Case Report, Int J Ophthalmic Pathol Vol: 0 Issue: 0
Complications Associated with the Surgical Techniques of Upper Eyelid Loading: A Clinicopathologic Study of 7 Explanted Gold Weight Lid Loads
Izabela NG*, Radoslaw R, Robert K and Marek R
Military Institute of Medicine, Poland
*Corresponding Author : Izabela Nowak-Gospodarowicz
Department of Ophthalmology, Military Institute of Medicine, 128 Szaserow St., 04-141 Warsaw, Poland
Tel: 0048 261 816 575
Fax: 0048 22 515 05 08
E-mail: istabel@wp.pl; inowak-gospodarowicz@wim.mil.pl
Received: June 14, 2017 Accepted: July 06, 2017 Published: July 13, 2017
Citation: Nowak-Gospodarowicz I, Rozycki R, Koktysz R, Rekas M (2017) Complications Associated with the Surgical Techniques of Upper Eyelid Loading: A Clinicopathologic Study of 7 Explanted Gold Weight Lid Loads. Int J Ophthalmic Pathol 6:3. doi: 10.4172/2324-8599.1000207
Abstract
Loading of the upper eyelid with gold weights is a well-established procedure for the correction of paralytic lagophthalmos. Only a few studies show the impact of the implant on the surrounding tissues. The aim of this case series is to present clinicopathological correlations in patients requiring implant removal due to post-surgical complications. There were 7 patients (2 men, 5 women aged 24 -79 years) from a sample of 64 with unresolved facial nerve palsy and exposure keratopathy treated with upper gold-weight lid-loading from 2009-2014. Causes of implant removal were: swelling and reddening of the eyelid followed by weight extrusion in 5 cases, 1 excessive ptosis, and 1 unsatisfactory cosmesis. The mean implant weight was 1.5 ± 0.3 g. Complications occurred within 5-92 months. All weights were surrounded by fibrous capsules with lymphocytic infiltrates CD3, CD4, CD8, and CD20 positive. We conclude that complications related to operation technique mimicked an allergic reaction to gold.
Keywords: Gold weights; Lid-loading; Allergy to gold; Exposure keratopathy; Lagophthalmos; Facial nerve palsy
Keymessages
Complications related to the operation technique may mimic symptomatic allergy to gold in the upper eyelid loading procedure. Meticulous fixing of the weight to the upper border of the anterior tarsus may outdistance it from the blood vessels of the lid margin and thereby minimize its potential impact on the surrounding tissues.
Introduction
Since its introduction in 1958 [1], upper gold-weight lid-loading has been an effective method for the correction of lagophthalmos in facial nerve palsy [2-4]. Among the relatively rare complications related to the procedure (including: weight extrusion, migration, bulging, induced astigmatism, under- or overcorrection and unsatisfactory cosmesis) only a few cases of non-infectious inflammatory response to gold have been reported [5-9]. Some complications are attributed to type IV hypersensivity reactions [5,8], but due to the small number of investigated cases, scarce histopathological examination, and limitations of screening technologies, there is still no direct confirmation of this hypothesis.
Case Report
This was a prospective, consecutive case series assessing clinical outcomes after upper gold-weight lid-loading as treatment for ocular complications of unresolved facial nerve palsy in years 2009-2014.
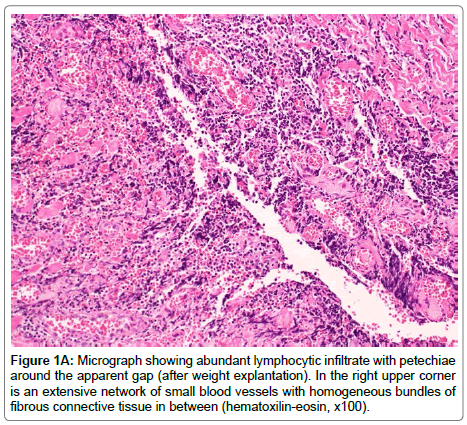
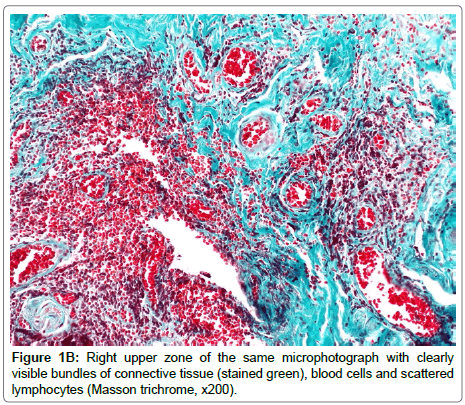
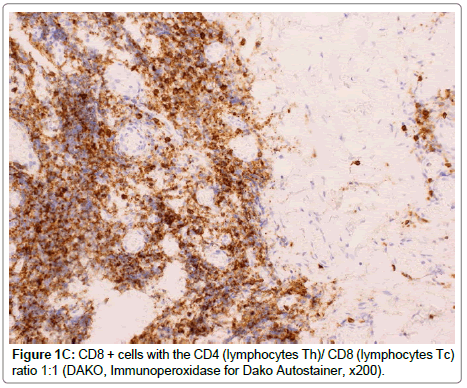
The weight with surrounding tissues was removed in 7 out of 64 cases. Each specimen was stained according to the following protocols: Mayer’s Hematoxylin and Eosin, Masson’s trichrome and using CD3, CD20, CD4, CD8 and Mac387 monoclonal antibodies (Table 1, Figure 1A-C). All patients had a negative history of metal allergies.
| Patient | Masson trichrome | CD3+ | CD20+ | CD4+ | CD8+ | Mac 387 | CD3:CD20 | CD4:CD8 |
|---|---|---|---|---|---|---|---|---|
| 1 | Fibrosis,fibrin, hemorrhages | 50 | 70 | 30 | 50 | Numerous, in clusters | 1:1,5 granulomatous |
1:1,5 |
| 2 | Fibrosis, hemorrhages | 60 | 60 | 40 | 20 | Numerous, in clusters | 1:1 non granulomatous |
2:1 |
| 3 | Fibrosis, hemorrhages | 50 | 30 | 40 | 70 | Numerous, in clusters | 1,5:1 non granulomatous |
1:1,5 |
| 4 | Fibrosis, fibrin | 70 | 20 | 30 | 10 | Sparse, dispersed | 4:1 non granulomatous |
3:1 |
| 5 | Fibrosis, hemorrhages | 40 | 30 | 40 | 40 | Numerous, in clusters | 1:1 non granulomatous |
1:1 |
| 6 | Fibrosis, fibrin | 50 | 50 | 50 | 70 | Sparse, dispersed | 1:1 non granulomatous |
1:1,5 |
| 7 | Fibrosis, hemorrhages | 50 | 40 | 70 | 70 | Numerous, in clusters | 1:1 non granulomatous |
1:1 |
Table 1: Histopathology.
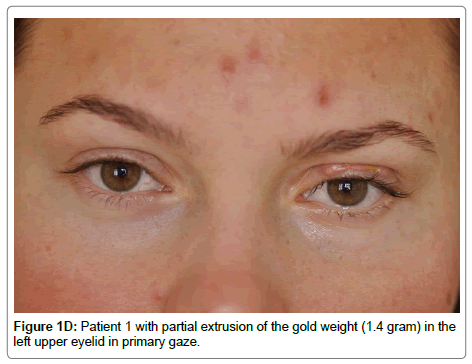
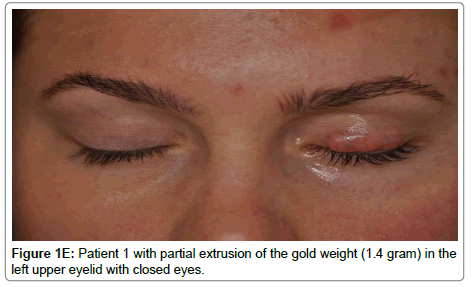
Patient 1
Presented with left fifth and seventh cranial nerve palsies (5th, 7th CNP) and weight extrusion (Table 2, Figure 1D,1E). Otherwise, she remained healthy.
| Patient | Age | Gender | Eye | Etiology | Weight [gram] |
Weight Manufacturer | Cause of removal | Complications free period [months] |
Corneal sensation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | F | L | ANS* | 1.4 | Upper Eyelid Implant, Heinz Kurz GmbH Medizintechnik, Germany | extrusion | 5(referral) | No |
| 2 | 35 | M | R | ANS* | 1.4 | domestic | extrusion | 92(referral) | No |
| 3 | 42 | M | L | ANS* | 1.2 | Contour™ Eyelid Implant, MedDev Corporation, USA | extrusion | 31(our surgery) | No |
| 4 | 37 | F | L | Trauma | 1.4 | Contour™ Eyelid Implant, MedDev Corporation, USA | cosmesis | 12(our surgery) | Normal |
| 5 | 60 | F | R | ANS* | 1.0 | Contour™ Eyelid Implant, MedDev Corporation, USA | extrusion | 29(our surgery) | No |
| 6 | 75 | F | L | HZO** | 1.8 | Contour™ Eyelid Implant, MedDev Corporation, USA | ptosis | 14(our surgery) | No |
| 7 | 79 | F | L | ANS* | 2.0 | domestic | extrusion | 8(referral) | No |
**HZO=Herpes Zoster Ophthalmicus
Table 2: Clinical data.
After implant removal, secondary gold weight implantation was performed with our standard 2-week postoperative course of topical antibiotic and steroid ointment (oxytetracycline with hydrocortisone, 4 qd). No further complications occurred.
Patient 2
Presented with right 5th, 7th CNP and gold weight extrusion after secondary lid-loading (Table 2). The patient’s other medical history was unremarkable.
After implant removal, the patient received a new 1.6 g gold weight, fixed to the anterior upper tarsal border with absorbable sutures (Vicryl 6.0). No complications occurred.
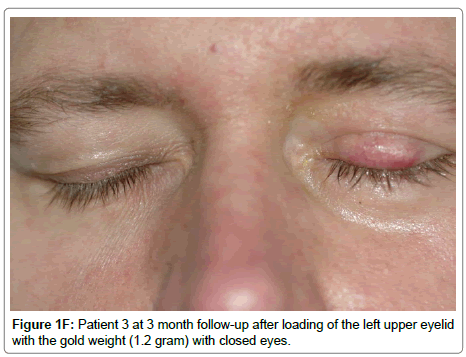
Patient 3
Was slightly hypertensive and presented with left 5th, 7th CNP and weight extrusion in the site of a previous tarrsorrhaphy performed for neurotrophic corneal ulceration (Table 2). The weight was fixed with absorbable sutures to the anterior tarsus, about 2 mm above the eyelash line. The eyelid was noticeably reddened on postoperative Day-1 (Figure 1F). After implant removal the eyelid was pale. Mullerectomy and levatoraponeurosis recession were performed to provide corneal protection. No other complications occurred.
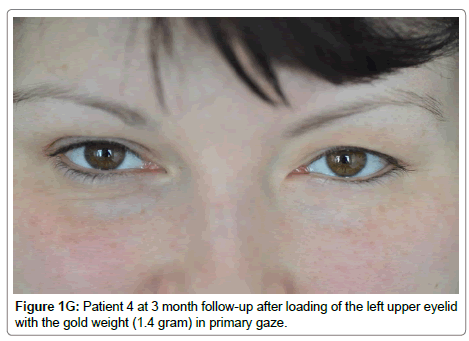
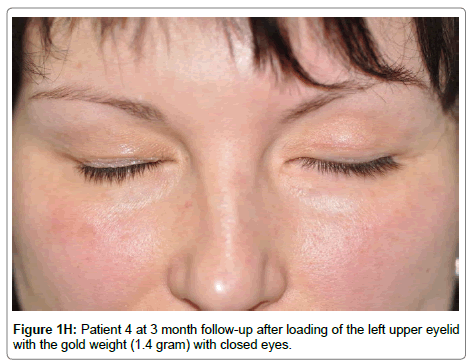
Patient 4
Presented with left 7th CNP (Table 2). She was healthy but tired of using topical moisturizers. Gold weight implantation was successful (Figure 1G, 1H). After initial enthusiasm due to a complete relief of symptoms, she started complaining of occasional drooping of the loaded eyelid in a downgaze position during postoperative Month 3. We removed the weight at the patient’s request. Eyelid closure is possible with some effort and the patient is asymptomatic.
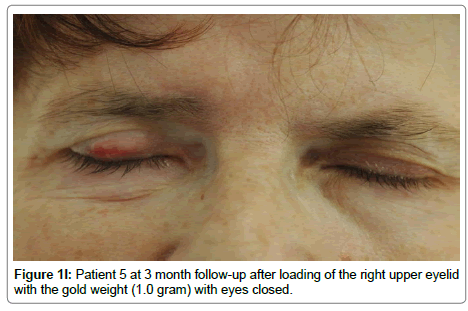
Patient 5
Was mildly hypertensive and presented with right 5th, 7th CNP and a corneal ulcer (Table 2). Urgent tarrsorrhaphy was performed and then we placed a gold weight into the pretarsal space, like in Patient 3. The clinical signs were similar (Figure 1I). The patient underwent secondary lid loading with the same weight fixed to the upper border of the tarsal plate with absorbable sutures. The eyelid was pale and no other complications occurred.
Patient 6
Presented with corneal neurotrophic ulcer and uveitis (Table 2) Except for mild hypertension, her past medical history was unremarkable. After conservative local and systemic antiviral treatment (Acyclovir) the symptoms of left 5th, 7th CNP persisted so we placed the weight on the upper tarsal border. The results were initially encouraging but after 3 months progressive ptosis occurred, which resulted in implant removal. The ptosis persists and the patient has refused any additional surgical intervention.
Patient 7
Was in general good condition and presented with left 5th, 7th CNP and weight extrusion (Table 2). After implant removal, eyelid closure was possible with some effort. The patient uses topical moisturizers. No additional surgical intervention is necessary.
Discussion
Following previous reports [3-6,10] our study confirms that gold implants induce a non-granulomatous chronic inflammatory reaction (Figure 1A, Table 1) of lymphocytes B (CD20+) and T (CD 3+) ( Figure 1C, Table 1) and local fibrosis, visualised by Masson trichrome (Figure 1B, Table 1). Macrophages (Mac 387) are crucial cells in an immune response, indicating that there was an active inflammatory process in Patients: 1,2,3,5 and 7, who had implant extrusions (Table 1).
In all patients with extrusions the implant was placed approximately 2 mm above the eyelash line and the major blood vessels, which are vital participants in inflammatory reactions and angiogenesis. Since we started suturing the weight to the bare anterior upper tarsal border with absorbable polyglactin sutures, we have had no extrusions, reddening, or vessels visible on the surface of the eyelid skin (Figure 1H). Both neovascularization visible in Patients 1,2,3,5,7 and significantly fewer vessels and hemorrhages in Patients 4 and 6 presenting no signs of extrusion, may confirm that implantation site plays a crucial role in tissue response to biomaterials [11]. Eosinophilic infiltrate, typical for allergic reactions as reported by Bair [6], was not noted in any sample. Given that the same weight implanted again in Patients 1 and 5 caused no adverse reactions after changing the operation technique and that histopathological findings revealed no difference between the different types of examined weights (Table 1), it seems that the implant itself did not influence the outcomes. A typical foreign-body giant cell reaction was observed only in Patient 1 close to the non-absorbable sutures (Table 1), similar to the results from other studies [6,10]. In contrast, however, absorbable polyglactin sutures used by Townsend [4], Doyle et al. and our ward, both seem to be sufficient to keep the implant in place until the fibrotic capsule formation stabilizes and seem not to induce any additional long-term reactions [5]. The significantly higher concentration of T helper cells (CD4+) in Patient 4 with isolated facial nerve palsy indicates that a lack of tissue innervation may inhibit T-helper mediated cell-to-cell interactions in the immune response.
Following our experience and Anderson’s study [11], we conclude that complications after upper gold-weight lid-loading related to operation technique may mimic an allergic reaction to gold. A symptomatic allergy to gold in the lid-loading procedure seems to be a rare complication, but it should be taken into account when taking a patient’s medical history.
Source(s) of Support: Young Scientist Dotation from Polish Ministry of Science and Higher Education (No. 200/2012)
References
- Illig KM (1958) Eine neue Operationsmethode gegen Lagophthalmus. Klin Monatsbl Augenheilkd 32: 410-411.
- Jobe RP (1974) A technique for lid loading in the management of the lagophthalmos of facial palsy. Plast Reconstr Surg 53: 29-32.
- Seiff SR, Sullivan JH, Freeman LN, Ahn J (1989) Pretarsal Fixation of Gold Weights in Facial Nerve Palsy. Ophthal Plast Reconstr Surg 5: 104-109.
- Townsend DJ (1992) Eyelid reanimation for the treatment of paralytic lagophthalmos: historical perspectives and current applications of the gold weight implant. Ophthal Plast Reconstr Surg 8: 196-201.
- Doyle E, Mavrikakis I, Lee EJ, Emerson R, Rainey AJ, et al. (2005) Type IV hypersensivity reactions to upper lid gold weight implants-Is patch testing necessary? Orbit 24: 205-210.
- Bair RL, Harris GJ, Lyon DB, Komorowski RA (1995) Noninfectious inflammatory response to gold weight eyelid implants. Ophthal Plast Reconstr Surg 11: 209-214.
- Dinces EA, Mauriello JA Jr, Kwartler JA, Franklin M (1997) Complications of gold weight eyelid implants for treatment of fifth and seventh nerve paralysis. Laryngoscope 107: 1617-1622.
- Björkner B, Bruze M, Möller H, Salemark L (2008) Allergic contact dermatitis as a complication of lid loading with gold implants. Dermatitis 19: 148-153.
- Iordanous Y, Evans B (2012) Noninfectious inflammatory reaction to a gold weight eyelid implant: A case report and literature review. Can J Plast Surg 20: 199-200.
- Schrom T, Taege C, Wolf G, Reinhardt A, Scherer H (2006) Histopathologie nach Implantation von Lidgewichten. HNO 54: 591-598.
- Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20: 86-100.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi