Research Article, J Virol Antivir Res Vol: 10 Issue: 1
Development of SARS-CoV-2 Circulating Immune Complex Candidate, (CRCX) as a New Promising Vaccine Eliciting Broad Immune Response, Alternative to Conventional Vaccine with Potent Protection against SARS-CoV-2
Salah Sherif1*, Mubarki Abdula2, Fahmy Walid3, Ghaleb Heider4 and Hamed Ehab5
1Department of Bacteriology, Cairo University, Cairo, Egypt
2Department of Hematology, Armed forces Hospital, Taminah, Saudi Arabia
3Department of Immunology, Al Azhar University, Cairo, Egypt
4Department of Pharmacology, Cairo University, Cairo, Egypt
5Department of Internal medicine, National Research Center, Giza Egypt.
*Corresponding Author: Salah Sherif, Department of Bacteriology, Cairo University, Cairo, Egypt; E-mail: sherif64@mail.com
Received date: 22 January, 2022, JVA-22-50117;Editor assigned date: 25 January, 2022, PreQC No. JVA-22-50117 (PQ);Reviewed date: 08 February, 2022, QC No. JVA-22-50117;Revised date: 22 March, 2022, Manuscript No. JVA-22-50117 (R);Published: 30 March, 2022, DOI: 2324-8955/jva.1000672
Citation: Salah S, Mubarki A, Fahmy W, Ghaleb H, Hamed E (2022) Development of SARS-CoV-2 Circulating Immune Complex Candidate, (Crcx) as a New Promising Vaccine Eliciting Broad Immune Response, Alternative to Conventional Vaccine with Potent Protection against SARS-Cov-2. J Virol Antivir Res 11:3.
Abstract
The emergence of Severe Acute Respiratory Syndrome Corona Virus (SARS-CoV) in 2003 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in late 2012 [1,2]. We need to develop a universal vaccine able to boost immunity to track the coronavirus if some modifications in its structure have occurred by itself, this is what we are dealing nowadays with a virus capable of mutating its structure, while the immunity is standing still paralyzed facing the virus. Here, we report the preclinical trials of (CRCx 3) and (CRCx 2) vaccine candidate in inducing a high level of positive neutralizing antibodies as well as a cellular immune response in animal model to provide protection against SARS-CoV-2. three-dose immunizations using 0.25 ml of (CRCx) vaccines with a 25 mm needle I/M for three successive injection 7days interval provided highly efficient protection against SARS-CoV-2. In addition, (CRCx) vaccines candidate exhibits efficient productivity and good genetic stability for vaccine manufacture. These results support the further evaluation of (CRCx) in a clinical trial.
Keywords: SARS-CoV-2 vaccine; CD8+cytotoxic T lymphocyte; CD4+helper cells; Neutralization antibodies; Spike(S) glycoprotein; Immune complex
Introduction
COVID-19 is caused by a new positive-strand RNA coronavirus (SARS-CoV-2), which belongs to the Coronaviridae family, along with the severe acute respiratory syndrome (SARS) and the Middle East Respiratory Syndrome (MERS) coronavirus [1]. Their genome encodes several major structural proteins and non-structural, including (E), membrane, Spike (S), envelope (M), and Nucleocapsid (N) proteins, approximately 16 nonstructural proteins (nsp1–16), and five to eight accessory proteins [2]. Among them, the S protein plays an essential role in viral attachment, fusion, entry, and transmission. The S protein is the common target antigen for antibodies and vaccine development. After SARS-CoV-2 infection, different categories of antibodies are circulating in serum as Immunoglobulin-G (IgG), Immunoglobulin-M (IgM) and Immunoglobulin-A (IgA) mainly targeting two viral proteins, the S protein, and the Nucleo Protein (NP). S protein is abundant and highly expressed however, due to its biological function; it seems to be unlikely that antibodies against NP have neutralizing activity. Most of the recent vaccines for COVID-19 that employ injection of viral antigens or viral gene sequences aim to induce neutralizing antibodies against the viral Spike protein (S), preventing uptake through the human ACE2 receptor and, therefore [3,4] blocking infection. Neutralizing antibodies elicited by prior infection or vaccination are likely to be key for future protection of individuals and populations against SARS-CoV-2. Moreover, passively administered antibodies are among the most promising therapeutic and prophylactic anti-SARS-CoV-2 agents [5]. However, the degree to which SARS-CoV-2 will adapt to evade neutralizing antibodies is unclear. The previous reports provided us the evidence that neutralizing antibodies are potent enough to prevent viral infection, and strongly suggest that neutralizing-antibody-based vaccines could provide effective protection against coronavirus and the antibodies can constitute a promising cornerstone for the efficacy of an effective vaccine against any viral infection. But we concluded that neutralizing antibodies could carry a pathogenic role controversial to its protective one and the coronavirus can use these nAbs to mask a proportion of corresponding antigens in immune-complex form (Ag/nAbs) antigen/neutralizing antibody for a long time preventing its attacks by CD8+cytotoxic T cells [6]. Based on our assumption we discussed the possibility of developing a new in vitro vaccine comprising of peptide combination Ag/non-specific Abs as extrinsic immune-complex (ICA, ICB and ICC) completely dissimilar than the existing intrinsic circulating immune complex that comprise coronavirus antigen (M, N and S and its specific neutralizing antibody as (IC1, IC2 and IC3) as circulating immune complex. (CRCx) vaccine candidate is an immune peptide combination that was created to act as a novel therapeutical intervention for curing and preventing coronavirus infection. By coupling (Ag/nonspecific Abs) in one form differs from the already existed intrinsic Circulating Immune-Complex (CIC) that share (Ag/specific Abs). The changes the self-tolerance refers to the lack of immune response to the intrinsic CIC as a result of central (thymic selections) or peripheral (lack of co-stimulation) tolerance education and we found that if we injected an extrinsic noncomplex trigger, to some extent as the same intrinsic CIC, can initiate specific immune recognition against the tolerated CIC, these interactions can trigger a series of immunoregulatory responses, involving both innate and adaptive immune systems. According to the previously, this novel immune peptide complex CRCx designed to stimulate the attention of CD8+T-cells towards the intruder extrinsic CIC and the intrinsic CIC, to put the CD8+T-cell in a state of confusion to distinguish between the existing CIC and the similar intruder noncomplex CIC, These interactions can trigger a series of immunoregulatory responses, involving both innate and adaptive immune systems and including cross-presentation of antigens, activation of CD8+T-cells and CD4+T-cells, phagocytosis, complement-mediated antibody-dependent cellular cytotoxicity (ADCC) and Complement-Dependent Cytotoxicity (CDC), finally the Cytotoxic cells choose to destruct both of them [7,8]. This type of vaccine can achieve longer-lasting immune response and a boostable memory response against coronavirus as showed in Figures 1 and 2. Here, we report the study of vaccine candidate (CRCx) and show that its potency and safety in preclinical studies warrants further clinical evaluation [9].
Vaccine design and production
We isolated SARS-CoV-2 antigens and its specific neutralizing antibodies to develop preclinical in vitro neutralization and challenge models for an inactivated SARS-CoV-2 vaccine candidate, three formulated vaccine candidates (CRCx) used in this study (Figure 1):
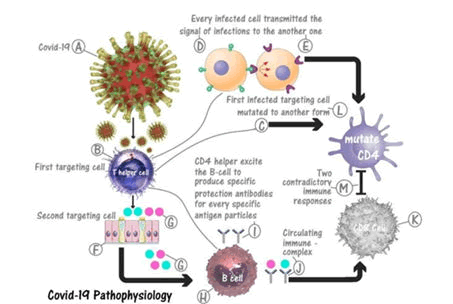
Figure 1: Describe the hypothetical mode of action for Coronavirus. were; (A) are the virus particles, (B) is CD4+T-cell as first susceptible cell, (C) shows the revolution of the CD4+T-cell to mutate to be unaccountable CD8+T-cell, (D) are the tendencies of the first susceptible infected cell to infects other healthy one and to be a newly form of CD8+T-cells, (E) is the newly formed infected first susceptible cell,(F) are the cells of the upper and lower respiratory tract as a second susceptible cells, (G) are the productions of the viral antigens particles from the second susceptible cells under the induction of first susceptible cells stimulus transmit ions signals, (H) CD4+T-cell stimulate the B-cells to produce negative neutralize coating antibodies to form complex with the antigens particles, (I) B-cells producing negative neutralizing antibodies,(J) are the formations of circulating immune complex comprising coronavirus antigens and its specific negative neutralizing antibodies, were (K) is the CD8+T-cells, (L) shows the inhibitors effect of these complexes in preventing the cytotoxic CD8+T-cells from attacking these viral particles, (M) is describe the interaction mechanisms that originate as a result of antagonists between the newly formed mutate CD4+T-cells and the normal CD8+T-cells (Figure 2).
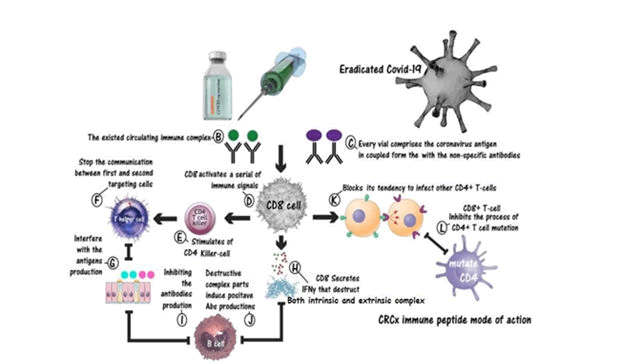
Figure 2: Shows the immune stimulating action of the CRCx3 vaccine. Were; a) is the CRCx immune peptide; b) Every vial comprises the coronavirus antigen in coupled form with the non-specific antibodies, c) The existed circulating immune complex; d) CD8+T-cell stimulated to induce a process of scanning and comparing between these non-complex combinations and the already existed during the vaccine injection, also CD8 stimulate a series of immune signals; e) Stimulation of CD4+Killer cell; f) Stimulation of CD4+Helper cell; g) CD4+T-cell send a signal that inhibit the formations of the coronavirus antigens; h) cytotoxic T-cells secrete IFNy that destruct the circulating immune complex and the intruder Non-complex one; i) The Destructive complex particles induce the positive antibodies productions from B-cells; j) Blocks the tendency of CD4 to infect other cell and (L) CD8+T-cells inhibits the process of CD4+T-cell mutation [10].
Vaccine A compositions: (crcx3)
Vaccine A formulated in three single dose vials containing 0.75 ml of injectable solution. Were a 0.75 ml of the first dose of (CRCx) vaccine contains 25 μg of SARS-CoV-2 spike protein (S1 subunit), and 40 μg of anti-nucleocapsid antibodies and Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride [11]. 0.75 ml of the second dose of (CRCx) vaccine contains 25 μg of nucleocapsid antigen, 40 μg of anti-membrane antibodies dissolved in human albumin, and Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride 0.75 ml of the third dose of (CRCx) vaccine contains 25 μg of membrane antigen and 40 μg of anti-spikes (S1 subunit) antibodies and Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride to be ready for immunizing the animal model groups with these non-immune-complexes to induce the production of positive antibodies. The vaccine does not contain any stabilizer or preservative [12].
Vaccine B compositions: (CRCx2)
Vaccine B formulated in two single doses vials containing 0.75 ml of injectable solution. Were a 0.75 ml of the first dose of (CRCx) vaccine (B) contains 25 μg of Spikes antigen (S1, S2 subunits), 40 μg of anti-spikes antibodies (S1, S2 subunits) and Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride. 0.75 ml of the second dose of (CRCx) vaccine (B) contains 25 μg of nucleocapsid antigen, 40 μg of anti-nucleocapsid antibodies Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride [13].
Immunogenicity of (CRCx)
Animal study
60 female and male mice of Six to eight-weeks-old, were housed in cages covered with barrier filters in an approved biosafety level 3, All animals participate in this research are in good health and are not involved in other experimental procedure [14]. All animals were allowed free access to water and diet and provided with a 12 h light/dark cycle (temperature: 18-28°C, humidity: 40%–70%). The mice were bred and maintained in specific pathogen free environment at the Laboratory Animal Center. they were classified into 12 groups five mice for each (the first six groups were designed to study the safety and efficacy of the vaccine candidate (A and B) as a preventive vaccine. group D as placebo, while the later six groups (E, F, G and H) were designed to evaluate the safety and efficacy of the vaccine candidate (A and B) as a therapeutic vaccine [15].
To assess the immunogenicity of (CRCx) as prophylactic vaccine candidate
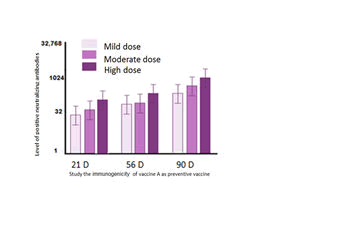
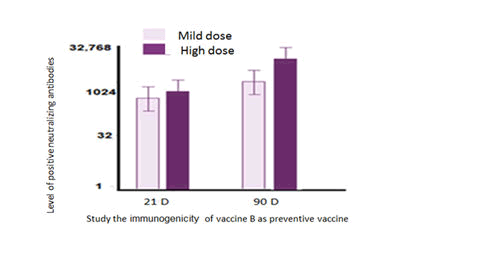
BALB/c mice were injected with different immunization programs and various doses (0.25, 0.35, 0.40 ml/dose) of vaccine A, B mixed with Fc of IgG mouse anti-Human IgM as adjuvant immunogen dissolved in human albumin, phosphate buffer and Na. chloride adjuvant. We study the immunogenicity for vaccine A (CRCx3). In the first-dose immunization group (vaccine A trail), mice were intraperitoneally administered a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) dose of (CRCx) at day 0, and the levels of neutralization antibody (NAb) at 7, 14, 21, and 28 days after injection were evaluated. Also mice were intraperitoneally administered a high (0.40 ml/dose) and low (0.25/dose) dose of (CRCx3) at day 0, second dose at day 7 and the levels of neutralization antibody (NAb) at 7, 14, 21, and 28 days after injection were evaluated, mice groups also were intraperitoneally administered a high (0.40 ml/dose) and low (0.25/dose) dose of (CRCx3) at day 0, second dose at day 7 and the third one at D14 the levels of neutralization antibody (NAb) at 7, 14, 21, and 28 days after injection were evaluated, Figure 3. We study the immunogenicity for vaccine B (CRCx2). mice were intraperitoneally administered a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) dose of (CRCx) at day 0, and the levels of neutralization antibody (NAb) at 7, 14, 21, and 28 days after injection were evaluated, also mice were intraperitoneally administered a high (0.40 ml/dose) and low (0.25/dose) dose of (CRCx3) at day 0, second dose at day 7 and the levels of neutralization antibody (NAb) at 7, 14, 21, and 28 days after injection were evaluated (figure 3).
Mouse neutralization antibody (NAb) levels with one-dose shot (D0) immunization of CRCx3 vaccine candidate (A). Mice were injected intraperitoneally or intramuscular with a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) at day 0 of vaccine candidate, and the NAb levels at 7 days, 14 days, 21 days, and 28 days after the first immunization were tested by the microtitration method (n=10). Mouse neutralization antibody (NAb) levels with two-dose shots immunization of CRCx3 vaccine candidate (A). Mice were injected intraperitoneally or intramuscular with a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) at day 0 and D7 of vaccine candidate, and the NAb levels at 7 days, 14 days, 21 days, and 28 days after the first immunization were tested by the microtitration method (n=10). Mouse Neutralization Antibody (NAb) levels with three-dose shots immunization of CRCx3 vaccine candidate (A). Mice were injected intraperitoneally or intramuscular with a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) at day 0, D7 and D14 of vaccine candidate, and the NAb levels at 7 days, 14 days, 21 days, and 28 days after the first immunization were tested by the microtitration method (n=10) [16] (Figure 4).
In group E and F, tested by the microtitration method (n=10). The level of NAb was evaluated at day at day 21, 28, 56 and 90. The group E showed marked progress in eliciting Nab with a high dose more than the titer of NAb in group F that immunized with mild dose. The results of vaccine A trails in transgenic mice model showed that the seroconversion rate in the high and middle, reached 100% at 21 days after immunization, and 60% with using low dose and the immunization effect was time dependent. The NAb levels at 7, 14, and 21 days in the moderate and high dose groups show significant variation, and a significant variation between 21 and 28 days was observed. In the high dose group, a significant variation only was observed between 14 and 21 days [17]. When we tested different immunization programs (D0, D7, and D14)/7D intervals in which three immunizations were applied at D0, D7, D14 intervals, respectively, were administered. The seropositivity of the high-, medium-, and low-dose groups from all three immunization programs reached 100% at 21 days after the third immunization and 76% after second immunization and 45% after first immunization. The immunogenicity of the three-dose immunization program was significantly higher than that of the one-dose and two dose immunization programs. The results showed that the three-dose (D0/D7/D14) immunization program resulted in higher NAb levels than the one and two dose programs in all groups at days 28 D moreover, we analyzed the NAb levels in mice with high, middle, and low doses of vaccine following the one-dose (D0), two-dose (D0/D21), and three-dose (D0/D7/D14) immunization programs and checked the NAb levels at 28 days after the first immunization to maintain the same starting and ending points. The results showed that the immunogenicity of the three-dose (D0/D7/D14) immunization program was higher than that of both the one-and two-dose programs. The results of vaccine B trails in mice model showed that the seroconversion rate in the high, reached 100% at 14 days after immunization, and 70% with using low dose and the immunization effect was time dependent [18]. The NAb levels at 7, 14, and 21 days in the high dose groups show no significant variation, and a significant variation between 21 and 28 days was observed. When we tested different immunization programs (D0, D7) 7D intervals in which two immunizations were applied at D0 and D7 respectively, were administered [19]. The seropositivity of the high and low dose groups from all two immunization programs reached 100% at 21 days after the second immunization and 55% after first immunization. That the seroconversion rate in the high and low-dose groups reached 50% at 7 days after immunization, and the immunization effect was time dependent. The NAb levels at 7, 14, and 21 days in the low dose group show no significant variation, whereas a significant variation between 21 and 28 days was observed. In the high-dose group, a significant variation only was observed between 7 and 14 days. At D24 (10 days after the third immunization with using vaccine A (first sex groups) and the second immunization with using vaccine B (second sex groups), all mice groups were intratracheally challenged with l06 TCID50 of SARS-CoV-2 per mice. Body temperatures of both the vaccinated groups and placebo group fluctuated within the normal range after virus challenge from 0 to 7 days post inoculation (dpi). Moreover, Blood was collected, and serum biochemical parameters were monitored at different time points after vaccination and challenge with living virus remained [20]. The protective efficacy of (CRCx3) and (CRCx2) against SARS-CoV-2 challenge at 14 days after third immunization was evaluated in transgenic mice. Changes in clinical signs (temperature, C) were recorded. Viral loads in throat (D) and anal (E) swabs obtained from mice at 3-, 5-, and 7-days post inoculation. Were determined by real-time PCR. All placebo mice showed and maintained a high viral load during the whole evaluation period after virus challenge by both throat and anal swabs. In contrast, the viral load in the throat swabs of the low-dose group peaked (6.10 log10 copies/mL) at 5 dpi and then decreased to 1.05 log10 copies/at 7 dpi, which was significantly lower than that of the placebo group. Among the five mice in the low-dose group, three showed a nondeductible viral load at 7 dpi. The throat swabs of all five mice in the high-dose group were negative for viral load. Moreover, no viral load was detected in the anal swabs of two (out of five) mice in the high-dose group. At 7 dpi, all animals were euthanized to determine the viral load in the lung tissue and for pathological examination. No mice in the low-dose and high-dose groups had a detectable viral load in any lung lobe, which was significantly different from the results in the placebo group. In the placebo group, a high viral load was detected in the left lower lung, right lower lung, and right accessory lung and the pathological histology analysis results showed severe interstitial pneumonia [21]. To note, only 3 of 7 sections of the lung lobes were detected to have infection in the placebo group, possibly because the virus infection in the lung lobes is dynamically changing. Furthermore, all mice that received vaccination showed normal lung with focal mild histopathological changes in few lobes, demonstrating the (CRCx) vaccination could efficiently block the infection of SARS-CoV-2 and COVID-19 disease in mice. At 7 dpi, the mice treated with placebo produced low-level NAb with a titer of 1:16, whereas the NAb levels of the vaccinated mice were highest at 1:2,048 (average 1:860) in the high-dose group and 1:1,024 in the low-dose group (average 1:512). Taken together, all these results demonstrated that both low-dose and high-dose (CRCx) conferred highly efficient protection against SARS-CoV-2 in mice [22].
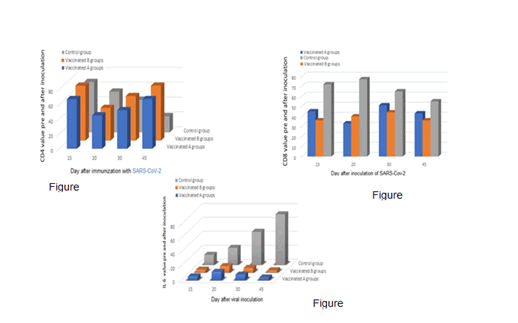
To assess the immunogenicity of (CRCx3) and (CRCx2) as therapeutic vaccine candidate: All mice groups (G, H, I, j, k and L) were intratracheally challenged with l06 TCID50 of SARS-CoV-2 per mice. Body temperatures of all groups and placebo group were measure after virus challenge from 0 to 7 days post inoculation (dpi), Blood was collected, and serum biochemical parameters and immunological markers were monitored at different time points, Human anti CD40, Anti-CD8, CMV IgG, PAI1 Human Plasminogen Activator Inhibitor 1 (PAI1), CD4 T-cell count, CD8 T-cell count, D-Dimer, Inflammatory array (quantitative) IL-1 alpha, IL-1 beta, IL-4, IL-6, IL-8, IL-10, IL-13, MCP-1, IFN-gamma, TNF alpha, C Reactive Protein, LDH Assay Kit/Cardiac Troponin I enzyme, Lactate Dehydrogenase, Serological detection for covid-19 IgG, IgM in serum samples, Nasotracheal swap for detection of Qualitative SARS-CoV-2 RNA, at D7 after virus inoculation and at D28 after immunization with (CRCx 2) and (CRCx 3) vaccine candidate, Mice of groups G, H and I were immunized intraperitoneally and intramuscularly with (CRCx3) at three doses (0.25, 0.35, 0.40 ml/dose using a syringe with a 25 mm needle with a high (0.40 ml/dose), middle (0.35/dose), or low (0.25/dose) dose of at day 7, second dose at day 14 and the last dose at day 21, 7D interval, while groups (J and K) were immunized intraperitoneally and intramuscularly with the (CRCx2) for two doses (0.25,0.40 ml/dose using a syringe with a 25 mm needle with a high (0.40 ml/dose) and low (0.25/dose) dose of at D7, second dose at day 14, 7D interval, group L as placebo. All the transgenic mice in groups (G, H, I, j, k and L) showed weight lost, became lethargic, and developed ruffled fur, a hunched posture, and rapid breathing. Signs of pneumonia marked elevation in body temperature, and mostly all animals showed nervous manifestations, a marked elevation in all biochemical and biological markers at the end of D6 after challenged with l06 TCID50 of SARS-CoV-2 [23]. The protective efficacy of (CRCx 3) and (CRCx 2) vaccine candidate against SARS-CoV-2 challenge at 14 days after second immunization was evaluated. In groups (G,H and I) with using of (CRCx 3) vaccine candidate, about 80% of viral signs and symptoms disappeared at D14 with using low and moderate doses, and reached to 100%, Viral loads in throat and anal swabs obtained from mice at D14 were decreased from 4.87 log10 copies/mL) at D7 to 0.5 log10 copies which was significantly lower than that of the placebo group (L) that recorded (6.4 log10 copies/mL) at D7, at the end of D28 no viral load was detected and all biochemical and biological markers dramatically decreased to above normal values. While the high dose group showed complete recover from all signs and symptoms, the viral load with real time PCR recorded (zero log10 copies/mL), marked adjusting for all biochemical and immunological markers at D21 to D28 [24]. with using of vaccine candidate (CRCx 2) in group I, J against SARS-CoV-2 challenge at 14 days after second immunization was evaluated, a significant positive result was detected directly from D7, all symptoms and signs completely disappeared with using high or low doses vaccine, no viral load was recorded at D14,21 and 28. All biochemical and immunological markers dramatically decreased to normal values with high dose group from D21 to 28 while the low dose group showed elevations in some immunological and biological markers at D28, the results in immunological markers were showed in (Figure 5).
We first performed a single intramuscular injection experiment in rats to evaluate the acute toxicity of CRCx. In this study, 20 rats were divided into two groups (n=10, 5/gender) and intramuscularly injected with 2x doses (0.6, 0.8, 1.20 ml/dose) of CRCx and physiological saline as the control. After inoculation, all rats were continuously observed for 14 days and euthanized at day 15 to assess systematic anatomy and for general observation [25]. No cases of death or impending death or obvious clinical signs were observed in any of the four groups over 14 consecutive days after vaccine inoculation. Moreover, there was no significant difference in weight or feeding state between the experimental groups and control groups. No histopathologic changes were observed after euthanasia. Notably, the Maximum Tolerated Dose (MTD) used for a single intramuscular injection in rats was 1.2 ml/rat, which is equivalent to 6 times the dose in humans, indicating the potential good safety of CRCx in humans [26]. Systemic anaphylaxis due to CRCx was subsequently evaluated by intramuscular and intravenous injections in guinea pigs. Twenty-four male guinea pigs were divided into 4 groups (6/group), a negative control group (physiological saline), a positive control group (human blood albumin, 20 mg/sensitization,40 mg/stimulation), a low-dose group (0.1x dose/sensitization, 0.2x dose/stimulation), and a high-dose group (1x dose/sensitization, 2x dose/stimulation). Sensitization was performed on D1, D3, and D5 [27]. The first stimulation (intravenous excitation via the foot) for 3 (out of 9) guinea pigs from each group was performed at D19, and secondary stimulation of the remaining animals of each group (6/9) was performed at D26. The results showed no abnormal reactions during the sensitization period by clinical observation and measurement of the body weights of the guinea pigs. No allergic reaction symptoms were found in the negative control group or experimental group on D19 or D26. The anaphylaxis of the positive control group was highly positive (1/6 animals were positive, 3/6 animals were strongly positive, and 2/6 animals were extremely positive). In sharp contrast, in the low and high-dose groups, no allergic reactions at D19 and D26 were found, and the allergic reactions [28].
Conclusion
The Development of preventive and therapeutic vaccine with high immunogenicity and safety is crucial for control of the global COVID-19 pandemic and prevention of further illness and fatalities. Different types of conventional vaccines inter the race aiming to gets a highly and safely results, one of them use whole viruses to trigger an immune response subunit vaccines use pieces of the pathogen often fragments of protein to trigger an immune response nucleic acid vaccines use genetic material either RNA or DNA to provide cells with the instructions to make the antigen. In the case of COVID-19, this is usually the viral spike protein. Once this genetic material gets into human cells, it uses our cells' protein factories to make the antigen that will trigger an immune response. The advantages of such vaccines are that they are easy to make, and cheap. Since the antigen is produced inside our own cells and in large quantities and the Viral vector vaccines also work by giving cells genetic instructions to produce antigens and it has been reported that the probabilities of reinfection after the use of vaccines with their diversity have not been resolved or prevented, also more reports were recorded the possibilities of growing a serious side effects after the first and second doses like; Thrombosis with Thrombocytopenia Syndrome (TTS) after Johnson and Johnson’s Janssen COVID-19 vaccination, myocarditis, or pericarditis among people ages 30 and younger who received COVID-19 vaccine. Most cases have been reported after mRNA COVID-19 vaccination (Pfizer-BioNTech or Moderna), particularly in male adolescents and young adults, in the United States from December 14, 2020, through October 6, 2021. During this time, VAERS received 8,638 reports of death (0.0021%) among people who received a COVID-19 vaccine, severe allergic reactions, including anaphylaxis, can occur after any vaccination. The most majority of approved vaccines were traditionally focused on the induction of strong protective neutralizing antibodies against the target pathogen, thus aiming to confer sterilizing immunity in vaccinated individuals and provide long-term immunity to protect the body from the risk of infection or recurrence. According to our postulation, the broadly neutralizing antibody that are generated during vaccination, exist in two forms, a bound positive form where it effectively masks a proportion of corresponding coronavirus antigen structural and a nonstructural protein in complex form prevents its elimination, controlling its activities and a free negative form which is a non-functional non neutralizing abs. This immune complex formation can explain the cause of persistence of the viral infection. The previous reports denoted to the importance of immune complex as inflammatory mediator’s stimulants, Immune-complex rises when the body's immune system generates antibodies against antigenic determinants of host or foreign substances that recognize and bind to the antigen molecules an immune-complex is formed which comprises this neutralizing Ag/Abs complex. Normally, insoluble immune complexes that are formed are cleared by the phagocytic cells of the immune system, but when an excess of antigen/antibody are present, the immune complexes are often deposited in tissues, where they can elicit complement activation, localized inflammation resulting in the generation of tissue lesions in a variety of autoimmune diseases, exacerbating disease pathology, we believe that our cells accumulate cumulative compounds or complex inside or outside it and these accumulations may increase to the point where the possibility of cellular asphyxiation may exist depending on the levels of accumulations, likening the perception which narrows the endothelium of arteries and veins leading to blood clots, this raise up to ask our self, why our cells produce these immune complexes and how these complexes could the main cause by which the coronavirus can target us? Seemingly our cells are on constant need for defense thus they produce many of emergency antibodies that becomes unconsciously excited due to the repetition of multiple stimulants, so immune cells initiate the production of these emergency negative antibodies to couple the stimulant to mitigate their impact but they do not produce these immune complex to get rid of the stimulant but unfortunately to create a bond which turns to a chronic state and the more the body is to produce these immune complex the more the severity of any invader will become, we all have different levels of these emergency antibodies which make us a possible target for any stimulant that might be viral, the essence will be to prevent the formation of those irrational form of complexes to prevent the viral existence. In our published data, we are trying to understand the behavior and the way by which the neutralizing antibodies works and the reasons behind the abilities of our humoral immune cells to produce these positive or negative neutralizing antibodies response, believing that difference between antibodies for the same antigen may be the corner stone for producing efficient vaccine and also the reason behind the failure of many vaccines in inducing long immunity. Here, we report development of SARS-CoV-2 immune complex vaccine, candidate CRCx vaccine that can induce high levels of positive neutralizing antibodies and cellular immunity in animal models. CRCx is an immune peptide combination candidate act as a novel therapeutic and preventive for SARS-CoV-2 infection. CRCx has two candidate vaccines (CRCx 3 and CRCx 2). CRCx 3 comprising three doses for 14 days/7 days interval and CRCx 2 that comprising two-dose/7 day’s interval; both can induce a highly efficient protection against SARS-CoV-2 in without observable ADE or immunopathological exacerbation. The vaccine candidate was found to significantly reduce or nullify viral loads and bronchoalaveolar affection in animal models challenged with SARS-CoV-2 14 days after receiving the third dose of the vaccine candidate, as attested by viral load measurement in bronchoalaveolar lavage fluid, nasal swab, throat swab, and lung tissues at 7 days post-infection in the immunized animals. Furthermore, no signs of pneumonia were detected in histopathological sections of the vaccinated and subsequently virus-challenged animals. In the absence of an effective antiviral drug against SARS-CoV-2, vaccines with good potency and safety will be needed to effectively establish immunity in population. Based on the result presented here, a Phase I clinical trial of CRCx is currently in progress.
Consent for Publication
All authors consent for publication and approved the manuscript.
Conflict Interests
The authors declare that they have no competing interests.
References
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID 19–11 March 2020.
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020)Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. lancet 395: 565-574.
[Crossref] [Google Scholar] [Pubmed]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF, et al. (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nature Rev Immunol 20: 363-374.
[Crossref] [Google Scholar] [Pubmed]
- Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, Rubio-Neira M, Guaman LP, et al. (2020) Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn microbiol Infectious dis 98: 115094.
[Crossref] [Google Scholar] [Pubmed]
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, et al. (2009) The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol 7: 226-236.
[Crossref] [Google Scholar] [Pubmed]
- Song W, Gui M, Wang X, Xiang Y (20189) Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS pathogens 14: e1007236.
[Crossref] [Google Scholar] [Pubmed]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, et al. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260-1263.
[Crossref] [Google Scholar] [Pubmed]
- Draft landscape and tracker of COVID-19 candidate World Health Organization. Accessed May 18, 2021.
- Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO, et al. (2021) SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 6: 1-7.
[Crossref] [Google Scholar] [Pubmed]
- Zhu J, Warren JD, Danishefsky SJ (2009) Synthetic carbohydrate-based anticancer vaccines: the Memorial Sloan-Kettering experience. Expert Rev Vaccines 8:1399-413.
[Crossref] [Google Scholar] [Pubmed]
- Kohama H, Umemura M, Okamoto Y, Yahagi A, Goga H, et al. (2008) Mucosal immunization with recombinant heparin-binding haemagglutinin adhesin suppresses extrapulmonary dissemination of Mycobacterium bovis bacillus Calmette-Guerin (BCG) in infected mice. Vaccine 26: 924-932.
[Crossref] [Google Scholar] [Pubmed]
- Bertoletti A, Ferrari C, Fiaccadori F, Penna A, Margolskee R, et al. (1991) HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci 88(23):10445-10449.
[Crossref] [Google Scholar][Pubmed]
- Cheng L, Ziegelhoffer PR, Yang NS (1993) In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci 90: 4455-4459.
[Crossref] [Google Scholar] [Pubmed]
- Jackson DA, Symons RH, Berg P (1972) Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc Natl Acad Sci 69: 2904-2909.
[Crossref] [Google Scholar] [Pubmed]
- Mackett M, Smith GL, Moss B (1982) Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci 79: 7415-7419.
[Crossref] [Google Scholar] [Pubmed]
- Arepally GM, Padmanabhan A (2021) Heparin-induced thrombocytopenia: a focus on thrombosis. Arterioscler Thromb Vasc Biol 41: 141-152
[Crossref] [Google Scholar] [Pubmed]
- Brodard J, Kremer Hovinga JA, Fontana P, Studt JD, Gruel Y, et al. (2021) COVID‐19 patients often show high‐titer non‐platelet‐activating anti‐PF4/heparin IgG antibodies. J thromb haemost 19: 1294-1298.
[Crossref] [Google Scholar] [Pubmed]
- Greinacher A, Selleng K, Mayerle J, Palankar R, Wesche J, Reiche S, Aebischer A, Warkentin TE, Muenchhoff M, Hellmuth JC, Keppler OT (2021) Anti-SARS-CoV-2 spike protein and anti-platelet factor 4 antibody responses induced by COVID-19 disease and ChAdOx1 nCov-19 vaccination.
- Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, et al. (2021) Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 6: 1202-1206.
[Crossref] [Google Scholar] [Pubmed]
- Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, et al. (2021) Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol 6: 1196-1201.
[Crossref] [Google Scholar] [Pubmed]
- Panagiotou OA, Kosar CM, White EM, Bantis LE, Yang X, et al. (2021) Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Internal Med 181: 439-448.
[Crossref] [Google Scholar] [Pubmed]
- Torjesen I (2021) Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ 15: 372.
[Crossref] [Google Scholar] [Pubmed]
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, et al. (2020) The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine United States, December 2020. Morb Mort Wkly Rep 69(50):1922.
[Crossref] [Google Scholar] [Pubmed]
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, et al. (2020) The advisory committee on immunization practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine United States, 2020. Morb Mort Wkly Rep 69: 1857.
[Crossref] [Google Scholar] [Pubmed]
- Lambert PH, Louis JA, Izui S, Kobayakawa T (1978) Relevance of polyclonal antibody formation to the development of auto-immunity in particular relation to murine lupus. 6th Int Convoc Immunol 12-15.
- Shands JW (1973) Affinity of endotoxin for membranes. J Infect Dis 128: 197-201.
[Crossref] [Google Scholar] [Pubmed]
- Perrin LH, Oldstone MB (1977) The formation and fate of virus antigen-antibody complexes. J Immunol 118: 316-322.
[Google Scholar] [Pubmed]
- Woodroffe AJ, Border WA (1977) Theofilopoulos AN, Götze O, Glassock RJ, Dixon FJ, Wilson CB. Detection of circulating immune complexes in patients with glomerulonephritis. Kidney Int 12: 268-278.
[Crossref] [Google Scholar] [Pubmed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi