of the stem cell self-renewal and pluripotency signalling pathways are directly regulated by the ECM, the mother parent of fibronectin and vitronectin. Although mESCs and hESCs have been shown to have different differentiation patterns [15,16], it would be interesting to study the role, if any, of integrin signalling in hESCs themselves.
This study observed delayed proliferation of cells that were cultured on vitronectin when compared to those on fibronectin. However, the population of cells at the end of the 3 weeks protocol were not that dissimilar the under the microscope. Although the vitronectin used in this study was truncated containing amino acids fragments 62-478 of the full length human vitronectin, recombinant truncated vitronectin has been shown to support cell survival and attachment better than the wild type [17]. The ‘slight peak’ of cell population at the end of the protocol was therefore not an anomaly. It has been shown previously that purified vitronectin is very effective in promoting cell growth and attachment [8]. Perhaps the efficacy of this vitronectin is due to the fact that it was grown in a different mouse myeloma cell line.
Other studies have been performed that support the findings of this study. Vitronectin has been shown to pluripotent human embryonic stem cells in the absence of serum [18] while fibronectin has been shown to support stem cell self-renewal for an extended period [19].
Conclusions
This study has shown that both fibronectin and vitronectin are capable of maintaining the undifferentiated state of stem cells after a few passages in culture. Although there was a slight delay in the propagation of cells in vitronectin, there was a dissimilar population of cells observed in both vitronectin and fibronectin cell cultures at the end of the 21-day protocol. Both substrates have the potential to support the in vitro expansion of hESCs for both research and clinical application purposes.
Acknowledgements
This work was undertaken using facilities of the Faculty of Science Unit, The University of Manchester, Manchester, United Kingdom.
Competing Interests
The author declares no competing interests.
Funding
This work was funded by Botswana International University of Science and Technology and the Government of Botswana.
References
- Cowan C, Klimanskaya I, McMahon J, Atienza J, Witmyer J, et. al (2004). Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med: 350:1353-1356.
- Martin G (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci; 78:7634-7638.
- Xu C, Inokuma M, Denham J, Golds K, Kundu P. et.al (2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol; 19:971-974.
- Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R (2005) Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng; 91:688–698.
- Lam A, Li J, Chen A, Birch W, Reuveny S (2015) Improved Human Pluripotent Stem Cell Attachment and Spreading on Xeno-Free Laminin-521-Coated Microcarriers Results in Efficient Growth in Agitated Cultures. Biores Open Access; 4:242-257.
- Gerecht S, Burdick J, Ferreira L, Townsend S, Langer R (2007) Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci; 104:11298–11303.
- Hayashi Y, Furue K (2016) Biological effects of culture substrates on human pluripotent stem cells. Stem Cells Int:5380560.
- Braam S, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, et.al (2008) Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells; 26:2257–2265.
- Hayashi Y, Chan T, Warashina M, Fukuda M, Ariizumi T, et.al (2010) Reduction of N-Glycolylneuraminic acid in human induced pluripotent stem cells generated or cultured under feeder- and serum-free defined conditions. PLoS ONE;5: e14099.
- Prowse A, McQuade L, Bryant K, Marcal H, Gray P (2007) Identification of potential pluripotency determinants for human embryonic stem cells following proteomic analysis of human and mouse fibroblast conditioned media. J Proteome Res; 6:3796–3807.
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J (2004) Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod; 70:837–845.
- Ilic D (2006) Culture of human embryonic stem cells and the extracellular matrix microenvironment. Regen Med; 1:95–101.
- Saksela O, Moscatelli D, Sommer A, Rifkin D (1988) Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol; 107:743–751.
- Yamada K, Even-Ram S (2002) Integrin regulation of growth factor receptors. Nat Cell Biol;4: E75–E76.
- Brons I, Smithers L, Trotter M, Rugg-Gunn P, Sun B, et.al (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature; 448:191–195.
- Tesar P, Chenoweth J, Brook F, Davies T, Evans E (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature; 448:196–199.
- Chen G, Gulbranson D, Hou Z, Bolin J, Ruotti V (2011) Chemically defined conditions for human iPSC derivation and culture. Nat Methods; 8:424-429.
- Manton K, Richards S, Van Lonkhuyzen D, Cormack L, Leavesley D (2010) A chimeric vitronectin: igf-I protein supports feeder-cell-free and serum-free culture of human embryonic stem cells. Stem Cells Dev; 19:1297–1305.
- Hughes C, Radan L, Betts D, Postovit L, Lajoie G (2011) Proteomic analysis of extracellular matrices used in stem cell culture. Proteomics; 11:3983-3991
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi