Research Article, J Spine Neurosurg Vol: 6 Issue: 3
Distinct Radioanatomic Features and Treatments for Spinal Epidural Arteriovenous Fistulae
Miyachi S1*, Mtsuo N1, Kawaguchi R1, Takayasu M1, Izumi T2, Matsubara N3, Ohnishi H3 and Hiramatsu R3
1Department of Neurosurgery and Neuroendovascular Therapy, Aichi Medical University, Yazakokarimata, Nagakute, Aichi, Japan
2Department of Neurosurgery, Nagoya University Graduate School of Medicine, Aichi, Japan
3Department of Neurosurgery and Endovascular Neurosurgery, Osaka Medical College, Osaka, Japan
*Corresponding Author : Dr. Shigeru Miyachi, M.D., Ph.D.
Neuroendovascular Therapy Center, Aichi Medical University, 1-1, Yazakokarimata,Nagakute, 480-1195 Aichi, Japan
Tel: +81-561-62-3311
Fax +81-561-63-2879
E-mail: neu137@osakamed.ac.jp
Received: May 27, 2017 Accepted: June 07, 2017 Published: June 15, 2017
Citation: Miyachi S, Mtsuo N, Kawaguchi R, Takayasu M, Izumi T, et al. (2017) Distinct Radioanatomic Features and Treatments for Spinal Epidural Arteriovenous Fistulae. J Spine Neurosurg 6:3. doi: 10.4172/2325-9701.1000271
Abstract
Spinal epidural arteriovenous fistula (SEDAVF) is a novel clinical entity that is distinct from spinal dural arterovenous fistula, although both diseases manifest as venous congestive myelopathy. We reviewed nine cases of SEDAVF to elucidate its pathogenesis and radioanatomic features and to optimize treatment strategy. All cases exhibited similar angioarchitecture, with a single shunt at the epidural venous plexus that drains into the intradural perimedullary vein and connects to the ventral spinal vein. Interestingly, all lesions were located on the ventral side of the lower lumber and sacral region, and shunts were located ventrally in association with the epidural pouch suspected to be isolated as extradural venous lake. This may be the result of the specificity of the feeding artery and a congenital shift in the distribution of the venous drainage system at the terminal film and conus. We explored two options for the treatment for SEADF: endovascular transarterial embolization and surgical shunt interruption. Among our patients, three were treated using the endovascular approach and six were treated with surgery. While both treatments were effective at improving symptoms, the delay between disease onset and diagnosis precluded a complete cure. Early diagnosis and adequate treatment based on precise knowledge of the angioarchitecture and pathogensis of SEDAVF is essential for improved outcomes.
Keywords: Spinal epidural arteriovenous malformation; Myelopathy; Angioarchitecture; Treatment; Embolization
Abbreviations
BBD: Bowel-Bladder Dysfunction; E: Endovascular Transarterial Embolization; S: Surgical Interruption Of The Shunt Evaluation Of Neurological Dysfunction; (Motor Dysfunction And BBD Was Based On The Aminoff-Logue Disability Scale); +++: Severe; ++: Moderate; +: Mild
Introduction
Spinal epidural arteriovenous fistula (SEDAVF) is a novel category of spinal arteriovenous shunt disease. Its clinical symptoms are similar to those of spinal dural AVF (SDAVF) resulting from congestive myelopathy, resulting in the frequent misdiagnosis of these diseases. Recent reports have revealed differences between these two disorders [1-5]. Typical and classical SDAVF is characterized by a single shunt on the dura at the penetration site of the emissary vein (EV) that drains into the spinal vein (SV). In contrast, SEDAVF is characterized by a single shunt at the epidural venous plexus that drains into the intradural perimedullary vein and connects to the spinal vein at the upper level. Here, we review several cases of SEDAVF to clarify its pathogenesis and radioanatomic features and discuss treatment strategies.
Materials and Methods
We treated 9 patients diagnosed with SEDAVF between 2007 to 2016 at Nagoya University, Osaka Medical College and Aichi Medical University hospitals. Cases of perimedullary AVF and paravertebral AVF were excluded. Patients with SDAVF were excluded based on meticulous radioanatomical study. The shunt point was defined by preoperative selective spinal angiography. In cases treated surgically, the diagnosis was confirmed in the surgical field.
Patients were treated with surgery or using an endovascular approach. Surgeries were performed in a hybrid operation room using angiography equipment. First, spinal angiography for the impaired artery was performed to confirm the target. Patients underwent laminectomy and microsurgical intradual interruption of the shunting vein by clip and/or coagulation while being monitored for somatosensory-evoked and motor-evoked potentials as well as using the ICG dying method. Complete obliteration of the shunt was confirmed via intraoperative selective angiography. Endovascular treatment was performed in the angiography room. After conventional spinal angiogram, a microcatheter was inserted into a feeder and advanced as close as possible to the shunt. A dilute n-butyl 2-cyanoacrylate (NBCA) mixture containing lipiodol was injected to penetrate the shunt point.
Results
Patients included six males and three females ranging in age from 49 to 84 years (mean age, 67.8 years). All patients presented with progressive myelopathy due to spinal congestion, including various degrees of paraparesis, monoparesis of the lower limb, sensory disturbance, and bowel/bladder dysfunction (Table 1). The degree of severity of these symptoms was evaluated based on the Aminoff-Logue Disability Scale [6]. The metameric level of the manifesting neurological symptoms largely corresponded to the extent of pathological change in cord swelling and hyperintensity determined by MRI T2-weighted imaging. The shunt point was located in the lumber region in seven patients (L1: 1; L2: 1; L3: 3; and L4: 4) and in the sacral region in two patients. Four of the lumber lesions were located on the left side. Eight of nine lesions were associated with the extradural pouch on the ventral side. Shunt flow penetrated the dura primarily at the ventral nerve root and drained into an abnormally expanded radiculmedullary/ perimedullary vein, then flowed upward on a ventral course and connected with the spinal venous system at the conus level. Six patients underwent direct surgical obliteration, and three received endovascular treatment. All shunts except one who underwent incomplete occlusion with failed embolization were successfully occluded. The symptoms improved to various degrees in seven patients; one patient experienced persistent deficits even after successful surgical obliteration.
| motorsensoryBBD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case No. | age | gender | laterality | shunt level | motor | sensory | BBD | treament | posope. clinical state |
| 1 | 84 | M | L | L2 | +++ | ++ | ++ | E | improvement |
| 2 | 65 | M | R | L3 | ++ | ++ | ++ | E | improvement |
| 3 | 65 | M | R | L4 | ++ | ++ | +++ | E | improvement |
| 4 | 49 | F | L | L1 | +++ | +++ | +++ | S | improvement |
| 5 | 73 | M | R | L3 | + | +++ | +++ | S | improvement |
| 6 | 69 | M | L | L4 | ++ | ++ | ++ | S | improvement |
| 7 | 70 | M | L | L3 | +++ | +++ | +++ | S | no change |
| 8 | 68 | F | R | sac | +++ | ++ | +++ | S | improvement |
| 9 | 67 | F | L | sac | ++ | ++ | +++ | S | improvement |
Table 1: Patient summary.
Representative Cases
Case 1
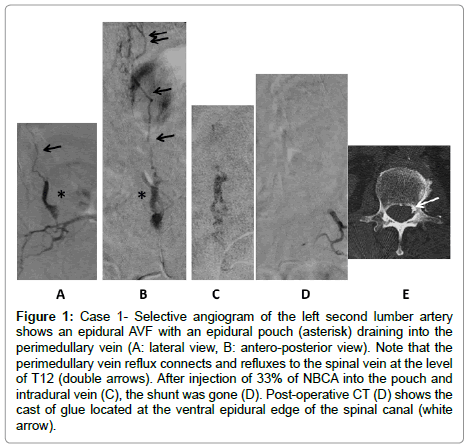
An 84-year-old male suffered from a progressive gait disturbance that was diagnosed as lumber canal stenosis. He could not stand unaided and presented paraparesis and dysesthesia of the lower limbs and severe bowel-bladder dysfunction. A spinal angiogram revealed an abnormal shunt at the level of left second lumbar artery. The radicular artery formed a pouch on the ventral side, and the shunt flow was directed through the abnormally developed perimedullary vein that was refluxed to the spinal veins at level T12. We navigated the microcatheter to the shunt point and injected a 33% NBCA mixture. Embolic material penetrated into the pouch and the intradural venous system. Postembolization angiogram indicated complete occlusion of the shunt. Marked improvement of the neurological symptoms was observed postoperatively, and the patient regained the ability to walk with a cane (Figure 1).
Figure 1: Case 1- Selective angiogram of the left second lumber artery shows an epidural AVF with an epidural pouch (asterisk) draining into the perimedullary vein (A: lateral view, B: antero-posterior view). Note that the perimedullary vein reflux connects and refluxes to the spinal vein at the level of T12 (double arrows). After injection of 33% of NBCA into the pouch and intradural vein (C), the shunt was gone (D). Post-operative CT (D) shows the cast of glue located at the ventral epidural edge of the spinal canal (white arrow).
Case 5
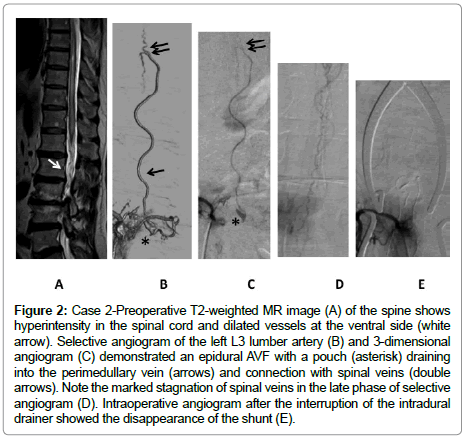
A 73-year-old male presented with a mild gait disturbance accompanied by storong hypalgesia of both legs with uriniary incontinence. Angiogram demonstrated a single shunt fed by the branch of the radicular artery at the right third lumber artery that connected to the perimedullary vein via a small extradural pouch on the ventral side. Upward perimedullary veins were connected and refluxed to the ventral spinal veins. After surgical interruption of the intradural entry of the drain, the patient’s sensory disturbance was much improved (Figure 2).
Figure 2: Case 2-Preoperative T2-weighted MR image (A) of the spine shows hyperintensity in the spinal cord and dilated vessels at the ventral side (white arrow). Selective angiogram of the left L3 lumber artery (B) and 3-dimensional angiogram (C) demonstrated an epidural AVF with a pouch (asterisk) draining into the perimedullary vein (arrows) and connection with spinal veins (double arrows). Note the marked stagnation of spinal veins in the late phase of selective angiogram (D). Intraoperative angiogram after the interruption of the intradural drainer showed the disappearance of the shunt (E).
Discussion
SEDAVF is a rare clinical entity among spinal arteriovenous shunt diseases, accounting for approximately 1.6% of all spinal arteriovenous malformations (AVMs) [7]. The clinical characteristics of SEAVF are highly similar to those of patients with SDAVF in terms of age at diagnosis, clinical manifestation, and MRI findings. Thus, diagnosis of SEAVF is very difficult and occasionally takes a long time to obtain the precise diagnosis.
The formation and progression of DAVF have been subject to much speculation. We support the hypothesis that over time, the external drainage routes at the initial shunt point occlude the emissary vein and venous sinus [8]. The resultant hemodynamic change leads to increasingly aggressive reflux into the cortical veins. As in cases of SDAVF, when the emissary vein is spontaneously occluded, the AV shunt begins to reflux into spinal veins, resulting in marked venous congestion. It is reasonable to conclude that SEDAVF progresses along a similar path. Rangel-Castilla et al. identified three types of SEDAVF: Type A, a SEAVF characterized by intradural refluxed drainage; and Types B1 and B2, which are SEAVFs characterized by a mass effect on the thecal sac and/or nerve roots, and SEAVFs in which no mass effect is observed [9]. According to this classification, SEDAVF may progress in stages to Type B2, then Type B1, and finally Type A. Clinical manifestations of SEAVF with neurological symptoms are typically Type A; all the patients in our series were classified as Type A.
SEDAVFs typically drain on the ventral side through a single radiculmedullary/perimedullary vein then causes reflux into the spinal veins. This pattern differs from that of SDAVF, which mainly refluxes to the posterior spinal veins though a direct connection. One of the causes of this distinction is the location of the feeder. Kiyosue et al. noted that the main feeder of SEDAVF is the dorsal somatic branch, which is located on the ventral side [5]. Furthermore, the large ventral emissary vein is mainly located at L2, where venous networks at the terminal film well typically develop on the ventral side and are rarely present dorsally [10].
Another characteristic that may be unique to SEDAVF is pouch formation at the extradural venous plexus. In these cases, the pouch might originally have served as a centrifugal drainage pathway but gradually occluded to form an isolated lake, as in cases of aggressive intracranial DAVF. The angioanatomic structure of the pouch and its maturation process correspond to the ventral type of DAVF at the cavernous sinus and anterior condylar confluence, based on the craniospinal epidural venous classification system [11,12]. The external venous plexus exists in the space between the dorsal vertebra and ventral dura matter but rarely extends laterally and dorsally, and its position may correlate with the ventral location of SEDAVF. However, the connection between the pouch and the intradual perimedullary veins is not always via the radicular vein but the rather per the dura, according to surgical data obtained for our series of patients. The pathological significance of these lacunar spaces and the mechanism of their formation and connection to the intradual venous system are complicated and remain controversial [13].
Previous studies of SEDAVF have included both congenital and acquired lesions [5,7,10]. It has been determined conclusively that paravertebral invasive AVF is located extradurally and drains to the external venous system, including the paravertebral venous plexus or azygos veins. However, these osseous or osetolytic high flow fistulae are often located in the cervico-thoracic area or exhibit a multi-metameric distribution, as in cases of neurofibromatosis type I or Cobb’s syndrome. These are completely congenital and genetic vascular disorders and as such should be differentiated from the cases of acquired developing SEDAVF that are the focus of this article.
SEDAVFs occasionally require rapid treatment when they are highly progressive and result in profound neurological deterioration. The goal of such treatment is to reliably occlude the shunt point or to drain the intradural space. For the treatment of SEDAVF, there are two useful options: a surgical and an endovascular approach. In our series, one case was treated with glue embolization, which failed to occlude the shunt completely. Fortunately, the decrease of shunt flow yielded a sufficient improvement in neurosurgical symptoms, although recurrence is highly likely. Previous reports have described the usefulness of endovascular embolization using liquid embolic materials [3,5,14]. Another report has recommended minimum invasive surgical treatment [15]. From a technical point of view, pinpoint and radical occlusion at the entry to the intradural drain is easier to achieve via surgical interruption than endovascular embolization because the glue used for embolization sometimes stops in the proximal feeder and fails to reach the shunt point. We therefore first attempt endovascular treatment as a less invasive therapy, and follow up with radical surgery in cases of failure.
Conclusion
SEDAVF exhibits peculiar radioanatomic features that differ from SDAVF, despite the similar clinical manifestations of the disorders. Most patients with SEDAVF in our series suffered from long-term progressive neurological symptoms due to misdiagnosis or the failure of the final diagnosis. SEDAVF is a radiologically treatable disease, although the rate of complete recovery is not high. Early diagnosis and adequate treatment based on a precise understanding of the angioarchitecture and pathogensis of SEDAVF is essential for improving patient outcomes.
Conflict of Interest
There is no conflict of interest for this paper in all authors.
References
- Silva N, Januel AC, Tall P, Cognard C (2007) Spinal epidural arteriovenous fistulas associated with progressive myelopathy. J Neurosurg Spine 6: 552-558.
- Krings T, Mull M, Bostroem A, Otto J, Hans FJ, et al. (2006) Spinal epidural arteriovenous fistula with perimedullary drainage. Case report and pathomechanical considerations. J Neurosurg Spine 5: 353-358.
- Nasr DM, Brinjikji W, Clarke MJ, Lanzino G (2017) Clinical presentation and treatment outcomes of spinal epidural arteriovenous fistulas. J Neurosurg Spine 26: 613-620.
- Burkhardt JK, Safaee MM, Clark AJ, Lawton MT (2017) Sacral epidural arteriovenous fistulas: imitators of spinal dural arteriovenous fistulas with different pathologic anatomy: report of three cases and review of the literature. Acta Neurochir 159: 1087-1092.
- Kiyosue H, Tanoue S, Okahara M, Hori Y, Kashiwagi J, et al. (2013) Spinal ventral epidural arteriovenous fistulas of the lumbar spine: angioarchitecture and endovascular treatment. Neuroradiology 55: 327-336.
- Aminoff MJ, Logue V (1974) The prognosis of patients with spinal vascular malformations. Brain 97: 211-218.
- Willinsky R, terBrugge K, Montanera W, Wallace MC, Gentili F (1993) Spinal epidural arteriovenous fistulas: arterial and venous approaches to embolization. Am J Neuroradiol 14: 812-817.
- Miyachi S, Izumi T, Matsubara N, Naito T, Haraguchi K, et al. (2011) Mechanism of the formation of dural arteriovenous fistula: the role of the emissary vein. Interv Neuroradiol 17: 195-202.
- Rangel-Castilla L, Holman PJ, Krishna C, Trask TW, Klucznik RP, et al. (2011) Spinal extradural arteriovenous fistulas: a clinical and radiological description of different types and their novel treatment with Onyx. J Neurosurg Spine 15: 541-549.
- Lasjaunias P, Berenstein A, Terbrugge K (2005) Spinal cord veins; Surgical Neuroangiography. Springer Velag, Berlin
- Geibprasert S, Pereira V, Krings T, Jiarakongmun P, Toulgoat F, et al. (2008) Dural arteriovenous shunts: a new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke 39: 2783-2794.
- Satomi J, Satoh K, Matsubara S, Nakajima N, Nagahiro S (2005) Angiographic changes in venous drainage of cavernous sinus dural arteriovenous fistulae after palliative transarterial embolization or observational management: a proposed stage classification. Neurosurgery 56: 494-502.
- Thron A, Krings T, Otto J, Mull M, Schroeder JM (2015) The Transdural Course of Radicular Spinal Cord Veins--A Microangiographical and Microscopical Study. Clin Neuroradiol 25: 361-369.
- Brinjikji W, Yin R, Nasr DM, Lanzino G. (2016) Spinal epidural arteriovenous fistulas. J Neurointerv Surg 2015: 012181.
- Najjar A, Zairi F, Sunna T, Weil A, Estrade L, et al. (2016) Minimally invasive approach for the treatment of lumbar epidural arteriovenous fistulas with intradural venous reflux. Neurochirurgie 62: 258-262.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi