Research Article, J Virol Antivir Res Vol: 8 Issue: 1
Concentrates from Aframomium melegueta K. Schum and Vernonia amygdalina Delile Inhibit Proliferation of Atypical Fowl Pox Virus in Embryonated Chick Eggs (Eces)
Afolami OI1*, Oladunmoye MK1, Chukwuedo AA2, Asala OO2, Nwagbo IO2, Oladejo BO1, Abe TO3, Akpa AR4, Aribisala JO1 and Owoyemi OO1
1Department of Microbiology, Federal University of Technology, P.M.B. 704, Akure, Ondo State, Nigeria
2Viral Research/Vaccines Production Division, National Veterinary Research Institute, Vom, Nigeria
3Department of Chemistry, Federal University of Technology, P.M.B. 704, Akure, Ondo State, Nigeria
4Department of Toxicology and Biochemistry, National Veterinary Research Institute, Vom, Nigeria
*Corresponding Author: Afolami OI
Department of Microbiology, Federal University of Technology, P.M.B. 704, Akure, Ondo State, Nigeria
Tel: +2347069438529
E-mail: afolamiolufemiifeoluwa@gmail.com or oiafolami@futa.edu.ng
Received: March 12, 2019 Accepted: March 28, 2019 Published: April 08, 2019
Citation: Afolami OI, Oladunmoye MK, Chukwuedo AA, Asala OO, Nwagbo IO, et al. (2019) Concentrates from Aframomium melegueta K. Schum and Vernonia amygdalina Delile Inhibit Proliferation of Atypical Fowl Pox Virus in Embryonated Chick Eggs (Eces). J Virol Antivir Res 8:1. doi: 10.4172/2324-8955.1000186
Abstract
This study evaluated the inhibition property of concentrates from Aframomium melegueta K.Schum and Vernonia amygdalina Delile against the proliferation of atypical Fowlpox virus strain (FPV Kabete) in Embryonated chick eggs (ECEs). We determined the effect of the concentrates on the propagation of the atypical FPV in ECEs via virus inhibition assay and linked the possible mechanisms by which compounds present in the concentrates were able to reduce the levels of cytopathic effect expressions caused by the atypical FPV in ECEs. The results showed that the concentrates had multiple inhibitory effects against the FPV while they showed mild toxicities in chick embryos with toxic threshold concentration set at 300 µM. In summary, the A. melegueta concentrate had stronger inhibitory effect against the FPV proliferation; it increased the average embryo weight (from 12.56 ± 1.61 g to 23.19 ± 1.79 g) and diminished the FPV Log10EID50 titer from 3.86 to 3.08. It also reduced the mean number of pock lesions from 18.28 ± 1.84 pfu/100EID50 to 2.19 ± 0.65 pfu/100EID50 and likewise reducing the mean pock diameter from 22.48 ± 2.74 mm to 4.89 ± 1.04 mm. Comparatively, the V. amygdalina concentrate showed lesser inhibitory effect than the A. melegueta concentrate; it increased the average embryo weight (from 12.56 ± 1.61 g to 19.49 ± 1.33 g), diminished the FPV Log10EID50 titer from 3.86 to 3.34, reduced the mean number of pock lesions from 18.28 ± 1.84 pfu/100EID50 to 6.85 ± 1.77 pfu/100EID50 and likewise reducing the mean pock diameter from 22.48 ± 2.74 mm to 8.96 ± 1.26 mm. This study showed the efficacy of the concentrates to inhibit the proliferation of the FPV in ECEs and that compounds contained in the concentrate can lead the discovery for biosynthesis of novel antipoxvirus inhibitors.
Keywords: Inhibition, Atypical FPV, Cytopathic effects, Proliferation, Propagation, ECEs
Introduction
Fowl pox virus belongs in in the sub family avipoxviridae and different studies on the virus have delineated its economic importance to domestic birds and its potential risk to man [1]. Studies on viruses in the sub family avipoxviridae have laid precedence for vaccine production, which limits the management of pox infections in birds to prevention via vaccination [2]. Fowl pox virus is primarily propagated on the chorioallantoic membrane (CAM) of Embryonated chick eggs (ECEs) or Chicken embryo fibroblasts and some wild type strains of the virus possess strong proliferative abilities, effecting mild to severe damage to visceral tissues [3]. Studies on Fowl pox virus have linked the ability of the virus to adsorb to specific animal tissues and propagate rapidly in the tissues, leading to formation of pock lesions and tissue aggregation [2]. In one of those studies reported the occurrence of an “atypical” Fowl pox virus (FPV) strain in Kabete, Kenya. The study showed that this strain of Fowl pox virus caused a rapid epithelial invasion, endotheliosis and lympho-proliferative damages in tissues of matured birds. Interestingly, other studies following this lead later confirmed that the atypical FPV might cause mild infections in human tissues since fragments of human reticulo-endotheliosis virus (REV) were discovered in the whole viral genome of atypical FPV [4]. Furthermore, [5] showed in his study that the lethally rapid pace of atypical FPV proliferation in different tissues makes the prospect of vaccine production quite uncertain and characterization of the atypical FPV genome confirmed that vaccinated birds may be at risk of infection since the atypical FPV has a shortened replication time and thus higher infectivity. Antiviral research involving the use of plant derived products has shown the potency of many plant species localized in different regions of the tropics. However, since most of these plants species are not ubiquitous and given their proven potency over past decades against viruses, it is expedient that continuous efforts be made to harness the full extent of their curative abilities [6]. Some classes of Phyto-compounds such as such as alkanols, base analogues, amides, polysaccharides, functional ketones and phenols have been proven drug leads against re-emerging infectious strains of viruses [7]. Scientists also have discovered that many of these phyto-compounds actively block viral replication, adsorption and or entry of viruses into specific host targets [8]. Furthermore, some studies on plant research have also shown that many of these phyto-compounds could trigger chemotactic factors and co-enzymes that may repress the proliferative abilities of viruses in susceptible tissues [9]. In western Nigeria, recent studies points to two local herbs Aframomium melegueta K.Schum and Vernonia amygdalina Delile as rich reservoirs of potent phyto-compounds that have wide range antimicrobial efficacies [10,11]. More so, a recent information in [12] showed that crude extract from V. amygdalina had antiviral effect against Newcastle Disease Virus strain (NDV N1E) while scant data exist on the antiviral efficacy of A. melegueta. With regard to our preliminary findings leading to this current study, we already obtained that the two most abundant compounds in the clarified concentrate from V. amygdalina were Phytol and a nucleoside analogue Methyl-2-O-benzyl-d-arabinofuranoside while the clarified concentrate of A. melegueta contain two abundant phenols (benzaldehyde-3-hydroxy-4-methoxy and butan-2-one-4- (3-hydroxy-2-methoxyphenyl). However, in this current study, we tested for the ability of the clarified concentrates from A. melegueta and V. amygdalina to inhibit the proliferation of atypical Fowlpox virus strain (FPV Kabete) in Embryonated chick eggs (ECEs). We determined to what extent the concentrates were able to halt the propagation of the atypical FPV in ECEs and linked the possible mechanisms by which compounds present in the concentrates were able to reduce the levels of cytopathic effect expressions caused by the atypical FPV in ECEs.
Materials and Methods
Preview on preliminary characterization of phyto-compounds in the clarified concentrates
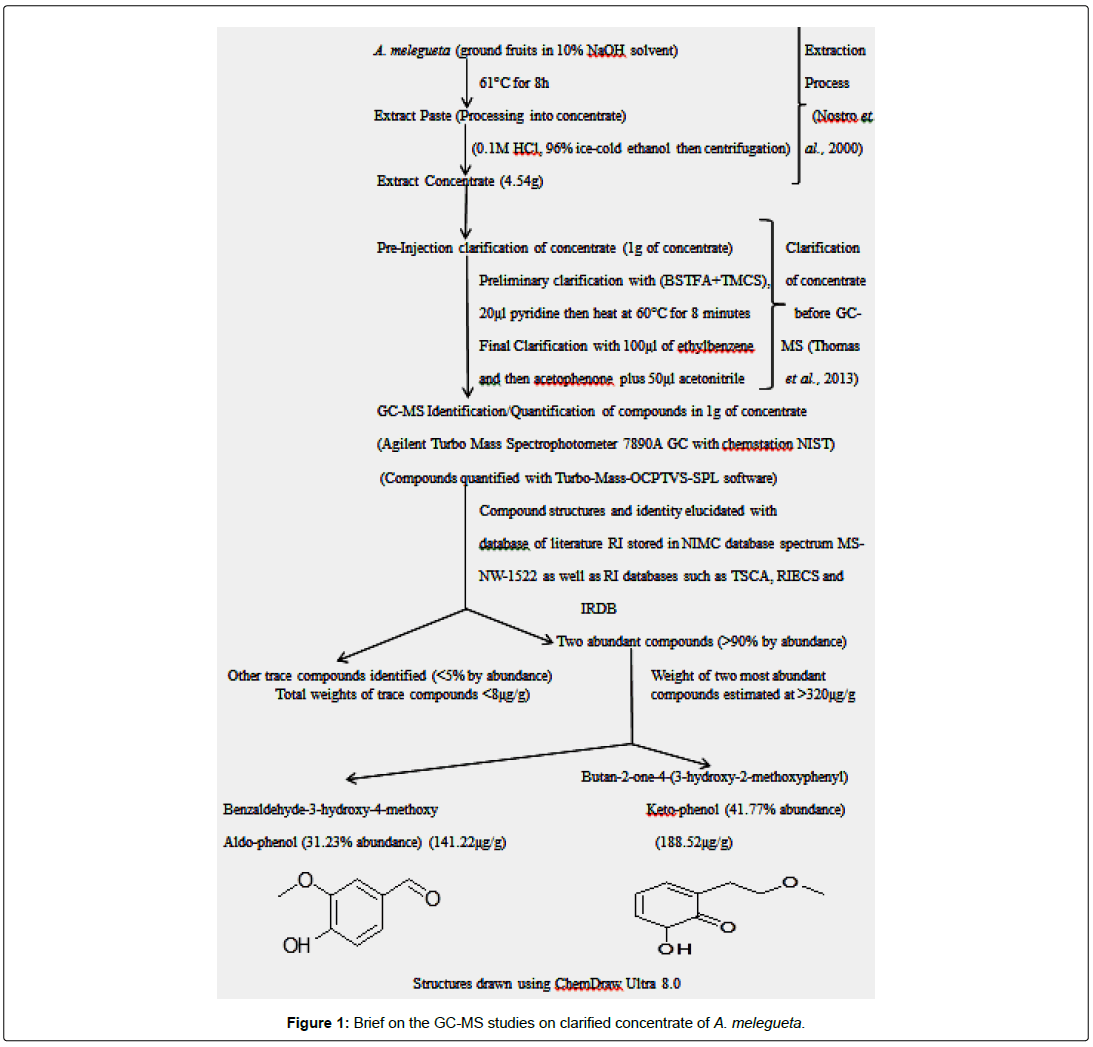
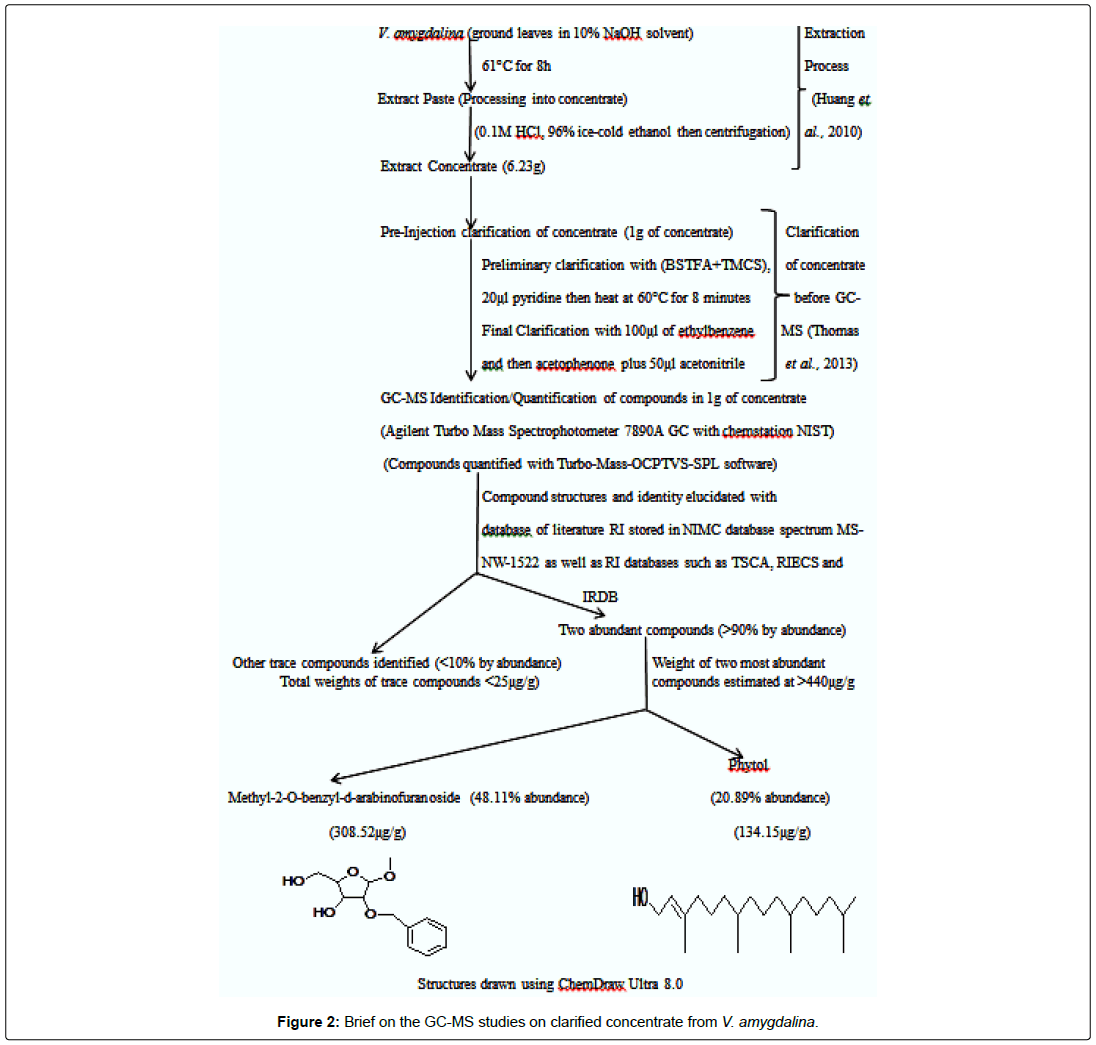
The concentrates from the test plants A. melegueta and V. amygdalina were obtained using procedures of [13]. After extraction, a protocol native to the Agilent Turbo Mass Spectrophotometer 7890A GC with chemstation NIST was used to clarify the concentrates obtained from the test plants [6]. Characterization of the phytocompounds contained in the clarified concentrates was carried out using Agilent Turbo Mass Spectrophotometer 7890A GC with chemstation NIST for Gas Chromatography-Mass Spectrometry (GC-MS) analysis. The two concentrates from the plants have been analyzed and clarified prior to antiviral assay. Full details of these procedures are already summarized in Figures 1 and 2.
Reconstitution of concentrate before antiviral assay
A 50 mM stock solution of the clarified concentrates were prepared by reconstituting 1 g of the each concentrate into a 20 ml of the diluent consisting of Penicillin, Streptomycin, Gentamycin, and Amphotericin B (PSGA X5) adjusted to pH 7.2. Resulting solutions were filtered with 0.45 μ membrane filters by the aid of suction pressure filtration into sterile vials and stored at -10°C until needed.
Virus source and egg cultures (ECEs)
The Viral Vaccines Production Division of National Veterinary Research Institute (NVRI), Vom provided the already determined 100EID50 (1/7200 ml-1) of the atypical strain of Fowlpox virus (FPV Kabete) used in this study while Embryonated Chick Eggs (ECEs) aged 13 days were from the poultry division of NVRI, Vom, Nigeria. The embryonated eggs were checked for their viability using flash point candling and viable eggs were punctured at the chorio-allantoic region and at a region about 3 mm below the air sac, using pressure from a vacuum pump (Eppendorf, Germany) to collapse the Chorioallantoic membrane (CAM) for inoculation of the concentrates. The determination of egg toxic threshold level, determination of embryo infectious dose (EID50) and inhibitory effects of the concentrates against proliferation of FPV Kabete in ECEs were carried out at viral research division of the NVRI, Vom, Nigeria.
Determination of toxic threshold of concentrates on ECEs
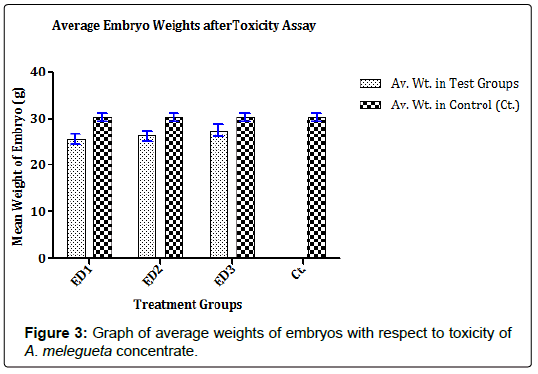
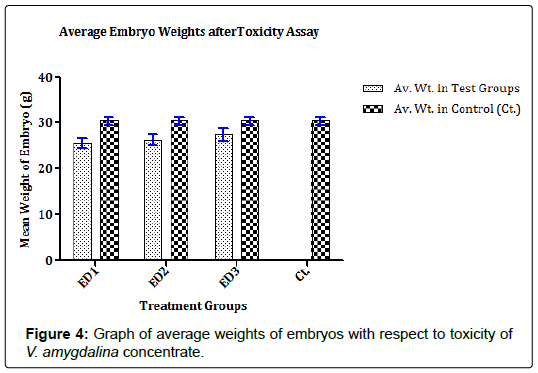
The toxic effects of concentrates on the chick embryo was determined to know the minimum toxic concentration that is safe for 13 days old ECEs. The 13 days old ECEs were divided into four groups of five (5) eggs per group and labeled appropriately in biosafety cabinets. The eggs were acclimatized in CO2 pression Incubator (Schumboldt CR-8V, Germany). From the 50 mM concentrate stocks prepared, three dilutions of the concentrates (450 μM, 300 μM, and 150 μM) were obtained. A 0.2 ml of each dilution was separately inoculated into allantoic cavity of the eggs in their treatment groups while control group received 0.2 ml PBS + PSGA X5 [12]. The inoculation sites were sealed with cutex and eggs were incubated for 120 h at 37 ± 1°C. The eggs were candled daily at the end of the incubation period, embryos were harvested. The toxic effects of the various dilutions from each concentrate on the ECEs were determined by examining embryos for deformities and determining the average weight of embryos in all the treatment groups [12].
Test for inhibition of concentrates against proliferation of FPV in ECEs
The inhibition effects of concentrates against FPV using ECEs was done according to [2,12]. From the toxic threshold test above, the minimum toxic concentration of concentrates was set at 300 μM and the 100 EID50 FPV stock solutions were prepared. Thirteen (13) days old ECEs were divided into six groups of fifteen (15) eggs each while the control group had six (6) eggs. The eggs were labeled and punctured inoculation sites were swabbed with 70% ethanol under biosafety cabinets. The inhibition assays were performed by first inoculating a 0.1 ml/300 μM of each concentrate into the ECEs via the CAM infection route. The eggs were incubated immediately after inoculation for at 37 ± 1°C for 90 minutes. This was to allow the concentrates to adsorb evenly into the CAM region of the eggs. At the end of 90 minutes, the 100 EID50/0.1 ml FPV was inoculated into the eggs. Inoculation sites on the eggs were sealed with cutex and eggs were incubated at 37 ± 1°C for 120 h. The eggs were candled daily and dead embryos were removed. After incubation, CAM and embryos were aseptically harvested from surviving eggs and embryos were examined for deformities. The CAMs were also examined for formation of pock lesions which were counted if present. The average weights of embryos from each group were determined and CAMs were checked for Cytopathic effects (CPEs).
Pock assay
Chorioallantoic membranes (CAMs) harvested from each treatment group were placed on sterile petri plates and treated them with 2 ml of the bacteriostatic 2X PSA (Penicillin, Streptomycin and Amphotericin B). After treatment, they were flushed thoroughly with 5 ml PBS and spread evenly on the petri plates using sterile forceps. A few drops of primary dye hematoxylin were added to the CAMs and after 5 minutes the dye was flushed off with PBS. A 2 ml of 65% ethanol was used to decolorize the dye and flushed off with PBS after 2 minutes. Finally, a few drops of eosin dye was added to the CAMs and flushed with PBS after 2 minutes. The eosin dye enhances visibility of the pock lesions against the blue background of the hematoxylin. The pock lesions on the CAMs were enumerated using the Scan through (ST) inverted microscope (0994-ZS-Weitzler) and pock diameters were measured across each treatment group.
Data analysis
Statistical tools in GraphPad Prism 5.0 were used to analyze the graph of pock counts and FPV EID50 titer, graph of average weights of embryos after treatment assays and graph of pock diameters across all treatment groups in this study. More so, the inhibition index of concentrates by dose dependent 50% inhibition concentration (IC50) were also determined using non-linear regression analyses in this study.
Results
Toxic threshold of concentrates in ECEs
The concentrates contained characterized compounds in high quantities as summarized in Figures 1 and 2. However, the major compounds in the concentrates had over 70% abundance by weight after compound recovery. The concentrate from V. amygdalina contained Phytol with 20.89% abundance (a recovery index of 134.15 μg/1 g) and a furan based nucleoside analogue Methyl-2-O-benzyld- arabinofuranoside having 48.11% abundance (recovery index of 308.52 μg/1 g). Correspondingly, two compounds most abundant in A. melegueta concentrate were benzaldehyde-3-hydroxy-4- methoxy having 31.23% abundance (recovery index of 141.22 μg/1 g) and butan-2-one-4-(3-hydroxy-2-methoxyphenyl) with 41.77% abundance (recovery index of 188.52 μg/1 g). The Figures 3 and 4 contain information on the toxic threshold of the concentrates and evaluations on the toxic effects of the concentrates on chick embryos were also represented as graphs of mean average weight in Figures 3 and 4.
Inhibition of FPV proliferation in ECEs by the concentrates
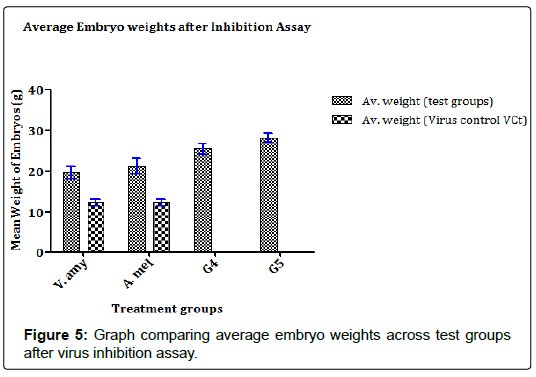
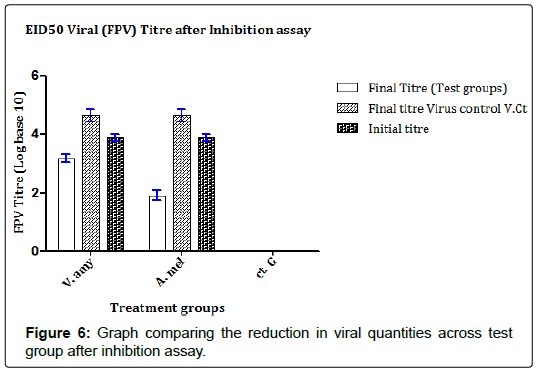
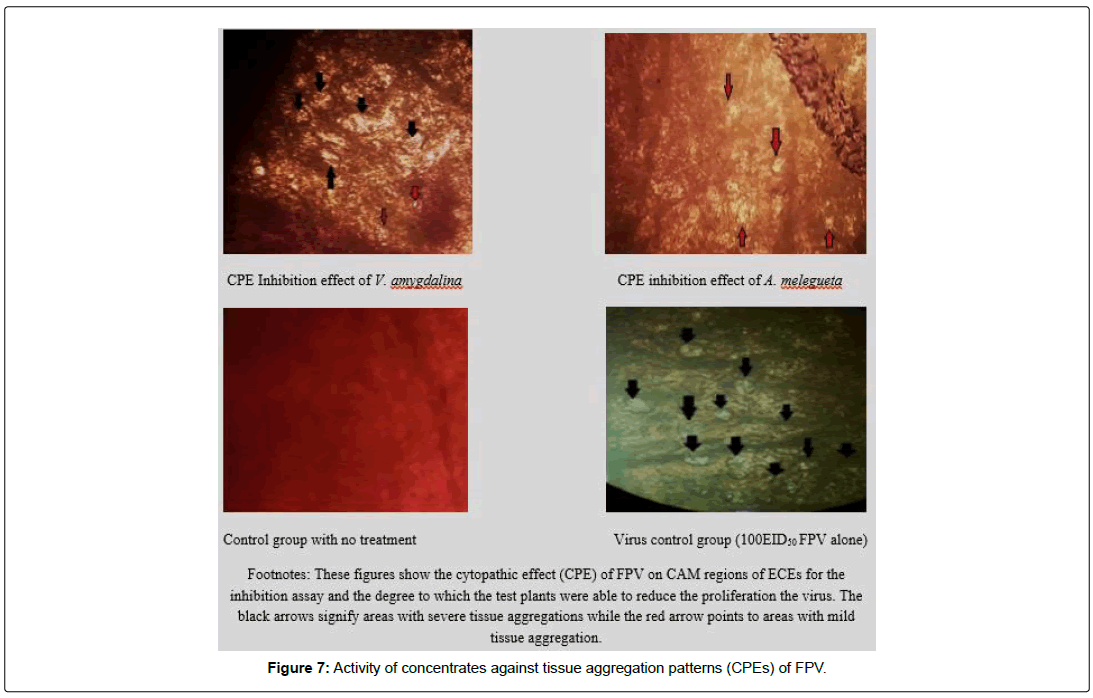
The results in Figures 5 and 6 showed that the concentrates were able to strongly inhibit the proliferation of the FPV in the chick embryos as evidenced in the weights of the embryos in test groups juxtaposed with the weight of embryos in the control group. In Figure 5, the A. melegueta concentrate reduced the lethality of the FPV more (Average embryo weight increased from 12.56 ± 1.61 g to 23.19 ± 1.79 g) than the V. amygdalina concentrate which marginally buoyed the average embryo weight loss from 12.56 ± 1.61 g to 19.49 ± 1.33 g. In the Figure 6 however, the virus titer determined after inhibition assay showed a similar trend with (Figure 5). The A. melegueta concentrate reduced the FPV load in the ECEs better than V. amygdalina after the inhibition assay. This shows that the both concentrates had strong anti-proliferative effects against FPV; however, the A. melegueta concentrate was more effective than V. amygdalina. The indices of FPV proliferation in ECEs are the cytopathic effects (CPEs) it produces in form of tissue aggregation. The (Figure 7) also showed that the two concentrates had varying activity against the proliferation of the FPV since there were regions of severe and mild tissue aggregations even in the test groups.
Reduction of FPV propagation in ECEs by the concentrates
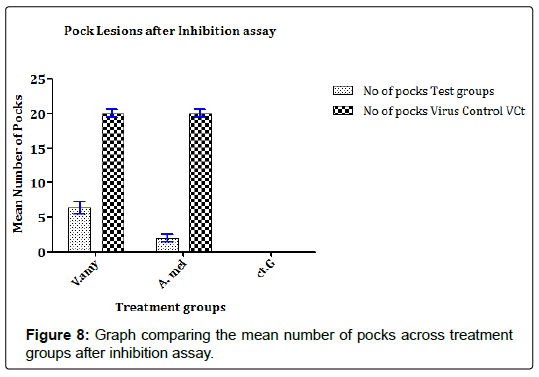
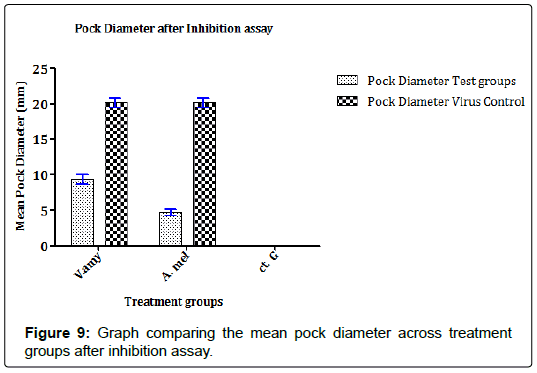
In previous studies that evaluate the propagation of FPV in ECEs, the findings mostly relate the establishment of infection in ECEs with pock formation and spread of pock lesions. Similarly, in this study, the results in Figures 8 and 9 showed that the concentrates were able to reduce the extent of FPV propagation either by the number of pock lesions present or if present, the diameter of the pock lesions. In Figure 8, it can be observed that concentrate from A. melegueta strongly reduced formation of pock lesions by the FPV (mean pock lesions reduced from 18.28 ± 1.84 pfu/100EID50FPV to 2.19 ± 0.65 pfu/100EID50FPV) compared to V. amygdalina concentrate that reduced pock lesions from 18.28 ± 1.84 pfu/100EID50FPV to 6.85 ± 1.77 pfu/100EID50FPV. In the Figure 9, the extents of FPV localization in ECEs were measured as a function of the spread of pock lesions. The diameter of the pock lesions detected after inhibition assay was in similitude with the information in Figure 8. Again the A. melegueta concentrate reduced the pock diameter (from 22.48 ± 2.74 mm to 4.89 ± 1.09 mm) better than V. amygdalina that reduced the pock diameter from 22.48 ± 2.74 mm to 8.96 ± 1.26 mm. This again points to strong antiviral potential of the concentrates in general and specifically, it shows that A. melegueta concentrate was more effective than V. amygdalina for inhibition of FPV in ECEs.
Discussion
The two prominent compounds in the V. amygdalina concentrate [furan-based nucleoside analogue Methyl-2-O-benzyld- arabinofuranoside and the long chain alcohol Phytol] might have some degrees of toxicity on embryos in the ECEs (Figure 3 or 4). Recent findings in [14] suggested that overexposure to furan-based nucleoside analogues might cause mild or chronic onset of neural adenopathy in Wister albino rats. The findings also maintained that cytotoxic damage may result from overload of chick fibroblast tissues with analogues of nucleoside analogue compounds as a result of lactic acidosis. Correspondingly, according to [15] proposed that many long chain alcohols such as phytol might still trigger mild damages to the host epithelial cells even at comparatively low doses; thereby making young tissues prone to shock. In similitude, the concentrate from A. melegueta also contained compounds triggered some level of toxicity observed in the results (Figures 3 and 4). The concentrate contained two major compounds [benzaldehyde-3-hydroxy-4-methoxy and butan-2-one-4-(3-hydroxy-2-methoxyphenyl)] for which specific findings in [16] showed that continuous uptake of plants derived phenols by young animal tissues inhibit protein translation activators and block ionic absorption potentials eventually leading to reduced tissue weight. Genotoxic damage to the visceral organs in test animals was also attributed to overdose of plant-derived phenols since the phenols interfere with tissue respiration and enzyme synthesis especially when above the threshold concentrations [17]. All these factors played a key role in informing why the dose administration for the concentrate toxicity assay was limited to a concentration of <500 μM.
Several factors account for the strong efficacy of the concentrates to inhibiting the FPV proliferation in ECEs and chief amongst them would be the direct impact the compounds in the concentrates had on the FPV. The findings in the report of [1] showed that plant phenols in the A. melegueta concentrate inhibit virus invasion of susceptible tissues by impairing cell surface attachment mechanisms in a pangenotypic manner; disrupting the tyrosine kinases on the viral envelope. Meanwhile, another observation in the study of [18] showed that phenols actively block the tailing enzymes of poxviruses, an important chemical factor needed for viral uncoating, hence, deactivation of the virions by cellular translocation. Conversely, [8] in their study described a more recent method by which phenols act against poxviruses. The study showed that phenols ionize surface receptors on host tissues to cause reduction in the ability of viruses to make direct attachment to host tissues. These might have accounted majorly as basis for the drastic reductions noticed in the FPV final titer, mean number of pock lesions and the pock diameter after inhibition assays since minimal damage was observed in the test groups (Figures 5 and 6). In a similar vein, [7] reiterated that many phenols often alter the formation of viral translation activation factor (eIF2α in case of FPV), essential for translation of new genes into viral proteins. This also led to the possibility that phenols might block the synthesis of protease promoters due to its oxidative mechanisms thereby weakening FPV virion assemblage. Alternatively, the benzylgroup attached to the two phenols considered in this study affords them the advantage of exposing FPV genome to genotoxic damage. They achieve this by blocking the production of protein kinase R (PKR) of FPV [7]. Although, the concentrate from V. amygdalina showed lesser potency for inhibition of the FPV proliferation than the A. melegueta concentrate. However, the concentrate still showed strong inhibition against the virus propagation and its proliferation in ECEs. The report of [19] showed that phytol in the concentrate blocks FPV transcription factors responsible for late protein glycosylation within the viral envelope. Alternatively, [9,19] described few points of note for antiviral mechanisms of nucleoside analogues. These studies showed that the nucleoside analogue Methyl-2-O-benzyld- arabinofuranoside in the concentrate inhibit the formation of concoctamer DNA complex essential for translation of new genes into viral proteins. This might lead the possibility that Methyl-2-Obenzyl- d-arabinofuranoside blocks the FPV late mRNA synthesis and then cause denaturation of transcripted genes. The same findings of [9] also raised the possibility that the nucleoside analogue in the V. amygdalina might have blocked the production of DNA polymerases in the FPV before the start of virus replication effectively ending the virus reproduction cycle abruptly. However, since the mechanisms responsible for antiviral activity of V. amygdalina concentrate are more suited for phases of virus cycle after viral adsorption to host tissues, it follows that the concentrate have lesser inhibition efficacy compared to the A. melegueta concentrate.
Conclusion
This current study has shown that the concentrates from V. amygdalina and A. melegueta showed strong inhibition potential against proliferation and propagation of FPV in ECE. This study also points to the need for further studies on the compounds characterized in the concentrates to deliver them effectively as antiviral targets for better inhibition of FPV in living tissues. Furthermore, from this study, we recommend the consumption of supplements from the plants V. amygdalina and A. melegueta for respondents already showing overt symptoms of poxvirus infection.
Conflict of Interest
There are no conflicts of interests.
Acknowledgement
We would like to acknowledge the technical support offered by the entire staff of the viral research division, viral vaccines production unit and poultry division of National Veterinary Research Institute, Vom, Nigeria.
Funding Source
The authors received funding support from the Nigerian Tertiary Education Trust Fund (TETFund) for this research (Grant ID VCPU/TETFund/155).
References
- Cupp J, Wanda P, Keith A, Snipes W (1975) Inactivation of the lipid-containing bacteriophage PM2 by butylated hydroxytoluene. Antimicrob Agents Chemother 8: 698-706.
- Nyaga P, Kaminjolo J, Mutiga R, Bebora C (1979) Occurrence of atypical Fowlpox virus in poultry farms in Kabete, Kenya. Avian Diseases23: 745-752.
- Reaves J, Li Y, Gao X, Hofman P, Davies V (2011) Variola and Monkeypox viruses utilize conserved mechanism of virion motility and release that depend on Abl and Src family tyrosine kinases. Journal of Virology32: 233-239.
- Singh P, Tripathy D (2003) Fowlpox virus infection causes a lympho-proliferative response in chickens. Viral Immunol 16: 223-227.
- Wang J, Meers D, Spadbrow P, Robinson F (2006) Evaluation of immune effects of Fowlpox vaccine strains and field isolates. Vet Microbiol 116: 106-119.
- Ekanem R (2018) Detection and quantification of potential bioactive compounds in the acetone extract of Citrullus lanatus (Melon) seed using GC-MS and FTIR 4 spectrometer. AJOCS 5: 1-5.
- Calland N, Sahuc M, Sandrine B, Pene V, Pierre B, et al. (2015) Polyphenols inhibit Hepatitis C Virus entry by a new mechanism of action. J Virol 89: 10053-10063.
- Colpitts C, Schang L (2014) A small molecule inhibits virion attachment to heparin sulfate- or sialic acid-containing glycans. J Virol 88: 7806-7817.
- Nunez M (2006) Hepatotoxicity of antiretrovirals: Incidence, mechanisms and management. J Hepatol 44: 132-139.
- Odetunde S, Adekola I, Avungbeto M, Lawal A (2015) Antimicrobial effect and phytochemical analysis of Aframomum melegueta on some selected bacteria and fungi. European J Biotech Biosci 3: 15-19.
- Nittya D, Suresh K (2015) A review on ethno-medicinal uses and pharmacology of Vernonia cinerea Less. Natural Product Research 29: 12-22.
- Faeji C, Oladunmoye M, Adebayo I, Adebolu T (2017) In ovo biological activities of Phyllanthus amarus leaf extracts against Newcastle disease virus. J Medicinal Plants R 11: 419-425.
- Farid A, Chemat G, Iman H (2012) Plant based natural products extraction: Concept and principles. Int J Mol Sci 13: 21-35.
- Jena A, Sachdeva R, Sharma A, Wanchu A (2009) Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: A 24-week prospective study. J Int Assoc Phys AIDS Care8: 318-322.
- Islam M, Streck L, de Alencar V, Cardoso W, Machado K, et al. (2017) Evaluation of toxic, cytotoxic and genotoxic effects of phytol and its nanoemulsion. Chemosphere 177: 93-101.
- Chow H, Cai Y, Hakim I, Crowell J, Shahi F, et al. (2003) Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate in healthy individuals. Clin Cancer Res 9: 3312–3319.
- Tapiero H, Tew K, Ba G, Mathe V (2002) Polyphenols: Do they play a role in the prevention of human pathologies? Biomed Pharmacother 56: 200-207.
- Prichard M, Shipman C (1996) Analysis of combinations of antiviral drugs and design of effective multidrug therapies. Antivir Ther 1: 9-20.
- Smith A, James W, Bruce G (2002) The formation and function transcription inhibitors of extracellular enveloped vaccinia virus. J General Virology 12: 22-27.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi