Case Report, J Regen Med Vol: 13 Issue: 1
Conservative treatment approach of Achilles tendon ruptures with orthobiologics: case series
Napoliane Santos1,2, Luyddy Pires1,2, Carlos Stéfano1,2, Alexandre Ribeiro1,2, Gabriel Silva Santos1,2*, Francisco Honorio1,2, Lucas Furtado da Fonseca1,2,3, Rafael Barnabe Domingues1,2,4, Raffael Marum Bachir5, Caroline Mayara Kavalco1, Rodrigo Vicente1, Palmerindo Mendonça1 , João Vitor Lana1,6, Jose Fabio Lana1,2,4,6
1Department of Orthopedics, Brazilian Institute of Regenerative Medicine (BIRM), Indaiatuba, SP, Brazil
2Regenerative Medicine, Orthoregen International Course, Indaiatuba, SP, Brazil
3Department of Orthopedics, Federal University of São Paulo (UNIFESP), São Paulo, SP, Brazil
4Anna Vitória Lana Institute, Indaiatuba, SP, Brazil
5Department of Orthopedics, Hospital IFOR, São Bernardo do Campo, SP, Brazil
6Medical Specialties School Center, Max Planck University Center (UniMAX), Indaiatuba, SP, Brazil
*Corresponding Author: Gabriel Silva Santos
Department of Orthopedics, Brazilian Institute of Regenerative Medicine (BIRM), Indaiatuba, SP, Brazil
E-mail: gabriel1_silva@hotmail.com
Received: : 22-Dec-2023, Manuscript No. JRGM-23-123266;
Editor assigned: 23-Dec-2023, PreQC No. JRGM-23-123266 (PQ);
Reviewed: 05-Jan-2024, QC No. JRGM-23-123266;
Revised: 08-Jan- 2024, Manuscript No. JRGM-23-123266 (R);
Published: 15-Jan-2024, DOI:10.4172/2325-9620.1000285
Citation: Santos GS, Santos N, Pires L, Stéfano C, Ribeiro A, et al. (2023) Bone Marrow Aspirate and Injectable Platelet Rich Fibrin for Achilles tendon Rupture. J Regen Med 13:1.
Copyright: © 2023 Santos GS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The Achilles Tendon (AT) is one of the strongest tendons in the body; it is also the most frequently ruptured tendon with increasing incidence. Unlike other tendons, AT ruptures are highly correlated with physical activity. In fact, more than 75% of AT ruptures occur during sportive maneuvers. AT rupture is a prevalent and debilitating condition linked to overuse injuries in the ankle and foot. Managing this condition is often complex due to the inadequate vascularity of tendons, relying on synovial fluid diffusion for nutrient supply. The weakened strength resulting from AT rupture can heighten the risk of further complications. While surgical interventions are commonly employed as the primary treatment, challenges may persist in the postoperative period. In contrast, therapeutic interventions with orthobiologics and shockwave therapy have demonstrated notable success in regenerative procedures. In this case series, 3 patients received multiple sessions of bone marrow aspirate, injectable platelet-rich fibrin and extracorporeal shockwave therapy. In the initial sessions, they already exhibited satisfactory healing results as assessed through functional measures, MRI findings, and pain scores. The patients successfully resumed sports activities without complaints, and follow-up MRI scans indicated evident signs of AT restoration. This case series highlights the safe and effective use of autologous orthobiologic products and shockwave therapy as viable alternatives for enhancing the healing process in musculoskeletal tissue injuries.
Methods: Patients received the following treatments: a single Bone Marrow Aspirate (BMA) and then Injectable Platelet-Rich Fibrin (i-PRF) injections fortnightly for 12 weeks, in addition to a weekly session of Extracorporeal Shock Wave Therapy (ESWT). The patients were reassessed at all follow-ups with physical evaluation and ultrasound examination. In addition, we also recommended lifestyle adjustments emphasizing the importance of sleep, diet and metabolism for better tissue recovery. At the end of 12 weeks, we requested a new magnetic resonance imaging of the left ankle for a comparative study, which revealed a significant improvement in the radiological findings. The results of this case report suggest that the application of orthobiologics plus ESWT expedites healing and rehabilitation time and reduces costs and risks inherent to the surgical procedure, which is particularly important in elderly patients and/or with co –morbidities. This approach may therefore represent a viable alternative for the accelerated recovery of musculoskeletal tissue injuries with safety and efficacy.
Keywords: Case Series; Achilles Tendon; Bone Marrow Aspirate; Platelet-Rich Fibrin; Shockwave Therapy; Regenerative Medicine.
Keywords: Case Series; Achilles Tendon; Bone Marrow Aspirate; Platelet-Rich Fibrin; Shockwave Therapy; Regenerative Medicine.
Introduction
The Achilles tendon is an important lower limb structure that assists in plantar flexion of the ankle, thus being one of the strongest tendons yet highly susceptible to injuries. It is formed by the congruence of the tendons of the medial and lateral gastrocnemius and soleus muscles, inserting into the posterior surface of the calcaneus [1].
Between the origin and insertion of the tendons that make up the calcaneus, the fibers rotate 90°, so the fibers of the gastrocnemius insert laterally, and those of the soleus medially [2]. In the topography of this rotation of tendon fibers, the most vulnerable area of the tendon is found, around 2 to 6 cm from its insertion in the calcaneus, where blood supply is deficient [3].
The AT may undergo biomechanical (degenerative) or biochemical (inflammatory) changes [4]. AT disorders are more common in individuals who participate in endurance sports that involve repetitive loading of the foot. The rising incidence of ruptures is related to an increase in the participation of the population in recreational and competitive sports and is therefore one of the most common orthopedic disorders in sports medicine [5].
Acute rupture is primarily related to sudden forced plantar flexion during weight bearing with the knee fully extended. Therefore, athletes who play sports that require explosive acceleration, sudden changes in direction or jumping and running are at greater risk [5,6]. Patients with an AT rupture usually describe a history of severe, sharp pain, mostly sport-related or in the middle portion of the AT comparable to the sensation of being shot or kicked above the heel. A popping sound is often described by the athlete. Additionally, physical dysfunction, weakness or stiffness of the affected ankle is commonly described. Patients with a partial tear do not usually complain of severe pain. Above all, patients may experience increased ankle swelling or dysfunction during weight bearing.
The diagnosis of an acute AT rupture is primarily based on clinical findings. However, the diagnosis may go unnoticed at the first medical appointment in up to 23% of cases. In most patients, dorsiflexion of the ankle is impaired, although some patients are still able to perform plantar flexion using the remaining intact tendons of the foot. Inspection and palpation may reveal swelling, bruising, and a gap along the course of the tendon. Ankle plantar flexion is normally decreased to such an extent which impedes heel lift [7]. To validate diagnosis, many physical examination maneuvers are described. The most sensitive and specific test is the (Simmonds-) Thompson test [8], whereby the patient is lying supine and the knee is maintained in 90° of flexion. Squeezing the calf leads to plantar flexion of the foot if the AT is intact. Decreased or absent plantar flexion is suggestive of a tear.
Although acute AT rupture treatment alternatives encompass conservative or surgical strategies, there is still no medical consensus regarding a “gold standard” alternative. Regardless, the treatment of choice should not aim to restore only tendon length and tension, but also the strength of the myotendinous unit in order to achieve satisfactory clinical function. Conservative treatment may be a viable alternative to surgery. In physically active individuals, surgical repair is preferred because of the onset of active mobilization and lower rates of re-rupture. However, clinical results and muscle atrophy are comparable to surgical treatment. In regards to the time required to return to activities (pre-injury conditions), the literature reports an average of 6 to 12 months. However, newer investigations regarding the use of orthobiologics, including platelet-rich plasma derivatives, have reported the possibility to return to activities in just 3 months after treatment [5].
Other authors evaluated the use of BMA and PRP in AT ruptures in the acute phase and observed that it positively affected tendon recovery in histological and biomechanical aspects [6]. Bone Marrow- Derived Mesenchymal Cells (BM-MSCs) and PRP may promote tendon recovery and increase its structural strength. It is thought that using MSCs may be more effective for tendon healing [9,10] but more advanced studies are still required. Other authors have demonstrated the strong immunomodulatory effects of MSCs as, after infusion, they reduce total mononuclear cell infiltration and promote a regenerative/ anti-inflammatory M2 macrophage phenotype in healing tendons [11]. (Aktas et al.) [12] have reported the ability of MSCs to increase IL-10 production whilst reducing IL-1α and IL-12 levels as well as the number of M1 macrophages (pro-inflammatory). MSCs are also shown to increase the number of M2 macrophages (inflammatory resolution) and anti-inflammatory factor IL-4 [12].
It is evident that macrophages and their M2 polarization driven by the immunomodulatory behavior of MSCs play a particularly important role in tissue regeneration. MSC-based products have been reported to produce long-lasting results, but the level of evidence is currently insufficient to draw definite conclusions.
Case report 1
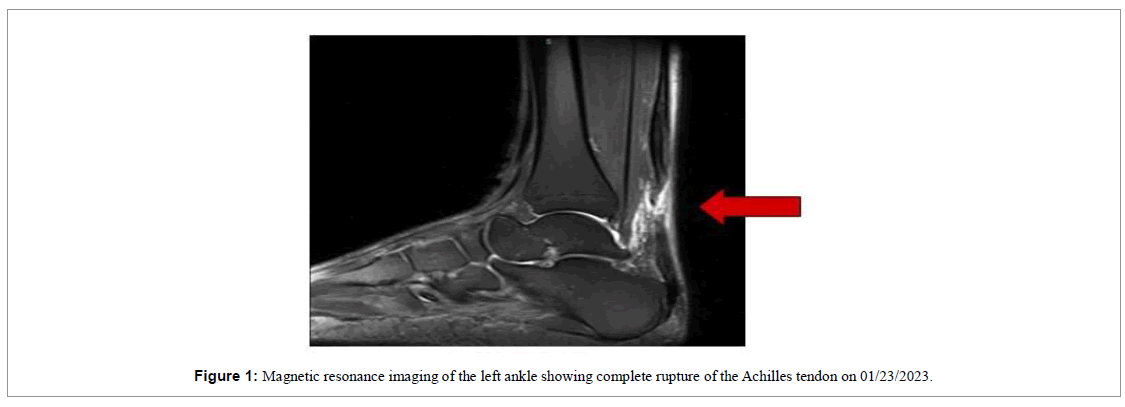
A 65-year-old male was playing tennis when he felt a “pop” in the lower portion of his left calf on January 21st 2023. He sought urgent medical care and the first physical examination revealed a positive Thompson test for complete rupture of the Achilles tendon, confirmed with an MRI scan (Figure 1). The first evaluation was carried out by an external orthopedist who indicated surgical treatment.
However, due to advanced age and fear of the risks involved in a surgical procedure, the patient sought our services at the Brazilian Institute of Regenerative Medicine (BIRM) on 01/25/2023 in order to try conservative treatment. The informed consent form was provided for the use of orthobiologics and shock wave therapy, as well as the consent form regarding the use of data for research purposes.
We carried out the patient’s evaluation for adjustment and guidance on improved metabolism, sleep, diet and rehabilitation, which are fundamental elements for optimized tissue regeneration. We apply a strict nutritional monitoring with an “anti-inflammatory” diet that excludes alcohol, sugar, wheat, gluten, industrialized products, milk and dairy products for 90 days. We also encourage the increased intake of vegetables and lean proteins.
In the first session, 15 ml of BMA from the posterior superior iliac crest was collected and mixed with 2 ml of hyaluronic acid (Synolis), 2 ml of Traumeel and 1 ml of Dexamethasone. 10 ml of this mixture was injected in the left AT and 10 ml in the contralateral tendon with ultrasound guidance. This procedure was followed by focal ESWT on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20.
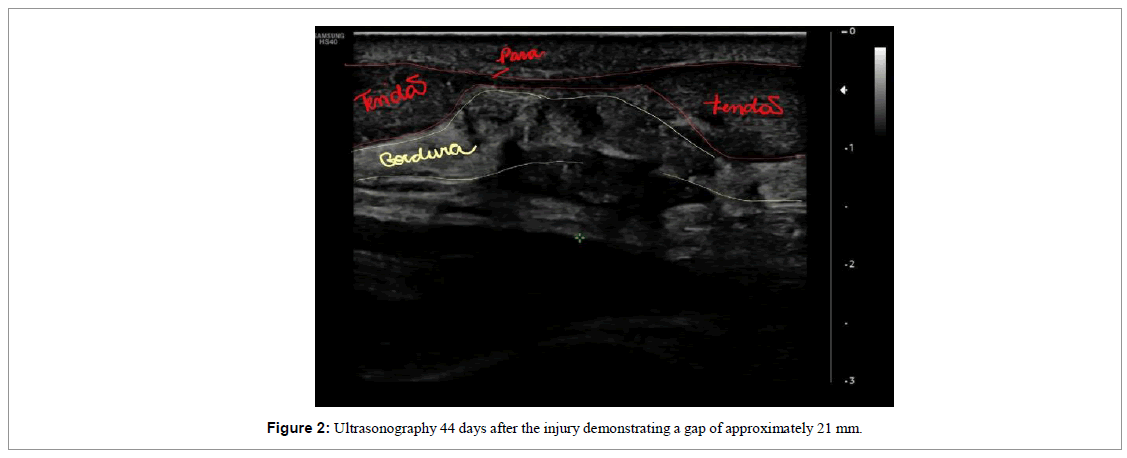
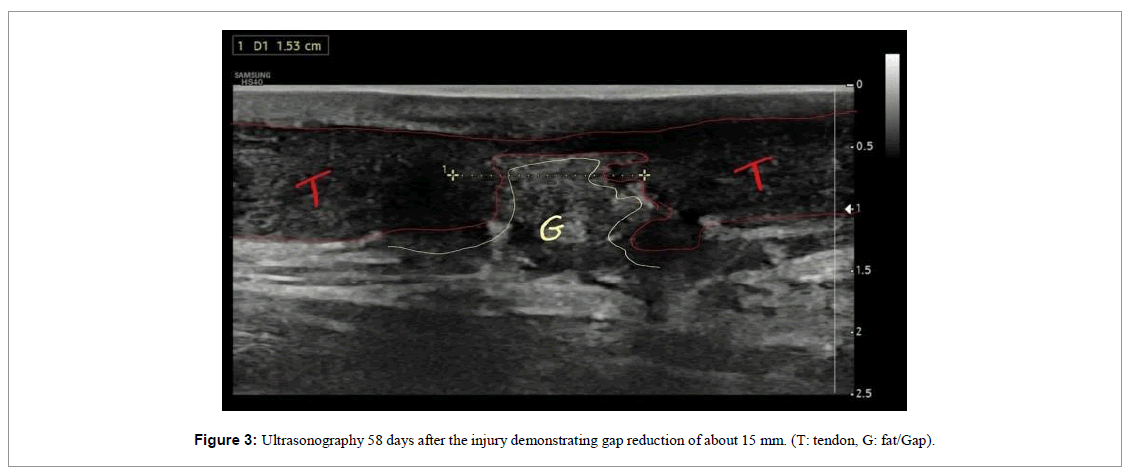
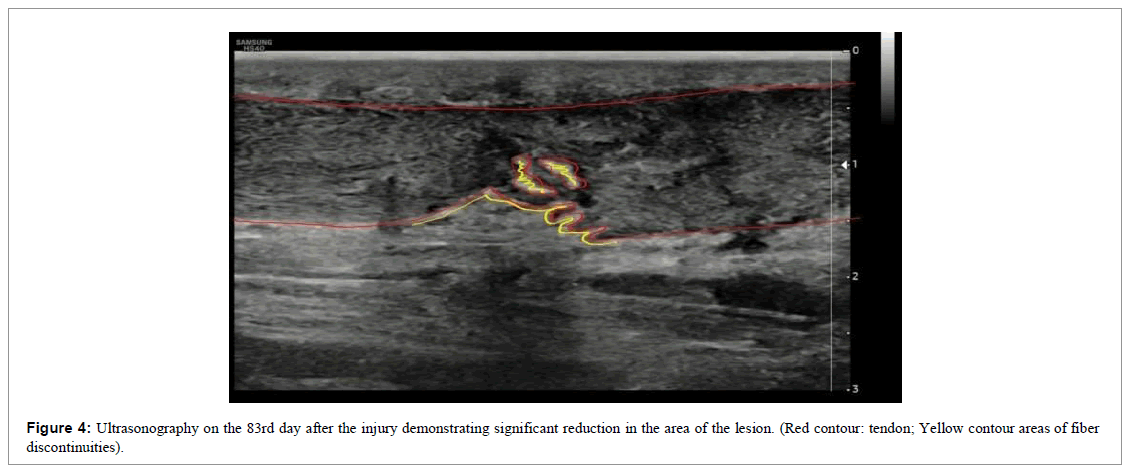
The patient was followed up through weekly appointments to assess function by physical examination, pain analogue scale (VAS) and ultrasound images (Figures 2-4).
We then performed 3 additional infiltration sessions with orthobiologics every two weeks with Injectable Platelet-Rich Fibrin (i-PRF) as mesotherapy procedures in order not to cause intratendinous lesions in the newly formed fibers. 80 ml peripheral blood was collected and transferred into sterile vacuum tubes without any anticoagulants or additives and centrifuged at 60 G for 5 min. 7 ml of plasma (i-PRF) were collected and mixed with 2.2 ml of Traummel and applied via mesotherapy technique beneath the dermis at the area of the injured tendon, followed by weekly focal ESWT treatment, for a total of 12 sessions.
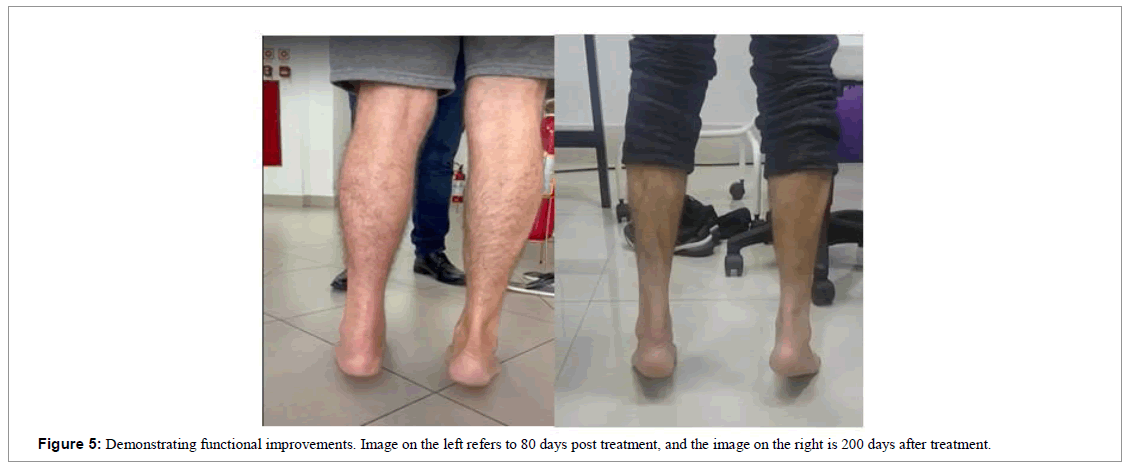
The patient used a foot immobilizer with a heel wedge to keep him in an equine position for the first 4 weeks. At 8 weeks we removed the immobilizer and the patient himself was already walking normally and showing strength in extension during physical examination of the ankle, with expressive signs of functional improvement (Figure 5).
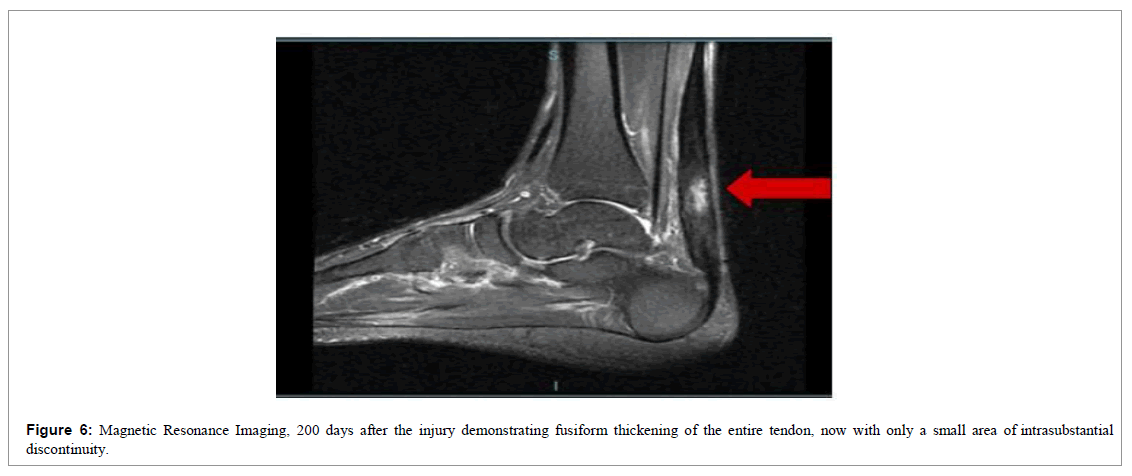
At the end of 12 weeks, we requested a new magnetic resonance imaging of the left ankle for a comparative study (Figure 6).
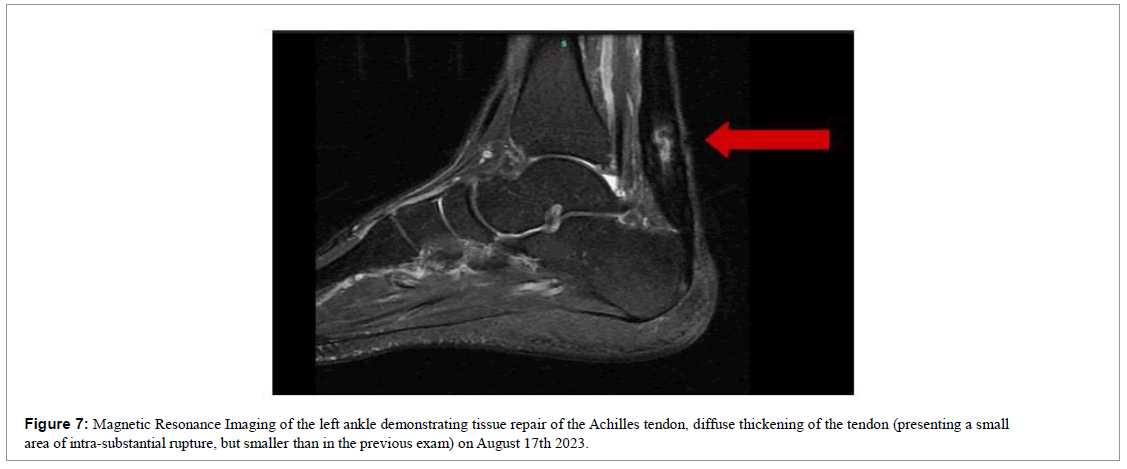
Six months after the injury, we requested a new magnetic resonance imaging for documentation purposes (Figure 7). Where the maintenance of improvement was evident, diffuse thickening of the tendon, improvement in architecture, realignment of fibers, observing only a small intrasubstantial discontinuity.
Results
Over the 12-week follow-up, functional improvement was evident in the clinical examination and in the tendinous fibers via ultrasound examinations performed during the reassessments (Figures 2-5). Tissue repair visualized by the ultrasound images was clear, where we observed the formation of new tendinous fibers. 20 days after the first intervention, there was a 60% improvement in pain and after 31 days there was a 100% improvement in the pain scale (VAS), which was maintained until the end of follow-up. On day 58, the patient reported a notable gain in range of motion and reduced swelling. At the end of 60 days, we released the patient for light physical activity in the water. At the end of 120 days the patient was already actively practicing water sports.
Case report 2
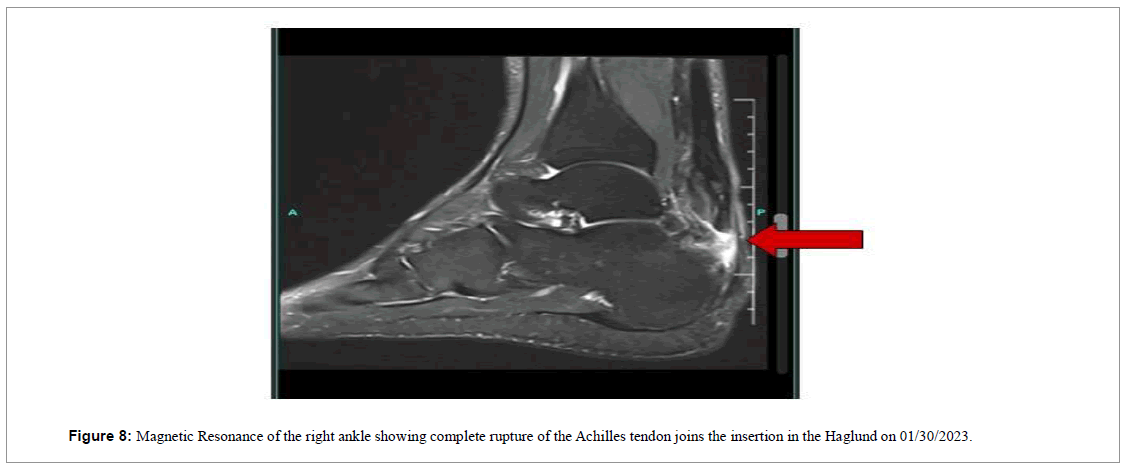
A 56-year-old male reported a sharp pain in the lower portion of the right ankle during a soccer match on 01/12/2023. He sought immediate medical care, being evaluated by a general practitioner with one X-ray scan. The diagnosis was registered as muscle distension and the patient was sent home. As the days passed, he realized that there was no improvement in the pain and swelling. He then consulted an orthopedist on 01/30/2023, who requested a magnetic resonance imaging (Figure 8).
MRI revealed extensive rupture/transfixation along the insertion in the Haglund and surgery was then scheduled for 02/24/2023. However the procedure was canceled the day before due to lack of materials, leading the patient to seek our services at the Brazilian Institute of Regenerative Medicine (BIRM), in order to try conservative treatment.
On 02/24/2023, the patient was evaluated by our team of physicians, and a positive Thompson test led to the conclusion of complete rupture of the AT.
The informed consent form was provided for the use of orthobiologics and shock wave therapy, as well as the consent form regarding the use of data for research purposes.
We carried out the patient’s evaluation for adjustment and guidance on improved metabolism, sleep, diet and rehabilitation, which are fundamental elements for optimized tissue regeneration. We apply a strict nutritional monitoring with an “anti-inflammatory” diet that excludes alcohol, sugar, wheat, gluten, industrialized products, milk and dairy products for 90 days. We also encourage the increased intake of vegetables and lean proteins.
In the first session, 15 ml of BMA from the posterior superior iliac crest was collected and mixed with 2 ml of hyaluronic acid (Synolis), 2 ml of Traumeel and 1 ml of Dexamethasone. 10 ml of this mixture was injected in the left AT and 10 ml in the contralateral tendon with ultrasound guidance. This procedure was followed by focal ESWT on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20.
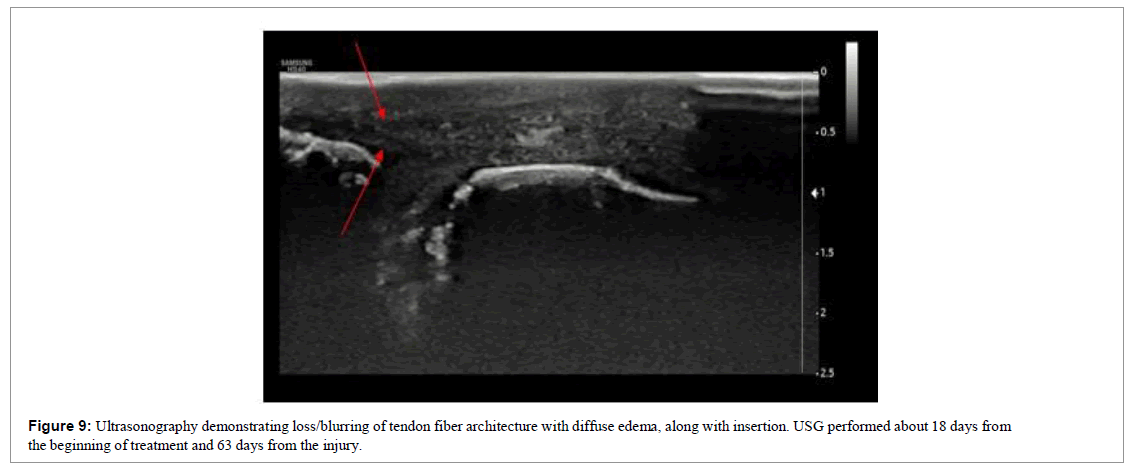
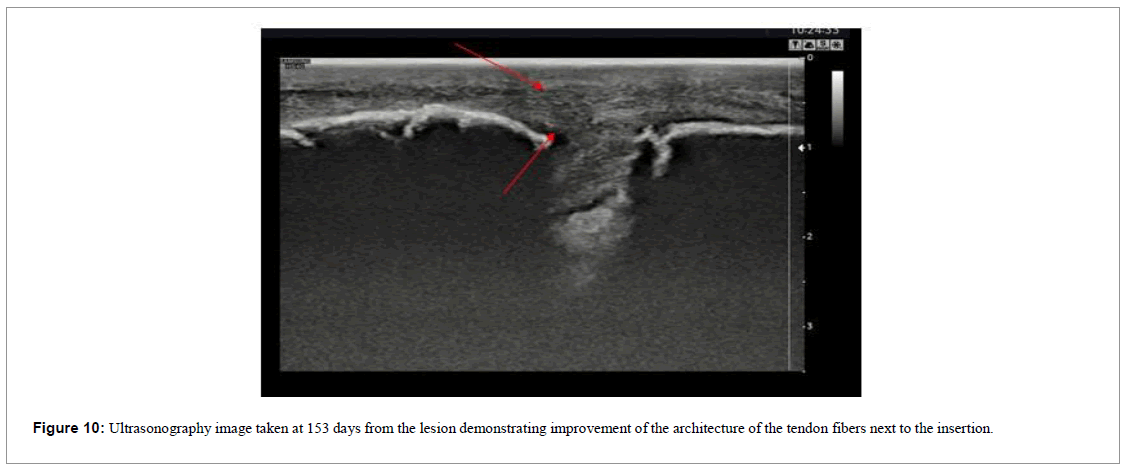
The patient was followed up through weekly appointments to assess function by physical examination, visual analogue scale (VAS) and ultrasound images (Figures 9 and 10).
This was a more complicated case because not only was the injury time longer, therapeutic intervention was initiated only 43 days post injury.
We then performed additional infiltration sessions with orthobiologics every two weeks with injectable platelet-rich fibrin (i-PRF) as mesotherapy procedures in order not to cause intratendinous lesions in the newly formed fibers. 80 ml peripheral blood was collected and transferred into sterile vacuum tubes without any anticoagulants or additives and centrifuged at 60 G for 5 min. 7 ml of plasma (i-PRF) were collected and mixed with 2.2 ml of Traummel and applied via mesotherapy technique beneath the dermis at the area of the injured tendon, followed by focal ESWT treatment.
Since this was a more serious case, during the evaluations we realized the need for more vigorous interventional strategies, so we performed 2 monthly BMA sessions (60 days after the beginning of the treatment). These BMA applications were interspersed with the fortnightly injections of i-PRF. ESWT was performed weekly, for a total of 18 sessions.
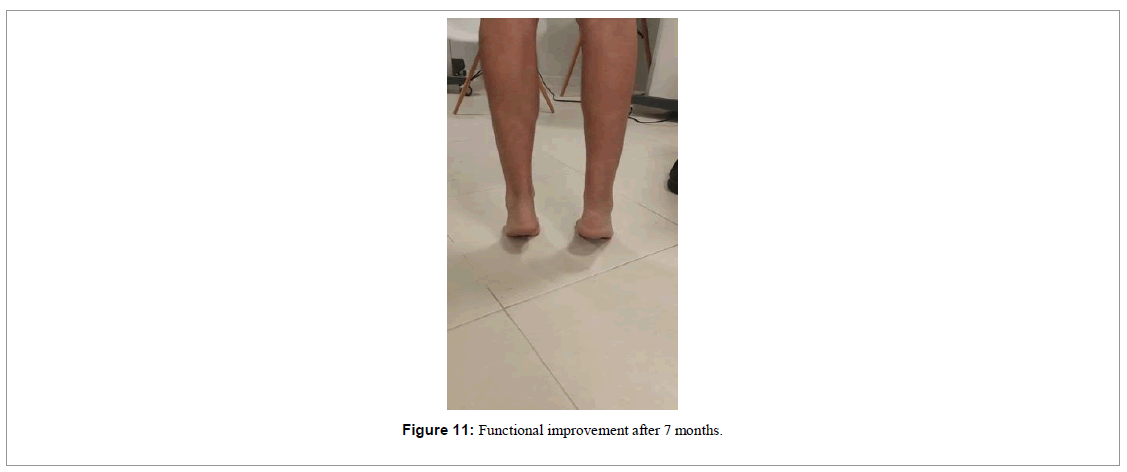
This patient also used a foot immobilizer with a heel wedge to keep him in an equine position for the first 4 weeks. At 12 weeks we removed the immobilizer and the patient himself was already walking normally, demonstrating strength during physical examination of ankle extension, with prominent signs of functional improvement (Figure 11).
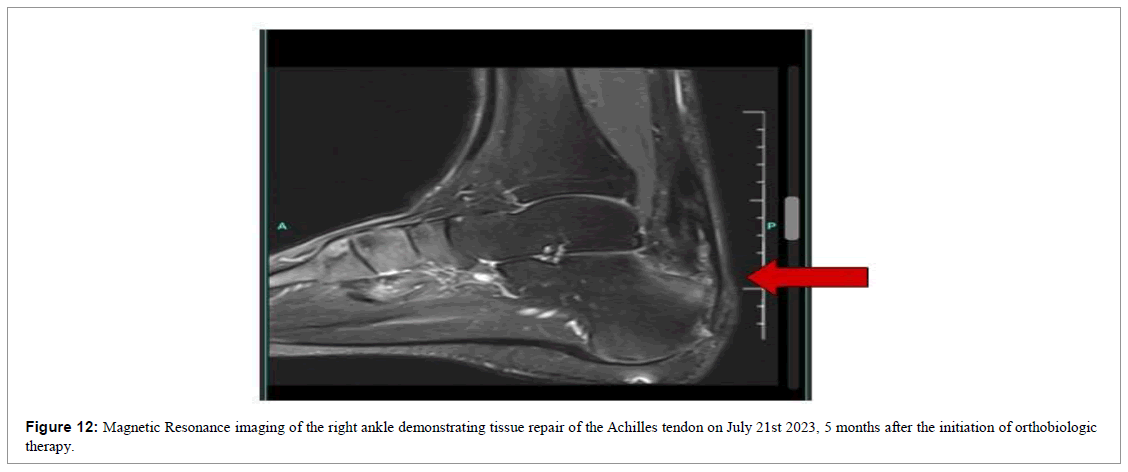
At the end of 23 weeks, we requested a new MRI scan of the left ankle for a comparative study (Figure 12), identifying improvement in the architecture and realignment of the tendon fibers.
Results
Over the 12-week follow-up period, functional improvement was achieved in clinical terms. Robust signs of tissue repair were visualized via MRI, where we observed the formation of new tendinous fibers. 40 days after the first intervention, there was a 60% improvement in pain and after 60 days there was a 100% improvement in the pain scale (VAS), which was maintained until the end of follow-up. Also, around the 60th day, the patient reported a notable gain in range of motion and reduced edema. This allowed him to once again wear tennis shoes and return to the gym for upper limb workouts; for the lower limbs he was permitted to engage in light exercises in the water, such as walking. At the end of 180 days, the patient was already practicing lower limb strengthening at the gym.
Case report 3
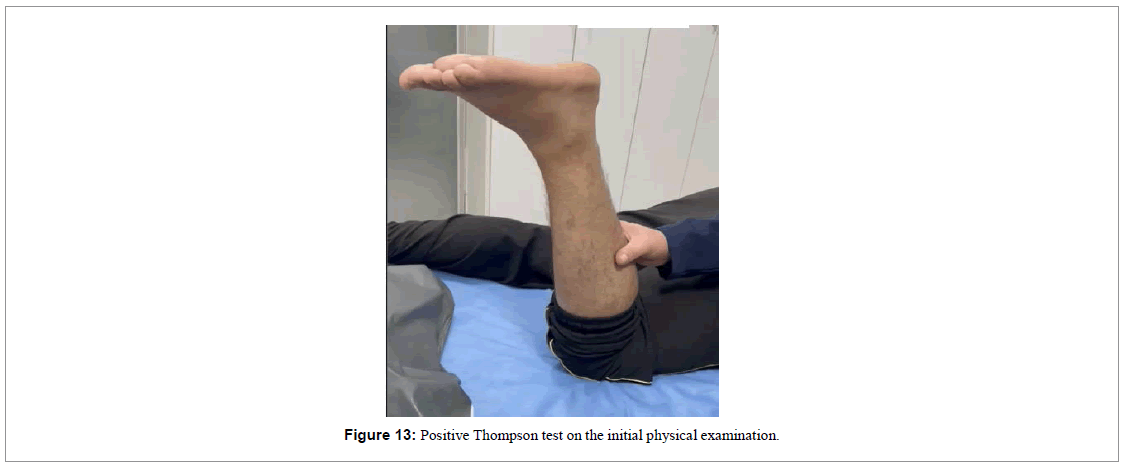
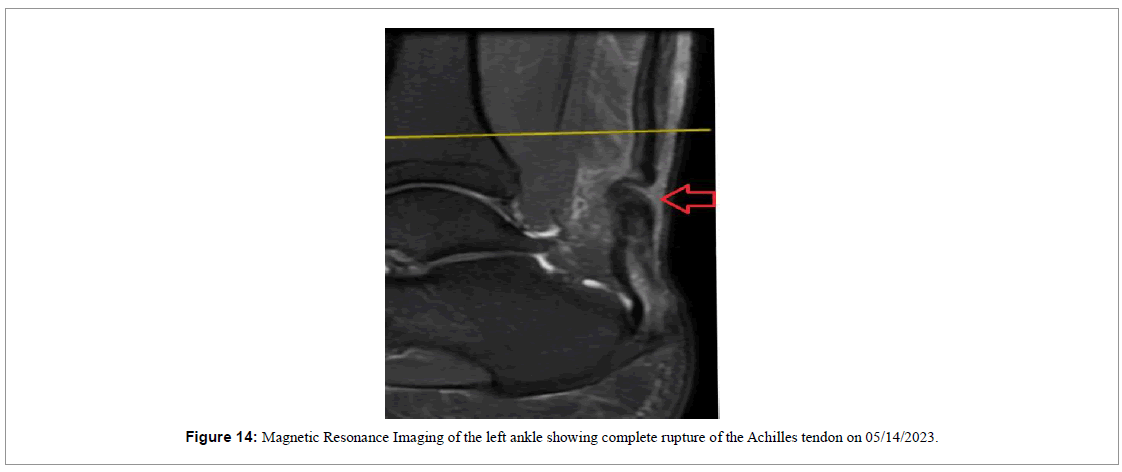
A 37-year-old male playing soccer experienced a sudden “pop” in the lower portion of his left calf on 05/14/2023 and visited the emergency room. During the initial physical examination on his medical attention, he exhibited a positive Thompson test indicating complete rupture of the Achilles tendon (Figure 13) which was later confirmed by a Magnetic Resonance Imaging (MRI) examination (Figure 14).
The patient then sought our clinic for further examination in order to treat his injuries and get rid of the pain. He accepted our recommendation of conservative treatment with orthobiologics and shockwave therapy, signing the informed consent form. We carried out the patient’s evaluation for adjustment and guidance on improved metabolism, sleep, diet and rehabilitation, which are fundamental elements for optimized tissue regeneration. We apply a strict nutritional monitoring with an “anti-inflammatory” diet that excludes alcohol, sugar, wheat, gluten, industrialized products, milk and dairy products for 90 days. We also encourage the increased intake of vegetables and lean proteins.
In the first session, 15 ml of BMA from the posterior superior iliac crest was collected and mixed with 2 ml of hyaluronic acid (Synolis), 2 ml of Traumeel and 1 ml of Dexamethasone. 10 ml of this mixture was injected in the left AT and 10 ml in the contralateral tendon with ultrasound guidance. This procedure was followed by focal ESWT on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20. on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20.
The patient was followed up through weekly appointments to assess function by physical examination, pain analogue scale (VAS) and ultrasound images (Figures 2-4).
We then performed 3 additional infiltration sessions with orthobiologics every two weeks with Injectable Platelet-Rich Fibrin (i-PRF) as mesotherapy procedures in order not to cause intratendinous lesions in the newly formed fibers. 80 ml peripheral blood was collected and transferred into sterile vacuum tubes without any anticoagulants or additives and centrifuged at 60 G for 5 min. 7 ml of plasma (i-PRF) were collected and mixed with 2.2 ml of Traummel and applied via mesotherapy technique beneath the dermis at the area of the injured tendon, followed by weekly focal ESWT treatment, for a total of 12 sessions.
The patient used a foot immobilizer with a heel wedge to keep him in an equine position for the first 4 weeks. At 8 weeks we removed the immobilizer and the patient himself was already walking normally and showing strength in extension during physical examination of the ankle, with expressive signs of functional improvement (Figure 5).
At the end of 12 weeks, we requested a new magnetic resonance imaging of the left ankle for a comparative study (Figure 6).
Six months after the injury, we requested a new magnetic resonance imaging for documentation purposes (Figure 7). Where the maintenance of improvement was evident, diffuse thickening of the tendon, improvement in architecture, realignment of fibers, observing only a small intrasubstantial discontinuity.
Results
Over the 12-week follow-up, functional improvement was evident in the clinical examination and in the tendinous fibers via ultrasound examinations performed during the reassessments (Figures 2-5). Tissue repair visualized by the ultrasound images was clear, where we observed the formation of new tendinous fibers. 20 days after the first intervention, there was a 60% improvement in pain and after 31 days there was a 100% improvement in the pain scale (VAS), which was maintained until the end of follow-up. On day 58, the patient reported a notable gain in range of motion and reduced swelling. At the end of 60 days, we released the patient for light physical activity in the water. At the end of 120 days the patient was already actively practicing water sports.
Case report 2
A 56-year-old male reported a sharp pain in the lower portion of the right ankle during a soccer match on 01/12/2023. He sought immediate medical care, being evaluated by a general practitioner with one X-ray scan. The diagnosis was registered as muscle distension and the patient was sent home. As the days passed, he realized that there was no improvement in the pain and swelling. He then consulted an orthopedist on 01/30/2023, who requested a magnetic resonance imaging (Figure 8).
MRI revealed extensive rupture/transfixation along the insertion in the Haglund and surgery was then scheduled for 02/24/2023. However the procedure was canceled the day before due to lack of materials, leading the patient to seek our services at the Brazilian Institute of Regenerative Medicine (BIRM), in order to try conservative treatment.
On 02/24/2023, the patient was evaluated by our team of physicians, and a positive Thompson test led to the conclusion of complete rupture of the AT.
The informed consent form was provided for the use of orthobiologics and shock wave therapy, as well as the consent form regarding the use of data for research purposes.
We carried out the patient’s evaluation for adjustment and guidance on improved metabolism, sleep, diet and rehabilitation, which are fundamental elements for optimized tissue regeneration. We apply a strict nutritional monitoring with an “anti-inflammatory” diet that excludes alcohol, sugar, wheat, gluten, industrialized products, milk and dairy products for 90 days. We also encourage the increased intake of vegetables and lean proteins.
In the first session, 15 ml of BMA from the posterior superior iliac crest was collected and mixed with 2 ml of hyaluronic acid (Synolis), 2 ml of Traumeel and 1 ml of Dexamethasone. 10 ml of this mixture was injected in the left AT and 10 ml in the contralateral tendon with ultrasound guidance. This procedure was followed by focal ESWT on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20.
The patient was followed up through weekly appointments to assess function by physical examination, visual analogue scale (VAS) and ultrasound images (Figures 9 and 10).
This was a more complicated case because not only was the injury time longer, therapeutic intervention was initiated only 43 days post injury.
We then performed additional infiltration sessions with orthobiologics every two weeks with injectable platelet-rich fibrin (i-PRF) as mesotherapy procedures in order not to cause intratendinous lesions in the newly formed fibers. 80 ml peripheral blood was collected and transferred into sterile vacuum tubes without any anticoagulants or additives and centrifuged at 60 G for 5 min. 7 ml of plasma (i-PRF) were collected and mixed with 2.2 ml of Traummel and applied via mesotherapy technique beneath the dermis at the area of the injured tendon, followed by focal ESWT treatment.
Since this was a more serious case, during the evaluations we realized the need for more vigorous interventional strategies, so we performed 2 monthly BMA sessions (60 days after the beginning of the treatment). These BMA applications were interspersed with the fortnightly injections of i-PRF. ESWT was performed weekly, for a total of 18 sessions.
This patient also used a foot immobilizer with a heel wedge to keep him in an equine position for the first 4 weeks. At 12 weeks we removed the immobilizer and the patient himself was already walking normally, demonstrating strength during physical examination of ankle extension, with prominent signs of functional improvement (Figure 11).
At the end of 23 weeks, we requested a new MRI scan of the left ankle for a comparative study (Figure 12), identifying improvement in the architecture and realignment of the tendon fibers.
Results
Over the 12-week follow-up period, functional improvement was achieved in clinical terms. Robust signs of tissue repair were visualized via MRI, where we observed the formation of new tendinous fibers. 40 days after the first intervention, there was a 60% improvement in pain and after 60 days there was a 100% improvement in the pain scale (VAS), which was maintained until the end of follow-up. Also, around the 60th day, the patient reported a notable gain in range of motion and reduced edema. This allowed him to once again wear tennis shoes and return to the gym for upper limb workouts; for the lower limbs he was permitted to engage in light exercises in the water, such as walking. At the end of 180 days, the patient was already practicing lower limb strengthening at the gym.
Case report 3
A 37-year-old male playing soccer experienced a sudden “pop” in the lower portion of his left calf on 05/14/2023 and visited the emergency room. During the initial physical examination on his medical attention, he exhibited a positive Thompson test indicating complete rupture of the Achilles tendon (Figure 13) which was later confirmed by a Magnetic Resonance Imaging (MRI) examination (Figure 14).
The patient then sought our clinic for further examination in order to treat his injuries and get rid of the pain. He accepted our recommendation of conservative treatment with orthobiologics and shockwave therapy, signing the informed consent form. We carried out the patient’s evaluation for adjustment and guidance on improved metabolism, sleep, diet and rehabilitation, which are fundamental elements for optimized tissue regeneration. We apply a strict nutritional monitoring with an “anti-inflammatory” diet that excludes alcohol, sugar, wheat, gluten, industrialized products, milk and dairy products for 90 days. We also encourage the increased intake of vegetables and lean proteins.
In the first session, 15 ml of BMA from the posterior superior iliac crest was collected and mixed with 2 ml of hyaluronic acid (Synolis), 2 ml of Traumeel and 1 ml of Dexamethasone. 10 ml of this mixture was injected in the left AT and 10 ml in the contralateral tendon with ultrasound guidance. This procedure was followed by focal ESWT on a Piezzo 2 device with 4000 pulses set at a frequency of 8 hz and intensity value of 20.
6 weeks later, the patient received biweekly infiltration sessions with i-PRF as mesotherapy technique. Injectable PRF was prepared by collecting 80ml of peripheral blood collected into sterile vacuum tubes without additives and a centrifugation parameter set at 60G for 5 minutes. As supplementary treatment, sessions of ESWT were conducted weekly, totaling 12 sessions.
The patient was reassessed at each visit through physical examinations, pain analogue scale and serial ultrasounds.
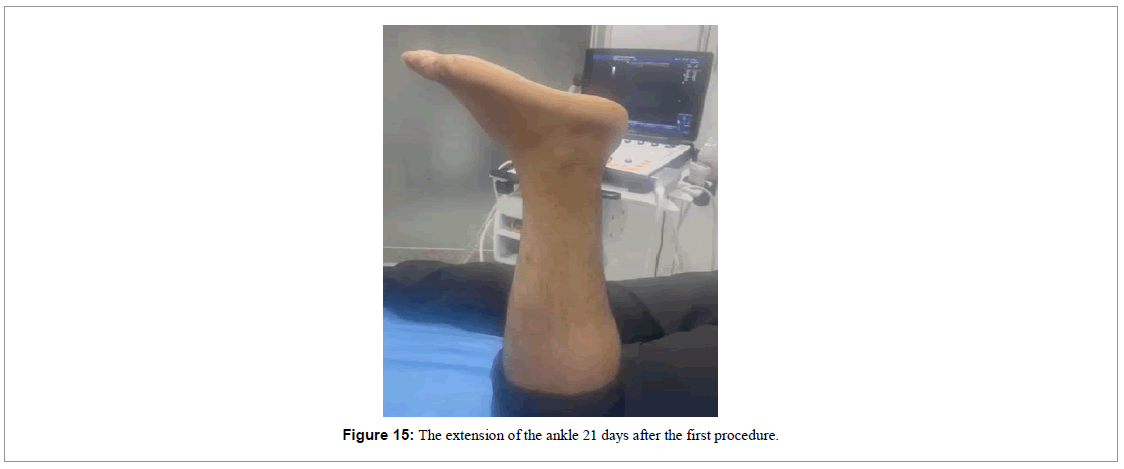
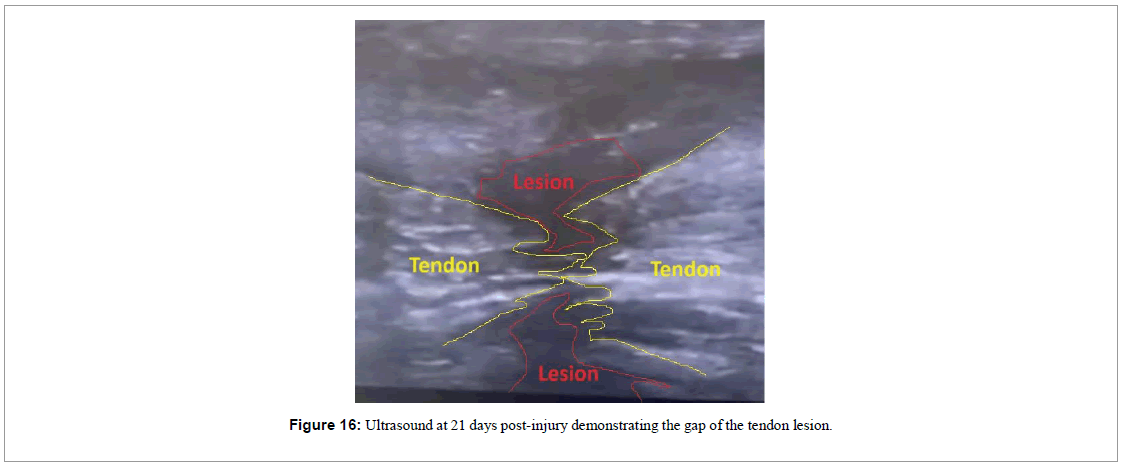
In the 21st day of treatment, the patient showed a slight movement of extension of his ankle (Figure 15). The ultrasound image showed a reduction of the gap between the two parts of the tendon lesion (Figure 16).
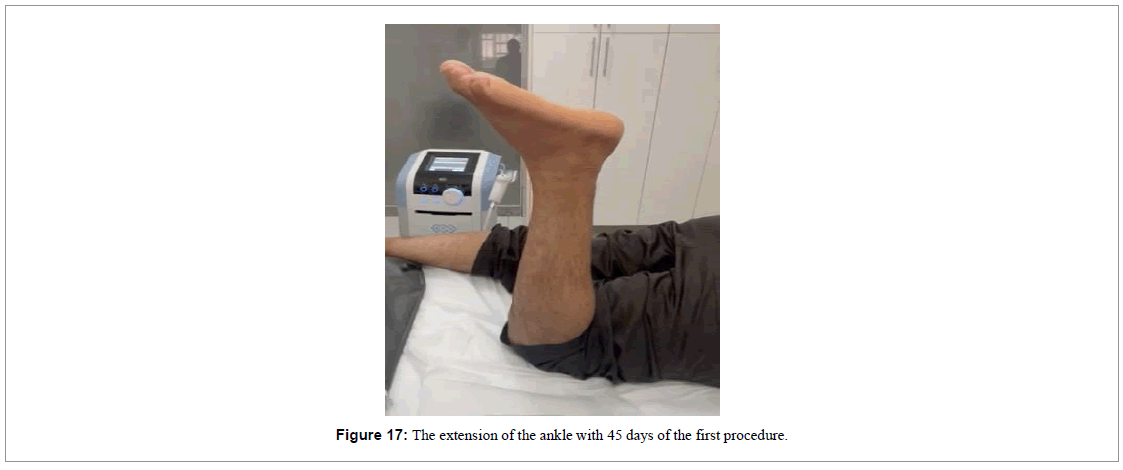
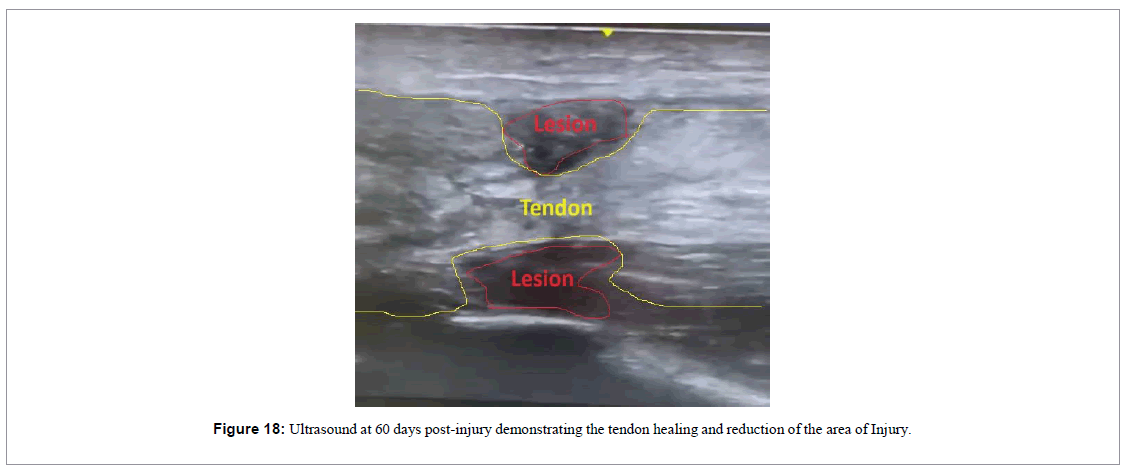
With 6 weeks of treatment, the movement of extension the ankle was nearly back to normal (Figure 17), and the ultrasound image demonstrated absence of the gap and presence of a new tendon-like tissue (Figure 18).
The patient used a foot immobilizer for 8 weeks to protect the injury and maintain the ankle in an equine position. When the immobilizer was removed, the patient was already walking normally, exhibiting ankle extension strength upon physical examination.
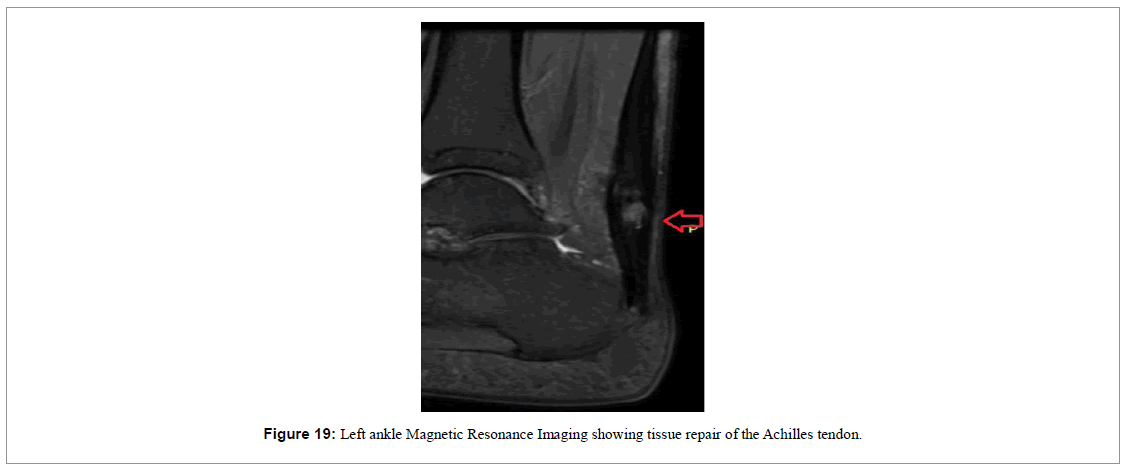
At the end of the 12-week treatment period, a follow-up MRI of the left ankle was requested for comparative analysis (Figure 19). The image showed the healing of the tendon with a central area of lesion and a global thickening of the tendon, demonstrating the healing process.
Results
Over the 12-week follow-up period, improvement was achieved in clinical terms. Clear signs of tissue repair were visualized via MRI, where we observed the formation of new tendon-like tissue. In just 6 weeks there was significant improvement in pain and function as range of ankle extension was nearly back to normal. This was maintained until the end of follow-up. The patient used a foot immobilizer for 8 weeks to protect the injury and maintain the ankle in an equine position. When the immobilizer was removed, the patient was already walking normally, exhibiting ankle extension strength upon physical examination. At the end of the 12-week treatment period, the follow- up MRI of the left ankle showed tendon healing with a central area of lesion and a global thickening of the tendon, sustaining evidence of the healing process.
Discussion
Achilles tendon ruptures are prevalent injuries in the lower extremities, often seen in athletes involved in activities that require repetitive jumping and running [13,14]. Addressing these injuries can be complex due to the tendons’ restricted blood flow, relying primarily on the diffusion of synovial fluid for nourishment [15]. As the tendon heals, it produces fibrous scar tissue that lacks the strength and flexibility of normal tissue [16]. The compromised strength heightens the potential for subsequent injuries and issues. Orthobiologic interventions are designed to reinstate the natural characteristics of the tendon, minimizing the chances of future injuries [17].
The best method for treating acute AT ruptures remains a subject of debate [18]. Traditional conservative care usually entails placing the ankle in a cast for around 6 weeks, starting with a plantar flexed stance and transitioning to a neutral position for the remaining period [18]. There are concerns regarding the effectiveness of conservative treatments, especially when there is no direct engagement with nearby structures. Conservative approaches have shown a greater likelihood of rupture in comparison to surgical methods [19]. However, it is important to consider that delayed healing can lead to calf weakness, and incomplete healing increases the risk of reruptures [18].
In healthy individuals, soft tissue injuries usually heal relatively quickly. However, the healing time can differ depending on injury severity and individual-specific factors [20]. In this case series, two patients were already above the age of 50 at the time of the incident, yet a rapid recovery was noted after one session of therapeutic intervention, as evidenced by MRI results and questionnaire feedback. While conventional treatments for AT ruptures typically include methods such as open surgery, these are not always effective and carry their own risks. Of the complications tied to AT ruptures, re-rupture stands out as the most critical post-treatment concern [21]. The repair process of injured tendons encompasses multiple crucial phases. These include the attraction and differentiation of progenitor cells, fibroblast proliferation, collagen synthesis, angiogenesis, and appropriate physiological loading [22]. Therefore, alternative methods like the use of orthobiologics might be beneficial for hastening tissue repair. Specifically, products derived from bone marrow have demonstrated potential as tools in regenerative medicine, applicable not only to musculoskeletal tissues but also to other organs across a range of diseases [23-25].
A promising alternative for treating AT ruptures is the use of the “BMA matrix,” as detailed by (Lana et al.) in 2021 [26]. This method harnesses bone marrow aspirate combined with hyaluronic acid to boost the crosslinking of fibrin fibers. This creates a biologically rich matrix that aids in the Achilles tendon’s healing process. This fibrin matrix acts both as a biological scaffold and a storehouse for cytokines and growth factors, further amplifying the activity of the MSCs found in the BMA. As highlighted before, MSCs have the intrinsic capability to self-renew and transform into various mesodermal lineage cells, encompassing bone, fat, cartilage, muscle, meniscus, and tendon [27]. Additionally, MSCs are vital in shaping the local tissue microenvironment via their autocrine and paracrine actions.
These cells present considerable benefits since they do not typically trigger strong immune reactions and can be easily extracted, which may enable allogeneic transplantation if necessary under isolated circumstances [26]. Nonetheless, the success of these treatments hinges largely on the capacity of MSCs to navigate to and engraft in the desired tissues. MSCs generally have a brief lifespan and ultimately get consumed by monocytes, which then prompt the generation of T-regulatory cells. This chain reaction plays a substantial role in the overall clinical outcome [26].
After tissue injury, local cells at the wound site secrete cytokines to lure MSCs to the affected area. Once summoned, MSCs generate molecular elements like Vasculo Endothelial Growth Factor (VEGF), Transforming Growth Factor Beta (TGF-β), Stromal-Derived Factor 1 (SDF-1), and Stem Cell Factor (SCF), to name a few. These growth factors empower MSCs to more effectively manage the healing journey. They deter fibrosis and apoptosis, curb excessive inflammation, and foster cell growth and differentiation via both paracrine and autocrine pathways [26]. It’s crucial to mitigate the persistent inflammation linked with tendon rupture, ensuring that the tendon has ample opportunity to adequately regenerate within a more conducive microenvironment.
Another molecular component found in the bone marrow is the Interleukin-1 Receptor Antagonist (IL-1Ra), which holds a pivotal position in the recovery journey. This robust cytokine serves as a competitive inhibitor. It connects to IL-1β and IL-1α receptors, subsequently blocking IL-1-driven catabolic activities and inflammation [26,28]. This might shed light on the notable pain relief experienced by our patients. IL-1Ra has the potential to decelerate the breakdown of the extracellular matrix, given that IL- 1β is known to stimulate the production of matrix metalloproteinase 3, tumor necrosis factor alpha, and prostaglandin E2. Furthermore, IL-1Ra is linked to the prevention of chondrocyte cell death and the suppression of collagen accumulation [26]. In fact, an animal study
[28] demonstrated that administering IL-1Ra can reverse alterations linked with established tendinopathy. In the context of tendon ruptures, the positive impacts steered by IL-1Ra become vital for reinstating tissue balance and enabling effective fiber repair. Recent studies using human models have unveiled that utilizing both BMA and BMAC (Bone Marrow Aspirate Concentrate) products bolsters safe and proficient tendon healing, eradicating pain and restoring function [29-31].
Other orthobiologic products rich in platelets and fibrin, such as autologous platelet concentrates, have garnered significant attention due to their positive impacts on various medical conditions. Platelet- Rich Plasma (PRP) and its derivatives like Platelet-Rich Fibrin (PRF) have shown promising results in both clinical and lab environments, especially in musculoskeletal conditions [32]. While PRP and PRF have unique features, they possess overlapping qualities. Both products, being rich in platelets, peripheral blood cells, and molecular constituents, activate a myriad of biological processes [32]. These range from modulating inflammation, enhancing neovascularization, and providing pro-anabolic stimuli that oversee cell attraction, growth, and differentiation [33]. The often-underappreciated fibrinolytic system is pivotal in musculoskeletal regenerative medicine, guiding proteolytic actions and supporting the congregation of inflammatory cells and MSCs in regeneration zones, encompassing bone and soft tissue [33]. PRP, while a strong bio-signaler, tends to disperse swiftly. In contrast, the fibrin in PRF provides a lasting structural framework, boosting cellular activities [32]. The injectable version of PRF (i-PRF) which we used in this case series may offer additional feasibility in treatment alternatives [34]. Injectable PRF is commonly produced in vacuum tubes devoid of any additives, a method aimed at stalling coagulation. Such a preparation gives medical professionals ample time to handle the orthobiologic specimen appropriately before proceeding with an intra-articular injection. Enhancing fibrin’s structural capacity with hyaluronic acid, another well-researched orthobiologic product, results in stronger fibrin polymerization. This amplification bolsters cell longevity, adhesion, movement, and expansion [32]. Injectable PRF can support the biological potential of BMA matrix, which may also explain the success we have had with orthobiologic therapy.
The other regenerative tool we employed was Extracorporeal Shockwave Therapy (ESWT). This modality stands out as a widely adopted non-invasive treatment approach in medicine for numerous diseases. Its potential applications in orthopedics and traumatology have been under investigation for decades [35]. The fundamental principle of ESWT is the generation and emission of acoustic waves, known as shockwaves [36]. These waves transport energy that can traverse bodily tissues. When these shockwaves pass through tissue, they can elicit both interstitial and extracellular reactions, which in turn yield a spectrum of therapeutic effects [37]. Notable among these are pain mitigation, enhanced vascularization, increased protein synthesis, cell growth, protection of nerve and cartilage cells, and the breakdown of calcium build-ups within musculoskeletal formations [35]. By orchestrating these beneficial responses, ESWT can facilitate tissue regeneration and provide pronounced pain relief, ultimately enhancing the recovery and functional outcomes of the damaged tissue. It is also important to note that radial shockwaves, in particular, can induce the proliferation and self-renewal of MSCs [38] and therefore improve clinical outcomes of certain musculoskeletal conditions. Given these attributes, ESWT presents immense promise as a feasible auxiliary tool in regenerative musculoskeletal medicine.
Conclusion
In this case series we demonstrate that autologous BMA and i-PRF products are a viable alternative for the accelerated recovery of musculoskeletal tissue injuries safely and effectively. The results of the functional evaluation, magnetic resonance imaging and ultrasonography were encouraging at the 12-week follow-up, showing significant signs of structural restoration of the AT. Our patients exhibited a positive reaction to the treatment, devoid of any grave side effects.
While numerous studies assess the potential of bone marrow- derived products for various tendon ailments, a distinct gap exists in clinical research specifically targeting the application of BMA products for AT ruptures. Nevertheless, given BMA’s track record in regenerative medicine for lower limb issues, we felt assured in our decision to use this orthobiologic approach. The outcomes exceeded our anticipations. Employing the “BMA matrix” resulted in marked reductions in pain, enhanced functional outcomes, and evident progress in soft tissue recovery following a single treatment session. By the 6-month mark post-treatment, the patient fully resumed all activities without experiencing pain or any physical hindrance. It’s imperative that future studies further delve into the underlying mechanics of this method.
References
- O'Brien M (2005) The Anatomy of the Achilles tendon. Foot Ankle Clin; 10(2):225-238.
- Tarantino D, Palermi S, Sirico F, Corrado B(2020) Achilles Tendon Rupture: Mechanisms Of Injury, Principles Of Rehabilitation And Return To Play. J Funct Morphol Kinesiol; 5(4):95.
- Schulze-Tanzil GG, Cáceres MD, Stange R, Wildemann B, Docheva D(2022) Tendon Healing: A Concise Review On Cellular And Molecular Mechanisms With A Particular Focus On The Achilles Tendon. Bone Joint Res;11(8):561-574.
- Ramires LC, Jeyaraman M, Muthu S, Shankar A N, Santos GS, et al.(2022) Application Of Orthobiologics In Achilles Tendinopathy: A Review. Life (Basel); 12(3):399.
- Morimoto S, Iseki T, Nakayama H, Shimomura K, Nishikawa T, et al. (2021) Return To The Original Sport At Only 3 Months After An Achilles Tendon Rupture By A Combination Of Intra-Tissue Injection Of Freeze-Dried Platelet-Derived Factor Concentrate And Excessively Early Rehabilitation After Operative Treatment In A Male Basketball Player: A Case Report. Regen Ther; 18:112-116.
- Darrieutort-Laffite C, Soslowsky LJ, Le Goff B(2020) Molecular And Structural Effects Of Percutaneous Interventions In Chronic Achilles Tendinopathy. Int J Mol Sci; 21(19):7000.
- Carmont MR, Silbernagel KG, Brorsson A, Olsson N, Maffulli N, et al. (2015) The Achilles Tendon Resting Angle As An Indirect Measure Of Achilles Tendon Length Following Rupture, Repair, And Rehabilitation. Asia Pac J Sports Med Arthrosc Rehabil Technol; 2(2):49-55.
- Douglas J, Kelly M, Blachut P(2009) Clarification Of The Simmonds–Thompson Test For Rupture Of An Achilles Tendon. Can J Surg; 52(3):E40.
- Kim SJ, Song DH, Park JW, Park S, Kim SJ(2017) Effect Of Bone Marrow Aspirate Concentrate-Platelet-Rich Plasma On Tendon-Derived Stem Cells And Rotator Cuff Tendon Tear. Cell Transplant; 26(5):867-878.
- Bachir RM, Zaia IM, Santos GS, da Fonseca LF, Boni G, et al.(2023) Bone Marrow Aspirate Concentrate Improves Outcomes in Adults With Osteochondral Dissecans of the Talus and Achilles Rupture. Arthroscopy; 39(3):881-886.
- Quintero D, Perucca Orfei C, Kaplan LD, de Girolamo L, Best TM, et al (2023) The Roles And Therapeutic Potentialof Mesenchymal Stem/Stromal Cells And Their Extracellular Vesicles In Tendinopathies. Front Bioeng Biotechnol; 11:1040762.
- Aktas E, Chamberlain CS, Saether EE, Duenwald‐Kuehl SE, Kondratko‐Mittnacht J, et al. (2017) Immune Modulation With Primed Mesenchymal Stem Cells Delivered Via Biodegradable Scaffold To Repair An Achilles Tendon Segmental Defect. J Orthop Res; 35(2):269-280.
- Li HY, Hua YH (2016) Achilles Tendinopathy: Current Concepts About The Basic Science And Clinical Treatments. Biomed Res Int; 2016:6492597
- Waldecker U, Hofmann G, Drewitz S(2012) Epidemiologic Investigation Of 1394 Feet: Coincidence Of Hindfoot Malalignment And Achilles Tendon Disorders. Foot Ankle Surg; 18(2):119-123.
- Fenwick SA, Hazleman BL, Riley GP(2002) The Vasculature And Its Role In The Damaged And Healing Tendon. Arthritis Res; 4(4):252-260.
- Yang G, Rothrauff BB, Tuan RS (2013) Tendon And Ligament Regeneration And Repair: Clinical Relevance And Developmental Paradigm. Birth Defects Res C Embryo Today; 99(3):203-222.
- Dhillon MS, Behera P, Patel S, Shetty V(2014) Orthobiologics And Platelet Rich Plasma. Indian J Orthop; 48(1):1-9.
- Utashima D, Matsumura N, Suzuki T, Iwamoto T, Ogawa K, et al.(2020) Treatment Of Acute Achilles Tendon Rupture. Clin Orthop Surg; 12(1): 1-8.
- Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, et al. (2005). Treatment Of Acute Achilles Tendon Ruptures: A Meta-Analysis Of Randomized, Controlled Trials. J Bone Joint Surg Am; 87(10):2202-2210.
- Guo SA, DiPietro LA(2010). Factors Affecting Wound Healing. J Dent Res; 89(3):219-229.
- Hanada M, Takahashi M, Matsuyama Y(2011) Open Re-Rupture Of The Achilles Tendon After Surgical Treatment. Clin Pract; 1(4): e134.
- Yuan T, Zhang CQ, Wang JH (2013) Augmenting Tendon And Ligament Repair With Platelet-Rich Plasma (PRP). Muscles Ligaments Tendons J; 3(3): 139.
- Bozdag-Turan I, Goekmen Turan R, Ludovicy S, Akin I, Kische S, et al. (2012) Intra Coronary Freshly Isolated Bone Marrow Cells Transplantation Improve Cardiac Function In Patients With Ischemic Heart Disease. BMC Res Notes; 5: 1-9.
- Chahla J, Alland JA, Verma NN (2018) Bone Marrow Aspirate Concentrate For Orthopaedic Use. Orthopaedic Nursing. Orthop Nurs; 37(6): 379-381.
- Vahidy FS, Haque ME, Rahbar MH, Zhu H, Rowan P,et al. (2019) Intravenous Bone Marrow Mononuclear Cells For Acute Ischemic Stroke: Safety, Feasibility, And Effect Size From A Phase I Clinical Trial. Stem Cells; 37(11): 1481-1491.
- Lana JF, da Fonseca LF, Azzini G, Santos G, Braga M, et al. (2021) Bone Marrow Aspirate Matrix: A Convenient Ally In Regenerative Medicine. Int J Mol Sci; 22(5): 2762.
- Purita J, Lana JF, Kolber M, Rodrigues BL, Mosaner T, et al. (2020) Bone Marrow-Derived Products: A Classification Proposal–Bone Marrow Aspirate, Bone Marrow Aspirate Concentrate Or Hybrid?. World J Stem Cells; 12(4): 241.
- Eskildsen SM, Berkoff DJ, Kallianos SA, Weinhold PS (2019) The Use Of An Il1‐Receptor Antagonist To Reverse The Changes Associated With Established Tendinopathy In A Rat Model. Scand J Med Sci Sports; 29(1): 82-88.
- Farina KA, Kandah BA, Sowers NM, Moore GA (2021) Bone Marrow Aspirate Concentrate Injection Of The Achilles Tendon In A Competitive Distance Runner. J Musculoskelet Res, 24(01), 2140004.
- Imam MA, Holton J, Horriat S, Negida AS, Grubhofer F, et al (2017) A Systematic Review Of The Concept And Clinical Applications Of Bone Marrow Aspirate Concentrate In Tendon Pathology. SICOT J; 3.
- Thueakthong W, de Cesar Netto C, Garnjanagoonchorn A, Day J, Friedman G, et al. (2021) Outcomes Of Iliac Crest Bone Marrow Aspirate Injection For The Treatment Of Recalcitrant Achilles Tendinopathy. Int Orthop; 45: 2423-2428.
- Lana JF, Purita J, Everts PA, De Mendonça Neto PA, de Moraes Ferreira Jorge D, et al. (2023) Platelet-Rich Plasma Power-Mix Gel (PMP)—An Orthobiologic Optimization Protocol Rich In Growth Factors And Fibrin. Gels; 9(7): 553.
- Dos Santos RG, Santos GS, Alkass N, Chiesa TL, Azzini GO, et al.(2021). The Regenerative Mechanisms Of Platelet-Rich Plasma: A Review. Cytokine; 144: 155560.
- Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, et al. (2017) Injectable Platelet Rich Fibrin (I-Prf): Opportunities In Regenerative Dentistry?. Clin Oral Investig; 21: 2619-2627.
- Simplicio CL, Purita J, Murrell W, Santos GS, Dos Santos RG, et al. (2020) Extracorporeal Shock Wave Therapy Mechanisms In Musculoskeletal Regenerative Medicine. J Clin Orthop Trauma; 11: S309-S318.
- Cheng JH, Wang CJ (2015) Biological Mechanism Of Shockwave In Bone. Int J Surg; 24: 143-146.
- Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, et al. (2014) Shock Wave Treatment Enhances Cell Proliferation And Improves Wound Healing By Atp Release-Coupled Extracellular Signal-Regulated Kinase (Erk) Activation. J Biol Chem; 289(39): 27090-27104.
- Zhang H, Li ZL, Yang F, Zhang Q, Su XZ, et al. (2018) Radial Shockwave Treatment Promotes Human Mesenchymal Stem Cell Self-Renewal And Enhances Cartilage Healing. Stem Cell Res Ther ; 9(1): 1-13.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi