Research Article, J Biodivers Manage Forestry Vol: 6 Issue: 4
Correlation of Leaf Area Index to Root Biomass in Populus tremuloides Michx supports the Pipe Model Theory
Caldwell BT* and O’Hara KL
University of California Berkeley, 130 Mulford Hall, Berkeley, CA 94720, USA
*Corresponding Author : Benjamin T. Caldwell
University of California Berkeley, 130 Mulford Hall, Berkeley, CA 94720, USA
Tel: +1.510.859.3328
E-mail: btcaldwell@gmail.com
Received: November 21, 2017 Accepted: December 22, 2017 Published: December 30, 2017
Citation: Caldwell BT, O’Hara KL (2017) Correlation of Leaf Area Index to Root Biomass in Populus tremuloides Michx supports the Pipe Model Theory. J Biodivers Manage Forestry 6:4. doi: 10.4172/2327-4417.1000186
Abstract
There is relatively little information about the root systems of mature trees. Ecophysiological theory predicts that the size and extent of mature root systems would be correlated with leaf area and aboveground biomass within groups such as species or populations. Using recent methods for root sampling that reduce bias and improve representativeness, we tested these hypotheses for quaking aspen (Populus tremuloides Michx.) in California. We found that for a representative sample of basal areas for this species, the leaf area index (LAI) was a highly significant predictor of both coarse and fine root biomass (adj. r2 of .79 and .59, respectively). Coarse root biomass was a highly significant predictor of fine root biomass (adj.r2 of .84). Results support the pipe model theory, and may prove useful for land managers interested in root systems for aspen stand regeneration or management for carbon sequestration.
Keywords: Tree roots; Ground penetrating radar; Ecophysiology; Pipe model theory; Carbon sequestration; Leaf area index; Populus tremuloides Michx
Introduction
The pipe model theory is a theoretical framework with implications for plant form and the connectivity between roots and leaves via the xylem [1,2]. Applications of pipe model theory to forest management and ecology range widely, but include estimating leaf area to guide management for timber, water quality, and complex stand structures, as well as measurement of growing space efficiency and total growing space occupancy [3,4]. As an ecophysiological metric, LAI (leaf area per ground surface area, a unitless measure) is a key descriptor in ecology. Furthermore, it is highly scalable from the ecosystem level (where it can be used to estimate net primary productivity and ecosystem transpiration) down to the stand level (where it can be used to manage growing space) [5,6]. Interestingly, while the prediction of leaf biomass and leaf area from sapwood area is well described, the obvious counterpart, prediction of the roots, is not. Root biomass measurement and prediction represent a key gap in our knowledge of forested ecosystems [7].
Functionally, coarse roots serve to buffer tree stems from windthrow [8,9]. They also serve as the site of fine root attachment, allowing roots to extend to greater distances and depths [10]. Finally, they are the location where most of the below-ground forest biomass is sequestered.
Quaking aspen (Populus tremuloides Michx.) is one of the most widely distributed tree species in the world [11]. It is at the southern and western extent of its range in California’s Sierra Nevada. The species is consequently very sparse in any given location in California, and restricted to relatively small stands on mesic, high-elevation sites. Nonetheless, quaking aspen plays an important role for wildlife, water quality and conservation, and floral diversity in this ecosystem, and has been a species of considerable interest for restoration and for study by ecologists [12-14].
Aspen is a fire-adapted species with the unusual ability to regenerate by suckering, or sprouting from its roots, after disturbance. This vegetative regeneration originates in the roots; suppressed hormonally in the presence of healthy leaves and stems, the roots will produce suckers if the above-ground component of the tree is damaged or removed [15]. This heavy reliance on vegetative regeneration also allows some aspen clones to achieve a superlative status among living organisms for age and size. Aspen may therefore include the largest and oldest organism [16,17].
It is little wonder then that the quaking aspen root system, as the site of vegetative regeneration, has been the object of serious study for several decades. Many studies have focused on description of the root system itself, using hydraulic excavation to reveal the extent and distribution of clonal root systems [18]. The authors of early studies noted that aspen roots are prolific, interconnected, and shallowly distributed. This information, combined with the observation that aspen clones are large and persistent, led to speculation that nutrient transfer might occur via the roots within multiple stems of a single clone. This was subsequently confirmed using chemical tracers, which indicated the transfer of water and carbohydrates between stems in a given clone via connected root systems [19].
More recent research has focused on aspen clone persistence in the face of rapid anthropogenic change of temperate ecosystems. Studies on these phenomena have focused on fire suppression and the impact of climate warming on water availability, as well as increased pressure from insects [13,20]. As land managers in the Sierra Nevada consider options for quaking aspen regeneration, one key issue is how to adapt regeneration, which historically was probably driven by fire, to management realities in which allowing or intentionally starting stand-replacing fires may not be an option [21]. A second consideration is the influence of climate change, and the consequent water stress in many western North American ecosystems [22]. Since a given clone is typically composed of multiple stems, and water stress is experienced by all stems due to interconnected roots, catastrophic water stress can affect many stems simultaneously, leading to so-called “Sudden Aspen Death” [13]. Aspen is therefore of considerable ecological interest, has been very competitive, and may be faced with a contracting range due to anthropogenic ecosystem impacts. Further study of aspen is thus both worthwhile for its unusual suite of physiological adaptations and timely as its range begins to shift north.
Understanding how quaking aspen leaves and roots respond to changes in historic disturbance regime is key information as land managers try and maintain aspen as an ecosystem component. A major gap in an ecophysiological approach is the ability to predict the size or the extent of the root biomass in question. Also lacking are regional models to estimate leaf area and biomass from commonly taken measurements like basal area (see a notable exception in Alberta [23]). From an applied standpoint, an understanding of aspen root and leaf connection and extent would improve rapid assessment of stand health. For aspen, the coarse roots are the sites of vegetative regeneration and a site of carbohydrate storage [15]. From a theoretical standpoint it would also be interesting to estimate the proportion of a stand belowground, in the context of optimal carbohydrate allocation theory [24]. Related to applications in restoration management, leaf area estimates can also be used for prediction of below-ground biomass and belowground carbon sequestration in an ecosystem [23].
The objective of this study was to test whether, in keeping with the pipe model theory, it is possible to model below-ground biomass in mature trees using above-ground biomass and leaf area. In contrast to previous work in this area we used ground-penetrating radar, in conjunction with root cores and soil pits, to estimate root biomass. We expected that leaf area would correlate well with fine root biomass, and that above-ground biomass could be used to model below-ground biomass with a high degree of fidelity. Finally, we examined tree crosssectional area at 1.37 m height as a proxy for conducting connective tissue to predict tree root biomass and leaf area.
Materials and Methods
Sampling locations
Three different sites in the southern and central Sierra Nevada on public and private lands were selected for sampling (Table 1 and Figure 1). Stand selection occurred with the intent to cover as broad a range of tree diameters and stand basal areas possible, as well as include sites that ranged from mesic to zeric. Plots were randomly located within sites. The number of plots in each site ranged from one to three, depending on the extent of aspen and the variability observed in each site. Plot sizes ranged from 0.02 to 0.04 ha depending on the size of the aspen stand. Within each plot, sampling was focused on measurements of leaf area, fine roots using root cores, and coarse roots using trenches and ground-penetrating radar.
| Site | Management history | latitude | longitude | soil order | suborder | elevation(m) |
|---|---|---|---|---|---|---|
| BM | Restoration thinning | 39.41 | -120.18 | Alifisols | Xeralfs | 1700 |
| LYBLM | Unmanaged | 39.67 | -120.15 | Mollisols | Xerolls | 1670 |
| SAG | Unmanaged | 39.43 | -120.28 | Alifisols | Xeralfs | 2500 |
| SN | Unmanaged | 37.61 | -118.83 | Mollisols | Xerolls | 2200 |
| SPI | Timber | 39.47 | -120.52 | Inceptisols | Unbrepts | 2100 |
Table 1: Sampling locations.
Each plot center was recorded using a WAAS-enabled GPS and marked with a tree tag or flagging. In each plot sampled, diameter at 1.37 meters (diameter at breast height or dbh) for all stems was measured using a diameter tape to the nearest mm. Stems were mapped using a laser rangefinder with a digital compass (Laser Ace TM). For a subset of stems, total height and height to the base of the live crown was also measured using the same instrument. Stems included in the subset were selected randomly, with at least five sampled in each plot. If there was a high degree of variance observed in the crown heights or diameter- height relationship, additional height measurements were taken.
Leaf area estimation
Individual trees were destructively sampled to develop leaf area prediction relationships. A branch summation approach [25,26] was used to subsample tree leaf area. However, sapwood was not measured because of the difficulty in determining sapwood/heartwood boundaries in aspen [27].
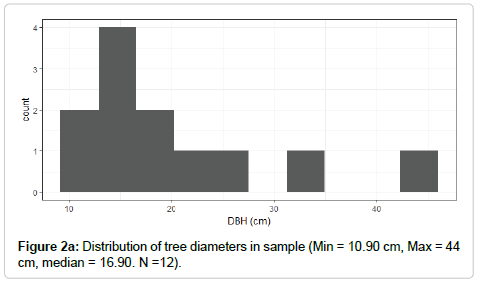
In the field, selected stems (n=12) were felled, and diameters were measured at ground level, at breast height, and at the crown base. Stems were selected to cover a range of tree sizes and crown positions (Figure 2a). Only healthy stems without major structural damage were selected for sampling. The crown was subdivided into thirds, and stem diameter was measured at the base of each third. Stem disks were removed on the main stem at one-third and two-thirds of the crown’s length. Total height and height at the base of the live crown was also recorded. The diameter at the base of every branch and branch length was recorded. At least three branches from each third of the live crown were removed from the stem. Leaves and petioles were stripped from the branch, bagged separately in paper bags, and placed on ice in a sealed container.
In the lab, a subset of all leaves was collected from each tree for scanning. The three branches from each third of the crown had all leaves removed, for a total of nine branches per tree. All leaves from the selected branches were scanned and the leaf area calculated using WinFolia, (Regent Instruments, Inc.). All leaves were weighed and then placed in a drying oven at 70°C. Dry mass was recorded after a stable mass was reached. Dry and wet mass for all leaves was recorded; measurements of the scanned subset of leaves were kept separate for subsequent analysis.
Coarse root biomass
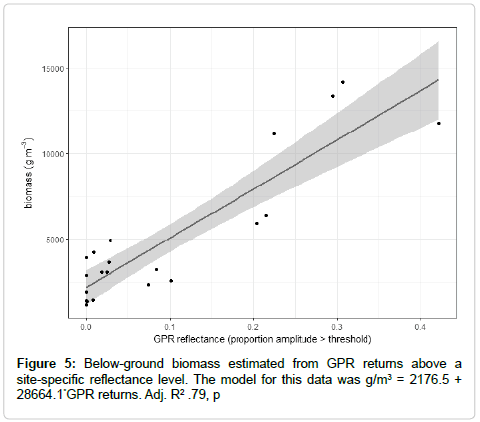
Methods for coarse root biomass measurement were modified from methods presented by Butnor et al. [28]. Coarse root biomass was estimated using a combination of indirect measurement of root biomass using ground-penetrating radar (GPR) and direct measurements from trenches in each plot. Returns from the radar were regressed on the measurements from the trenches to determine the relationship between the GPR returns and root biomass. Returns were also visualized in three dimensions in R [29-31] to aid interpretation of data (Figure 2b). The more extensive measurements from GPR were then used to estimate root biomass for that area.
Fine root biomass
Fine root biomass (diameter <5 mm) was measured in each site using root cores (n = 140) taken at 10, 20 and 30 cm depth. Root cores were extracted using a quantitative corer and brought back to the lab for analysis [32].
Modeling and Analysis
Leaf area
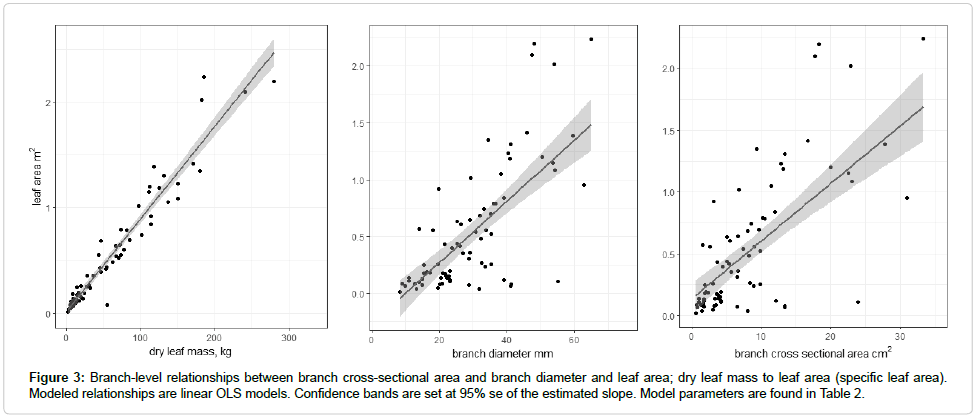
Specific leaf area for each branch was the ratio of leaf area and leaf mass. A regression between branch leaf area and branch cross-sectional area was developed for all trees (Figure 3). Inclusion of branch length did not result in significant improvement in the models, so only crosssectional area was used. Whole-tree leaf area was the sum of measured leaf area (on sample branches) and estimated leaf area for each tree. Tree cross-sectional area was then used to predict tree leaf area. All analysis was conducted in R [29]. Non-linear model forms were explored, but ultimately all models selected were ordinary least squares (OLS) regressions analyzed using the lm() function in R’s base package.
Fine root estimation
Rather than exhaustively extracting the fine root biomass from every root core, root biomass for each root core was estimated by determining the best model form for the cumulative biomass of roots extracted from the core over time [33]. To do this, a subsample of root cores were extracted at set time intervals until root biomass was exhausted in each. The models were then tested for goodness of fit to the cumulative extraction over time from the cores. At 40 minutes per core, the amount of biomass predicted by the logistic model had an acceptable level of error compared to the actual biomass extracted (<10%, no detectable bias). For the rest of the cores, roots were extracted for 40 minutes, and a logistic model fit to the cumulative biomass extracted to arrive at the root biomass in each core. Since the asymptote of a logistic function approaches infinity, a cutoff time was used when the derivative of the slope was <1% [33]. Cores that had model slopes, intercepts or standard errors that were outliers were removed, for a sample size of 118. Fine root biomass was calculated for a per-area basis (density) using the volume of the core extracted.
Belowground biomass estimation using above-ground data
Multivariate models to predict fine and coarse biomass with measured above-ground metrics were tested, and the most significant variables used to reduce the models to ones with the best predictive power. Linear relationships were explored between variables of interest using visual inspection of the data to remove outliers and determine whether the assumptions of OLS regression were met [34]. R base and the ggplot2 package were the primary tools used for this analysis [29,31]. Once the model form and assumptions were confirmed, adjusted r2 and p values were used to assess the utility and significance of tested correlations, again using R base. First-order polynomials were found to best explain the relationship between tested dependent and independent variables, and were used throughout the analysis. While multivariate models were tested, in all cases there was a single strong predictor used in the best model.
Results
Leaf area estimation
The specific leaf area relationship found for stems was approximately 8.77 ± 0.30 m2kg-1. The relationship between wet and dry leaf biomass exhibited very little variation, with a slope of 0.5 and an intercept very close to zero (adj. r2 = 0.93, highly significant relationship (Figure 3).
The estimated relationship between branch leaf area and cross-sectional area was
LAbr=.12 + .05ABr (1)
Where
LAbr: Branch leaf area (m2)
ABr: Branch cross sectional area (cm2)
Branch-level leaf area was moderately well predicted by branch cross-sectional area (Table 2 and Figure 3).
| Adj. R2 | DF | SE (m) | Intercept | slope | p-value | |
|---|---|---|---|---|---|---|
| Leaf area ~ CSA | 0.538 | 75 | 0.0053 | 0.12 | 0.05 | 1.95E-14 |
Table 2: Leaf area model at the branch level. The model uses leaf area in terms of meters squared as the dependent variable. The independent variable is branch crosssectional area. It is in terms of cm2.
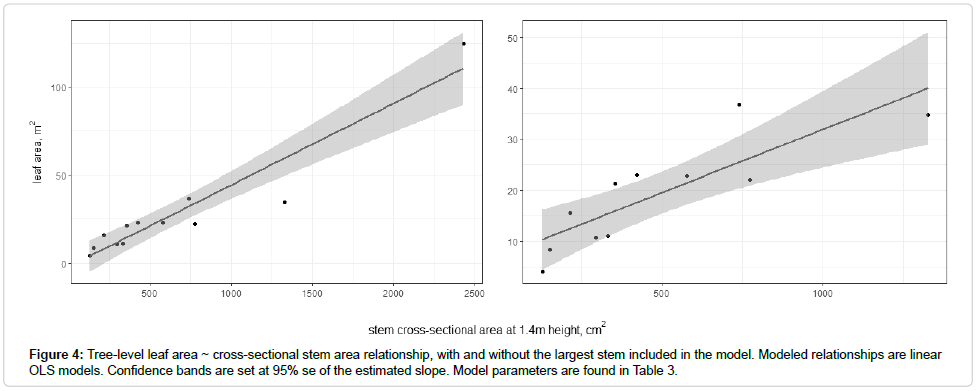
Several different independent variables were explored to determine which provided the best estimate of leaf area (based on a comparison of adj. r2 values and visual examination of the regressions and residuals). We used tree cross-sectional area at breast height to estimate the tree-level leaf area. For the entire dataset, the predictor for leaf area was the tree cross-sectional area, with an adjusted r2 of .89. Inclusion of tree height in the model increased the residual standards error and lowered the coefficient of determination; therefore, only tree cross-sectional area was retained as an independent variable. LAt to cross-sectional area at 1.37 m height was estimated to be
AL=-2.065+0.05BA (2)
Where AL: tree projected leaf area (m2)
BA: tree cross-sectional area (cm2)
The largest stem in the sample was approximately twice as large by diameter as the next-largest stem. Without inclusion of the largest stem, the model estimated was
AL=7.175+0.03BA (3)
Inclusion of the largest stem in the dataset did not result in a dramatically different model estimate over the range of values in the sample and subsample (Figure 4). The best fit models and those considered most ecologically meaningful are presented in Table 3, with their appropriate descriptive statistics. A model which used diameter at base of live crown as the independent variable is presented for comparison because, while it often is the metric with the best predictive power, it did not prove to be in this case (Figures 5 and 6).
| Adj. R2 | DF | SE(m) | Intercept | slope | p-value | |
|---|---|---|---|---|---|---|
| Leaf area~CSA | 0.89 | 10 | 0.0049 | -2.065 | 0.05 | 2.57 E-06 |
| Leaf area~ DBH | 0.82 | 10 | 0.4257 | -31.950 | 3.05 | 3.07E-05 |
| Leaf area~ d.BLC | 0.56 | 10 | 1.2600 | -45.680 | 4.88 | 0.00318 |
| Leaf area~ DBH w/o stem 12 | 0.68 | 9 | 0.3193 | -6.380 | 1.49 | 0.00117 |
| Leaf area~CSA w/o stem 12 | 0.60 | 9 | 3.1662 | 7.175 | 0.03 | 0.00127 |
Table 3: Tree-level models for leaf area. All models are in terms of leaf area in meters squared. The three independent variables are diameter at breast height and diameter at base of the live crown for all stems, and diameter at breast height with stem 12 removed from the analysis. All independent variables are in terms of cm.
LAI
For the five plots in which aspen was predominant, LAI for the aspen stems ranged from 1.6 to 2.5, and for all species from 1.6 to 9.5 (Table 4).
| Plot | LAI |
|---|---|
| 1 | 1.047458 |
| 2 | 1.64398 |
| 3 | 2.216311 |
| 4 | 2.21727 |
| 5 | 2.518608 |
Table 4: LAI for five aspen stands mean = 1.9, sd=.59.
Coarse root biomass
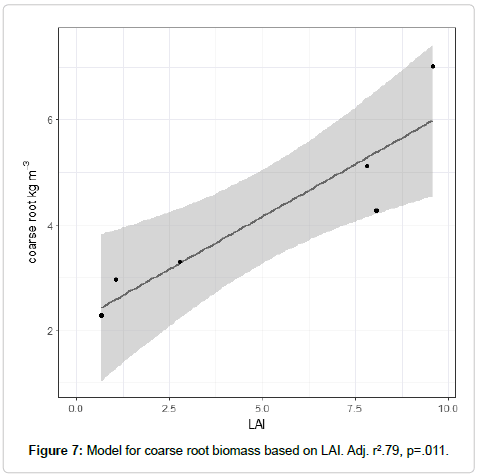
Basal area and LAI were very strong predictors of coarse root biomass. Adjusted r2 exceeded .79 for these models (Table 5 and Figure 7). GPR scans of the soil show a highly interconnected mat of roots between quaking aspen stems, confirming results from other studies that relied on excavation.
| model | adj.r2 | p value |
|---|---|---|
| Coarse Root~basal area | 0.896 | 0.00267 |
| Coarse Root~LAI | 0.791 | 0.01108 |
| Fine Root~coarse root | 0.8405 | 0.00639 |
| Fine Root~basal area | 0.6185 | 0.00728 |
| Fine Root~LAI | 0.5895 | 0.00955 |
Table 5: Model significance and fit for fine and coarse roots to selected aboveground metrics.
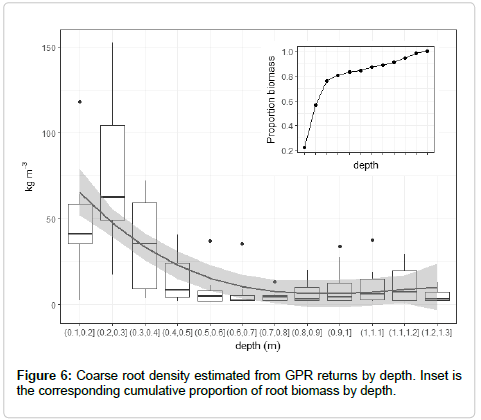
Root biomass was observed to be concentrated in the upper 50 cm of the soil, with approximately 80% of total biomass found in the top 50 cm, on average; root biomass decreased unimodally with depth (Figure 6).
Fine root biomass
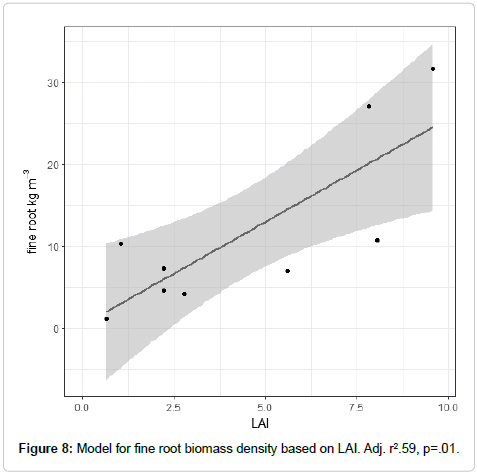
LAI was a highly significant predictor of fine root biomass in these ecosystems, and predicted the majority of the variation in fine root biomass (Table 5 and Figure 8).
Discussion
Specific leaf area
There have been very few previous studies that have reported values for specific leaf area for quaking aspen. Bond-Lamberty et al. [35] reported SLA of 5.82 ± 1.91 m2kg-1 in northern Manitoba, Canada; Kaufmann and Troendel [36] reported 10.97 m2kg-1 (no reported variance) near Fraser, Colorado. Our mean value of 8.77 ± .30 m2kg-1 falls within this range. The more extreme climatic conditions and shorter growing season in Canada may have resulted in lower SLA.
Leaf area
While some studies have found that including crown parameters improved tree-level estimates of leaf area using sapwood cross-sectional area or total cross-sectional area in other species [37,38], these terms did not improve models tested in this study. One possible inference is that the relationship between leaf area and the stem cross-sectional area is relatively constant across different light environments for quaking aspen. This may be a result of the relatively low tolerance to poor light conditions of quaking aspen, which has been observed to decline rapidly when overtopped in the Sierra Nevada [20]. Populus tremula L., a closely related species from Europe, Asia and North Africa, has been noted to change leaf inclination to manage water stress and light availability [39], but the species does not change the physiology of its leaves in response to the light regime, as many shade-tolerant species do. As a consequence, quaking aspen probably exhibits less plasticity in the mass ratios of its leaves than many of the firs (Abies sp.) studied by Kaufmann et al. [40]. Data on quaking aspen physiology is relatively scarce compared to other congenerics. As there has been relatively little published on the above-ground adaptability of quaking aspen to different water and light conditions, this area of study deserves more attention.
Kaufmann and Troendle [36] presented leaf area - sapwood relationships from quaking aspen in Colorado, but did not report diameters for the stems measured. Assuming the range of diameters sampled was comparable as the present study, the leaf area estimates from that study and this one are at least on the same order of magnitude and range. In the most directly comparable study, Kaufmann et al. [40] reported a leaf area – basal area relationship for destructively sampled aspen in Colorado. They reported a quadratic model for aspen leaf area as explained by basal area. Their results were complicated, however, by the fact that large aspen stems in different site conditions in their study appeared to exhibit very different basal area/leaf area relationships. Two large stems on ‘moist south slopes’ had leaf area/basal ratios approximately four times that of three other stems in the same size range located in a cooler more xeric sites. Kaufmann et al. reported that the stems on the xeric sites indicated a quadratic model form, and the other two stems a linear form to the model. They attributed that difference to water availability and excluded the two stems that suggested a linear model from their analysis. The quadratic model developed by Kaufmann et al. exhibited symptoms of over-fitting, however, and inspection of their graph suggests that an asymptotic model may have been more appropriate. It is unclear whether the linear model form that we report would hold for quaking aspen on xeric sites. As most quaking aspen stands in the Sierra Nevada are found on mesic sites, however, a linear model seems to best explain the diameter-SLA relationship for Californian aspen. Specific application of the pipe model theory indicates sapwood cross-sectional area would provide better predictions of tree leaf area. Sapwood/heartwood differentiation is very difficult in aspen and previous studies have used tree diameter or basal area [41]. The difference in sapwood to heartwood ratios, particularly in larger trees, is a likely source of error in predicting leaf area using only basal area or diameter.
LAI for sites sampled was relatively low compared to aspen sites in Alberta, Canada and Northern Wisconsin [23,42] which may lend support to the contention that sites where aspen is found in the Sierra may be marginal for the species. Low LAI values may indicate poor site quality that may not be able to support vigorous growth. Whether this is due to fire suppression [43], climate effects [13,44], or some other cause is unclear. It may simply be that sites in the Sierra Nevada, located at higher elevations on average and with lower water availability than those sampled by Ruark et al. and DesRochers and Lieffers [45,46] have always been marginal for quaking aspen. More data would thus be needed if there is a convincing case that quaking aspen is under unusual stress on these sites. LAI and SLA, as well as the root/shoot ratio for the stand, would be excellent physiological metrics to compare in order to begin to make that case [17].
Finally, we note that the model presented in this study between basal area and LAI is based on a limited number of stems. While it was not possible in this study, inclusion of more stems in the sample, perhaps by pooling data with other studies, would likely improve the accuracy of the model and would be a useful line of research in the future.
Root biomass and distribution: the efficacy of GPR
Coarse root density (kg m-3) peaked in biomass at 20-30 cm below the ground’s surface, with a monotonic decline in root density with increasing depth thereafter (Figure 6). GPR readings were able to obtain a reliable signal relatively deep in this soil profile, reaching a median depth of 1.7 m. In the Sierra Nevada, quaking aspen generally occurs on mesic sites, and this was also the case in this sample. Superficial availability of water may explain some of this pattern of root concentration on the soil surface. However, quaking aspen is generally considered a shallow-rooted species, with excavations on sites across North America confirming a shallow root distribution [11]. For example, excavated aspen in Alberta had root biomass only to 70 cm [23] while excavation in Utah revealed the majority of the root biomass concentrated in the upper 1.5 meters of the soil [47].
Fine roots
Fine root biomass was highly variable both within sites and between sites in Sierran ecosystems. Biomass estimated at the stand level was comparable to other values in the literature [48-50].
Model efficacy and comparisons to other studies
Based on this analysis, above-ground metrics, particularly basal area and LAI, do provide useful empirical models for estimating belowground biomass. Although sampling took place across a range of site conditions, there are likely regional and climatic effects that were not captured in this study.
A study of quaking aspen in Alberta reported a strong relationship between leaf area and root biomass [23], which we also found on our sites in the Sierra Nevada. The slope of the models was quite different, however, at 3.58 in Alberta and 1.27 in the Sierra, an indication that the LAI: coarse root relationship, at least, is regional. More importantly, the relationship is probably dependent on the disturbance history of the stand, as a stable relationship may be achieved between LAI and root biomass once the stand matures. The LAI present in quaking aspen immediately after stand-replacing disturbance is strongly influenced by stand health and carbohydrate storage prior to disturbance. DesRocher and Lieffer’s [46] study was in a stand that had recently undergone a stand replacement disturbance. Since LAI of young sprouts and LAI of mature stems probably have different relationships to root biomass (i.e. root biomass might in fact be a better predictor variable for LAI in recently-disturbed aspen stands) the relationship may not be comparable. As a result, the relationship in Alberta is more indicative of a stand immediately post-disturbance that maintained much of its predisturbance root biomass.
Fine roots are consistently hard to estimate using above-ground metrics [51-53]. Some new explanatory variables such as site class, and novel metrics such as a per-tree estimate of fine roots have been tried with greater success [51,54]. The strength of the relationship between LAI and fine roots in these Sierran stands was thus unusual. While the goodness of fit of the LAI to fine root model was somewhat lower than for other models used, this model explained more variation in the data than other models in the literature which have sought to predict fine root biomass empirically [51,55]. A variety of above-ground metrics are used in the major studies on fine root modeling above, but LAI estimated from below-ground metrics has not previously been attempted. Two important factors in the strength of the leaf area to fine root model are the physiology of the species and the spatial and temporal availability of the water resources to the trees in the stand. The physiology of the species will affect the form and the coefficient of the model used; for example, in a species with poor regulation of water loss would expect to allocate relatively higher fine root biomass per unit mass of leaf area compared to a species with better regulation of water loss. The species’ efficacy at water uptake, of course, would also affect that ratio.
If, however, trees on a given site are accessing groundwater at depths below those sampled for fine roots (often only sampled to 30 cm) then the principal function of superficial fine roots on that site may be the uptake of nutrients from the litter and organic soil. In locations with seasonal precipitation, root distribution and physiology would be predicted to optimize needs across the year or multiple years. Whatever the case, understanding water availability in time and space should make significant progress towards landscape-scale empirical leaf area/ fine root models when comparing the leaf area/fine root relationships of similar species.
Root/shoot ratio
The measured coarse root/shoot ratio for Sierran sites in this study ranged from 0.3 to 1.9, with the highest ratio in a recently disturbed, nearly pure quaking aspen stand. In general, we found the highest root/ shoot ratios in stands where aspen dominated by basal area, and where there had been recent disturbance. This is an expected consequence of quaking aspen’s suckering regeneration strategy: aspen roots persist after disturbance when the above-ground parts of the stand may be destroyed. The surviving root system provides the regeneration mechanism and carbohydrate reservoirs for new suckers after disturbance [56,57]. Other studies have found similarly high or higher root/shoot ratios in aspen, particularly in stands with active suckering [23].
The root-shoot relationship for aspen may also reflect the longterm carbohydrate balance in the stand, conifer competition and disturbance history, and the water balance for the whole aspen stand, as aspen roots graft [13,46].
Root biomass estimation and measurement continues to present a significant challenge in forested systems. The resources required to obtain unbiased data to a reasonable degree of precision are large. Consequently, these data are relatively limited. The results obtained here support the utility of the new techniques and methods to measure and estimate root biomass, including the use of GPR. They also support the efficacy of modeling fine and coarse root biomass using above-ground data, as a way to further improve estimates of NPP and biomass at large spatial extents.
Aspen regeneration and stand health assessments
In California, quaking aspen has been the subject of considerable concern as a species potentially in decline as a result of climate change and fire suppression [11,13,58]. Aspen, a shade intolerant, short-lived, and disturbance-adapted species are often overtopped by conifers in the absence of fire and clones may eventually become carbohydrate-starved and unable to regenerate after disturbance [20]. Aspen roots in declining stands are observed to exhibit decay and decline in root/shoot ratio as compared to healthy stands [13,46]. Encroachment in aspen stands by conifers is easily observed using imagery or a standard timber inventory. GPR and biomass surveys will probably have less application in this field. The one exception might be an assessment of root biomass coupled with measurements of carbohydrate reserves in roots to determine whether aspen is likely to regenerate vigorously after disturbance.
Implications for Modeling Root Biomass at the Landscape Level
Ground penetrating radar has applications for estimation and refinement of root shoot ratios, particularly in forested ecosystems such as quaking aspen where roots are fairly shallow. While GPR has limits in terms of the penetration depth, it also has clear advantages in its non-destructive nature and potential to allow repeated measurements in the same area.
The strong relationship between aspen LAI and below-ground biomass lends support for modeling above- and below-ground relationships to arrive at better estimates of below-ground biomass and root/ shoot allocation at the stand and landscape level. If similar relationships could be developed for other forested ecosystems, they would provide a basis to improve broad-scale below-ground biomass estimates across ecosystems. Challenges include the measurement or estimation of below- ground biomass of deeply rooted trees, as the theoretical lower limit for GPR measurement of something the size of a root is in the range of 4 meters deep under ideal conditions. There are also significant technical challenges to measuring LAI precisely with existing remote sensing techniques, or applying values estimated from direct measurement to a landscape scale [27]. Nonetheless, these methods provide advantages over excavation or the application of general root/shoot relationships based on very small, possibly biased samples. They also represent an opportunity to advance the field of allocation theory and estimates of the carbon reservoir in below-ground biomass. GPR scans should be used in combination with whole-tree root excavations and excavation at the plot level to overcome the shortcomings of each method used in isolation.
Conclusions
Quaking aspen leaf area shows good correspondence to both fine and coarse root biomass. Coarse root biomass is a key factor in aspen regeneration, and fine root biomass of key importance in water uptake and availability. The possibility of an assessment of root biomass using leaf area allows scalable assessments, from those based on ground-based measurement to those based on remotely-sensed imagery. Additionally, these results confirm the predictive capabilities of using basal area or tree diameter to predict leaf area in quaking aspen. These options add to the toolbox of land managers and those interested in forecasting the future effects of climate changes on production, carbon dynamics, and stand dynamics of quaking aspen in the Sierra Nevada.
Acknowledgements
We thank the National Science Foundation and Valentine Eastern Sierra Reserve for funding this research, and the USDA National Institute for Food and Agriculture (NIFA).
References
- Shinozaki K, Yoda K, Hozumi K, Kira T (1964) A quantitative analysis of plant form-the pipe model theory-futher evidence of the theory and its application in forest ecology. Japanese Journal of Ecology 14: 133-139.
- Dean TJ, Roberts SD, Gilmore DW, Maguire DA, Long JN, et al. (2002) An evaluation of the uniform stress hypothesis based on stem geometry in selected North American conifers. Trees - Structure and Function 16: 559-568.
- Waring R, Thies W, Muscato D (1980) Stem growth per unit of leaf area: a measure of tree vigor. Forest Sci 26: 112-117.
- O’Hara K (1988) Stand structure and growing space efficiency following thinning in an even-aged Douglas-fir stand. Can J For Res 18: 859-866.
- Vertessy RA, Benyon RG, O’Sullivan SK, Gribben PR (1995) Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Physiol 15: 559-567.
- O’Hara KL, Valappil NI (1999) Masam - a flexible stand density management model for meeting diverse structural objectives in multiaged stands. Forest Ecol Manag 118: 57-71.
- Waisel Y, Eshel A, Kafkafi U (2002) Plant Roots. The Hidden Half. New York, New York: Marcel Dekker, USA.
- Coutts MP (1983) Root architecture and tree stability. Plant Soil 188: 171-188.
- Mitchell SJ (2012) Wind as a natural disturbance agent in forests: a synthesis. Forestry 86: 147-157.
- Pregitzer KSK (2002) Fine roots of trees–a new perspective. New Phytologist 154: 267-270.
- Shepperd WD, Rogers PC, Burton D, Bartos DL (2006) Ecology, biodiversity, management, and restoration of aspen in the Sierra Nevada. Gen Tech Rep RMRS-GTR-178 Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station 122, USA.
- Berrill J-P, Dagley CM (2012) Geographic Patterns and Stand Variables Influencing Growth and Vigor of Populus tremuloides in the Sierra Nevada (USA). ISRN Forestry 2012: 1-9.
- Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, et al. (2012) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the United States of America 109: 233-237.
- Berrill JP, Dagley CM, Coppeto SA, Gross SE (2017) Curtailing succession: Removing conifers enhances understory light and growth of young aspen in mixed stands around Lake Tahoe, California and Nevada, USA. Forest Ecol Manag 400: 511-522.
- Frey BR, Lieffers VJ, Landhausser SM, Comeau PG, Greenway KJ (2003) An analysis of sucker regeneration of trembling aspen. Can J For Res 33: 1169-1179.
- Mitton JBJB, Grant MC (2009) Genetic variation and the natural history of quaking aspen. Bioscience 46: 25-31.
- Lieffers VJ, Landhäusser SM, Hogg EH (2001) Is the wide distribution of aspen a result of its stress tolerance? Sustaining aspen in western landscapes. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 311-324.
- Barnes BVB (1966) The clonal growth habit of American aspens. Ecology 47: 439-447.
- Byle NV De (1964) Detection of functional intraclonal aspen root connections by tracers and excavation. For Sci 10: 386-396.
- Shepperd WDWD, Bartos DL, Mata SA (2001) Above-and below-ground effects of aspen clonal regeneration and succession to conifers. Can J For Res 31: 739-745.
- Krasnow KD, Halford AS, Stephens SL (2012) Aspen restoration in the Eastern Sierra Nevada: effectiveness of prescribed fire and conifer removal. Fire Ecology 8: 104-118.
- Alexander L, Allen S (2013) Summary for Policymakers. In Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press, UK.
- DesRochers A, Lieffers VJ (2001) Root biomass of regenerating aspen (Populus tremuloides) stands of different densities in Alberta. Can J For Res 31: 1012-1018.
- Bloom AAJ, Mooney HA, Chapin F (1985) Resource limitation in plants - an economic analogy. Annual Review of Ecology 16: 363-392.
- Kenefic LS, Seymour RS (1999) Leaf area prediction models for Tsuga canadensis in Maine. Can J For Res 29: 1574-1582.
- O’Hara KL., York RA (2014) Leaf area development and crown architecture in a giant sequoia spacing study. Forest Science 60: 776-783.
- Bréda NJJ (2003) Ground-based measurements of leaf area index: a review of methods, instruments and current controversies. J Exp Bot 54: 2403-2417.
- Butnor JR, Doolittle JA, Johnsen KH, Samuelson L, Stokes T, et al. (2003) Utility of ground-penetrating radar as a root biomass survey tool in forest systems. Soil Sci Soc Am J 67: 1607-1615.
- R Development Core Team (2017) R: A Language and Environment for Statistical Computing.
- Adler D, Murdoch D (2013) Rgl: 3D visualization device system (OpenGL).
- Wickham H (2009) Ggplot2: elegant graphics for data analysis.
- Ryals R, Silver WL (2013) Effects of organic matter amendments on net primary productivity and greenhouse gas emissions in annual grasslands. Ecol Appl 23: 46-59.
- Metcalfe DB, Williams M, Aragão L, da Costa CL, de Almeida SS, et al. (2007) A method for extracting plant roots from soil which facilitates rapid sample processing without compromising measurement accuracy. New Phytol 174: 697-703.
- Quinn GP, Keough MJ (2002) Experimental Design and Analysis for Biologists, 1st ed. New York, NY: Cambridge University Press, UK.
- Bond-Lamberty B, Wang C, Gower S (2002) Aboveground and belowground biomass and sapwood area allometric equations for six boreal tree species of northern Manitoba. Can J For Res 32: 1441-1450.
- Kaufmann M, Troendle C (1981) The relationship of leaf area and foliage biomass to sapwood conducting area in four subalpine forest tree species. Forest Science 27: 477-482.
- Nowak J (1996) Estimating leaf area and leaf biomass of open-grown deciduous urban trees. Forest Science 42: 504-507.
- Dean TJ, Long JN, Smith FW (1988) Bias in leaf area—sapwood area ratios and its impact on growth analysis in Pinus contorta. Trees-Structure and Function 2: 104-109.
- Niinemets Ü (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research 25: 693-714.
- Kaufmann M, Edminster CB, Troendle CA (1982) Leaf area determinations for subalpine tree species in the central Rocky Mountains. Rocky Mountain Forest and Range Experiment Station, Fort Collins, CO RN 238: 7.
- Asherin LA, Mata SA (2001) Basic tree-ring sample preparation techniques for aging aspen. USDA Forest service proceedings RMRS-P-18: 347-354.
- Ruark G, Bockheim J (1988) Biomass, net primary production, and nutrient distribution for an age sequence of Populus tremuloides ecosystems. Can J For Res 18: 435-443.
- North M, Innes J, Zald H (2007) Comparison of thinning and prescribed fire restoration treatments to Sierran mixed-conifer historic conditions. Can J For Res 37: 331-342.
- Frey BR, Lieffers VJ, Hogg EHT, Landhäusser SM (2004) Predicting landscape patterns of aspen dieback : mechanisms and knowledge gaps. Can J For Res 34: 1379 -1390.
- Ruark GA, Martin GL, Bockheim JG (1987) Comparison of constant and variable allometric ratios for estimating Populus tremuloides biomass. Forest Science 33: 294-300.
- DesRochers A, Lieffers VVJ (2001) The coarse root system of mature Populus tremuloides in declining stands in Alberta, Canada. Journal of Vegetation Science 12: 355-360.
- Gifford G (1966) Aspen root studies on three sites in northern Utah. American Midland Naturalist 75: 132-141.
- Vogt K, Vogt D, Moore E, Fatuga B (1987) Conifer and angiosperm fine-root biomass in relation to stand age and site productivity in Douglas-fir forests. The Journal of Ecology 75: 857-870.
- Gower S (1987) Relations between mineral nutrient availability and fine root biomass in two Costa Rican tropical wet forests: a hypothesis. Biotropica 19: 171-175.
- Vogt K, Moore E (1983) Conifer fine root and mycorrhizal root biomass within the forest floors of Douglas-fir stands of different ages and site productivities. Can J For Res 13: 429-437.
- Chen W, Zhang Q, Cihlar J, Bauhus J, Price DT (2004) Estimating fine-root biomass and production of boreal and cool temperate forests using aboveground measurements: A new approach. Plant and Soil 265: 31-46.
- Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O’Hara J, et al. (1996) Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant and Soil 187: 159-219.
- Kurz W, Beukema S, Apps M (1996) Estimation of root biomass and dynamics for the carbon budget model of the Canadian forest sector. Can J For Res 26: 1973-1979.
- Vanninen P, Mäkelä A (1999) Fine root biomass of scots pine stands differing in age and soil fertility in southern Finland. Tree physiol 19: 823-830.
- Finér L, Ohashi M, Noguchi K, Hirano Y (2011) Factors causing variation in fine root biomass in forest ecosystems. Forest Ecol Manag 261: 265-277.
- Landhäusser SM, Lieffers VJ (2002) Leaf area renewal, root retention and carbohydrate reserves in a clonal tree species following above-ground disturbance. Journal of Ecology 90: 658-665.
- Shepperd WD, Smith FW (1993) The role of near-surface lateral roots in the life cycle of aspen in the central Rocky Mountains. Forest Ecol Manag 61: 157-170.
- Krasnow KD, Stephens SL (2015) Evolving paradigms of aspen ecology and management : impacts of stand condition and fire severity on vegetation dynamics. Ecosphere 6: 1-16.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi