none, J Clin Exp Onco Vol: 12 Issue: 5
Dysregulation of Mitochondrial Homeostasis and Mitophagy in Cancer Stem Cells: A Novel Approach For Cancer Therapy that Targets Cancer Stem Cells
Hari Prasad Sonwani1*and Aakanksha Sinha2
1Department of Pharmacology, Apollo college of Pharmacy Anjora Durg C.G, India
2Department of Pharmaceutics, University institute of Pharmacy, Raipur, Chhattisgarh, India
*Corresponding Author: Hari Prasad Sonwani
Department of Pharmacology, Apollo
college of Pharmacy Anjora Durg C.G, India
E-mail: harisonwani10@gmail.com
Received date: 13 November, 2023, Manuscript No. JCEOG-23-119946;
Editor Assigned date: 15 November, 2023, PreQC No. JCEOG-23-119946 (PQ);
Reviewed date: 30 November, 2023, QC No. JCEOG-23-119946;
Revised date: 07 December, 2023, Manuscript No. JCEOG-23-119946 (R);
Published date: 14 December, 2023, DOI: 10.4172/2324-9110.1000371.
Citation: Sinha A, Sonwani HP (2023) Dysregulation of Mitochondrial Homeostasis and Mitophagy in Cancer Stem Cells: A Novel Approach For Cancer Therapy that Targets Cancer Stem Cells J Clin Exp Oncol 12:5
Abstract
Cancer Stem Cells (CSCs) linked to chemoresistance and disease recurrence are the major obstacles that presently stand in the way of the effectiveness of current cancer treatment choices, notwithstanding the potential of cancer medicine. Better adaptability allows CSCs to develop and survive under stress associated with the tumor microenvironment. This is mostly due to processes related to mitochondrial dynamics, including the fission–fusion cycle and mitophagy. In addition, mitophagy and mitochondrial biogenesis work together to preserve mitochondrial homeostasis in CSCs, which is essential for the cells’ growth and upkeep as well as the control of the metabolic transition from glycolysis to oxidative phosphorylation. In this review, we go over the various facets of mitophagy, mitochondrial homeostasis, and mitochondrial dynamics and how they affect the behavior of CSCs during the development of cancer. Furthermore, the effectiveness of employing anti-CSC medications to pharmacologically target these cellular processes when combined with the chemotherapeutic medications that are currently on the market increases the patient’s chance of surviving aggressive cancer kinds.
Keywords: Cancer stem cells; Mitochondrial fission;
Mitochondrial fusion; Mitochondrial; Retrograde response;
Oxidative phosphorylation; Targeted cancer therapy
Introduction
Mitochondria is known as the “Cellular powerhouse,” mitochondria produce ATP through a process called Oxidative Phosphorylation (OXPHOS) [1]. They also work together to control a variety of cellular processes, including calcium homeostasis, cellular respiration, and programmed cell death [2,3]. Additionally, mitochondria enable tumor heterogeneity and cancer stemness by enabling cancer cells to proliferate and survive under various physiological and metabolic stressors [3,4]. Tumor-initiating cells, or Cancer Stem Cells (CSCs), are a sub-population of cancer cells that resemble stem cells in certain ways. These include the ability to self-renew and differentiate like normal stem cells, as well as a greater propensity for dormancy and resistance to treatment [5,6]. The quantity, shape, related signaling pathways, and turnover of injured mitochondria were all changed by CSCs. to reduce the generation of ROS and the apoptotic induction. It facilitates their growth and survival under harsh Despite the promise of cancer medicine, the main barriers to the efficacy of existing cancer treatment options are Cancer Stem Cells (CSCs) and their association with chemo-resistance and disease recurrence. Increased flexibility enables CSCs to grow and endure in the stressful conditions of the tumor microenvironment. The fission–fusion cycle and mitophagy, two mechanisms linked to mitochondrial dynamics, are primarily to blame for this. Furthermore, mitochondrial biogenesis and mitophagy cooperate to maintain mitochondrial homeostasis in CSCs, which is necessary for the growth and maintenance of the cells as well as the regulation of the metabolic switch from glycolysis to oxidative phosphorylation. In this overview, we discuss the many aspects of mitochondrial dynamics, homeostasis, and mitophagy and how these impact CSC behavior throughout cancer development.
Additionally, the efficacy of using anti-CSC drugs to pharmacologically target these biological functions stress settings, indicating one of the main causes of therapy failure. A constant cycle of fusion and fission occurs during mitochondrial dynamics, changing the morphology of the organelles from long, linked mitochondria (mitochondrial fusion) to rounded, fragmented mitochondria (mitochondrial fission). These dynamic changes result in the mixing and swapping of the mitochondrial content as well as cristae modification, which controls mitochondrial respiration and enables CSCs to respond to stress related to energy shortage, ultimately resulting in their survival [7]. Mitophagy, on the other hand, entails the direct turnover of damaged mitochondria [8]. This process keeps a balance with mitochondrial biogenesis to sustain mitochondrial homeostasis in CSCs and positively regulates CSC populations by directly increasing their stemness, growth, and proliferation [9,10]. Additionally, a decrease in mitochondrial in head or neck and lung cancer, mass also distinguishes CSCs from non-CSC counterparts [11,12].
In CSCs, dysregulation in any of these pathways that results in the accumulation of defective mitochondria causes cell death through apoptosis. Due to their demand for bioenergetics, CSCs prefer OXPHOS metabolism over glycolytic metabolism and mitochondrial dynamics are crucial in regulating this metabolic flipping [13]. Therefore, this field becomes more intriguing when the mitochondrial regulatory mechanisms that govern the quantity and cellular bioenergetics of CSCs as well as their impact on the present treatment regimen are identified. Medications that target these mitochondria-centric processes are thought to be viable anti-CSC medications that can reduce the complexity of relapses and treatment resistance. Thus, we have covered a variety of topics related to mitochondrial dynamics, mitophagy, and homeostasis in the mitochondria altering the behavior of CSCs in the genesis of cancer. By pharmacologically addressing these cellular processes, CSCs’ drug sensitivity is increased, resulting in novel cancer therapies that specifically target CSCs. Additionally, we have talked about how effective combination therapy is at improving patient survival for aggressive cancer types when anti-CSC medicines and other well-known chemotherapeutic agents are used (Figure 1).
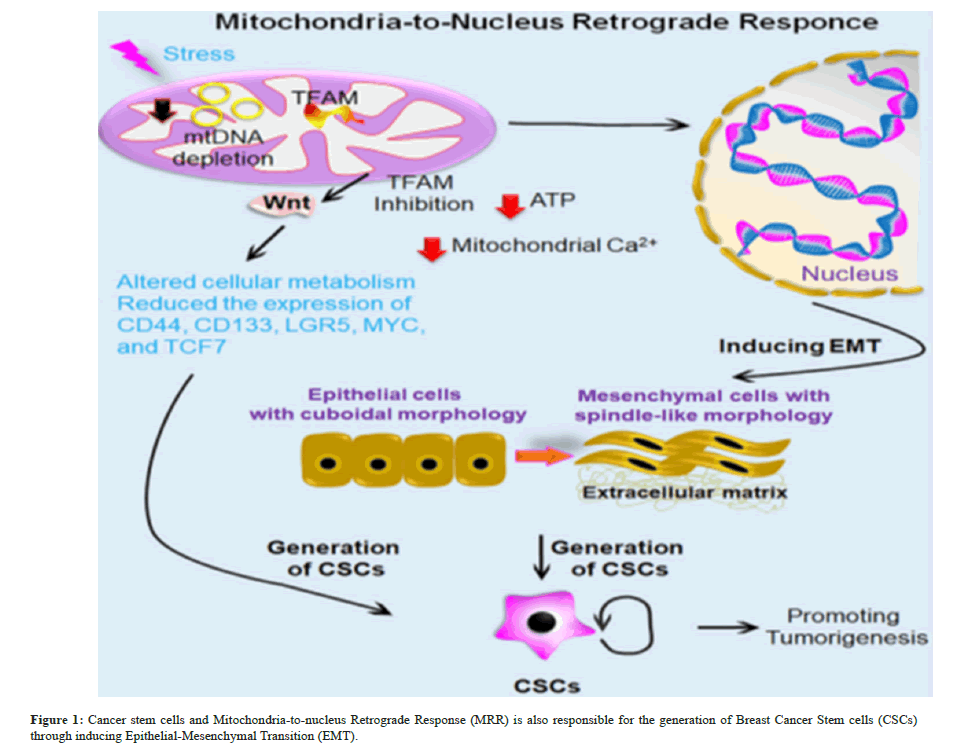
Reduction in mtDNA content activates calcineurin-mediated mitochondrial retrograde signalling pathway, which triggers EMT-like reprogramming to altered morphology as fibroblasts, loss of cell polarity, contact inhibition with increased migratory, and invasive phenotype. However reinstating the mtDNA content or inhibiting CnAa mRNA reversed the effect, suggesting the mechanistic role of retrograde signalling in Epithelial-Mesenchymal Transition (EMT). Mitochondrial Retrograde Response (MRR) also promotes tumorigenesis in colon cancer through regulating Wingless-related Integration Site (WNT) signaling. Moreover, Mitochondrial Transcription Factor A (TFAM) inhibition altered cellular metabolism and reduced the expression of WNT target genes associated with CSCs, such as CD44, CD133, LGR5, MYC, and TCF7 in colon cancer.
CSCs and the retrograde response of Mitochondria-Tontonuleus CSCs are an undifferentiated mass of cells that are found in the tumor tissue and have characteristics similar to those of stem cells, including the capacity for self-renewal and differentiation (Figure 1) [6,14]. Various approaches based based on the distinct characteristics of CSCs have been created to identify and enhance the CSC population in various cancer types. The methods are further divided into two categories based on the criteria for isolation. (i) Surface marker-based isolations like Fluorescence Activated Cell Sorting (FACS) and Magnetic Activated Cell Sorting (MACS) and (ii) Functional assay-based isolations (like spheroid formation assay, colony formation assay, Aldehyde Dehydrogenase (ALDH) activity, and side population assay) [15]. CSCs express distinct sets of surface markers according on their origin. Pancreatic CSCs, for instance, show various potential surface markers, including as CD44+, CD24+, and Epithelial Specific Antigen (ESA)+ CD133+, CXCR4+ ALDH1 high and c-Met+ [16-19]. Pancreatic cancer and CSCs continue to be a major source of disagreement because it appears that as the tumor grew, stem cell niche factor reliance decreased.
According to Seino et al., GATA6 is a crucial regulator of epithelial differentiation in this context as well as a regulator of WNT dependency in Pancreatic Ductal Adenocarcinoma (PDAC) [20]. Interestingly, none of the suggested surface markers can definitively separate a pure population of pancreatic CSCs, yet they are nonetheless widely employed since they provide solid evidence of a high level of pancreatic CSC enrichment. In addition to these surface markers, other signaling pathways such the Mammalian Target of Rapamycin (mTOR) and Nodal/Activin pathways control the stemness and ability of pancreatic CSCs to self-renew [21,22]. Because of their increased ATP Binding Cassette Subfamily G Member 2 (ABCG2) expression, CSCs show resistance to the majority of chemotherapeutic medications. (BCRP), which causes cancer cells to release their cytotoxic medications [23]. Similar to this, overexpressing ATP-binding Cassette Sub-family B Member 1 (ABCB1) Multidrug Resistance Protein 1 (MDR1) in CD44+ pancreatic CSCs resulted in resistance to gemcitabine; however, pharmacological blockage of the ABC transporter with verapamil restored the susceptibility of these resistant cells to gemcitabine [24].
Through signal transduction or membrane contact sites, mitochondria interact with other subcellular organelles, including peroxisomes, the ER, and the nucleus, to regulate immunological response, energy metabolism, and cellular turnover [25]. The Mitochondrial Retrograde Response (MRR), which occurs during stress, is the process by which mitochondria begin retro communicating with the nucleus [26]. The nucleus and mitochondria’s functional connection regulates the biosynthesis and operation of mitochondria. Three categories of mitochondrial stress are directly activated by retrograde signaling that promotes cellular transformation and cancer cell survival, such as calcium-calcineurin-mediated retrograde signaling, ROS-activated signaling, and Unfolded Protein Response (mtUPR)-activated signaling [27]. It’s interesting to note that via causing EMT, the MRR is also in charge of producing breast CSCs. Reduced levels of mitochondrial DNA (mtDNA) cause a calcineurin-mediated mitochondrial retrograde signaling pathway to be activated, which in turn causes human mammary epithelial cells to undergo EMT-like reprogramming to change their morphology into fibroblasts, lose their polarity, and experience contact inhibition. These changes result in an increased migratory and invasive phenotype.
Retrograde signaling may play a mechanistic role in EMT, as suggested by the effect that was reversed by increasing the amount of mtDNA or blocking CnAa mRNA [28]. By controlling WNT signaling, mitochondrial retrograde signaling also encourages the growth of tumors in colon cancer. Additionally, suppression of in colon cancer, WNT target genes such CD44, CD133, LGR5, MYC, and TCF7 were expressed less often and cellular metabolism was changed by Mitochondrial Transcription Factor A (TFAM) [29]. Another explanation for the diverse populations of cancer cells found inside a single pancreatic cancer tumor is EMT. According to Salnikov et al., hypoxia causes EMT in all tumor cell types and metastasis in pancreatic cancer stem-like cells [30]. In general, knowledge of the intricate mitochondrial biology of CSCs might be beneficial in creating a successful therapeutic strategy to eradicate this extremely drug-resistant tumor population that causes tumor relapse.
Before mitophagy begins, a necessary process in CSCs is the modulation of mitochondrial dynamics. Mitochondrial dynamics is a multiphase cellular process that maintains the functionality of the mitochondrial network by continuously varying the number and shape of mitochondria through a fusion and fission cycle [31]. The equilibrium between mitochondrial fission-fusion processes may be upset in cells under any kind of metabolic or external stress, which might impact the development and course of various cancer types [32]. Furthermore, the subcellular distribution of mitochondria and their capacity to regulate metabolic homeostasis and cellular energy are highly correlated with cancer metabolism, making them an invaluable resource and a special method for locating and eliminating CSCs. For instance, more broken-up mitochondria are observed during cell division or apoptosis-mediated cell death however in the event of food scarcity, elongated mitochondria are observed [33,34].
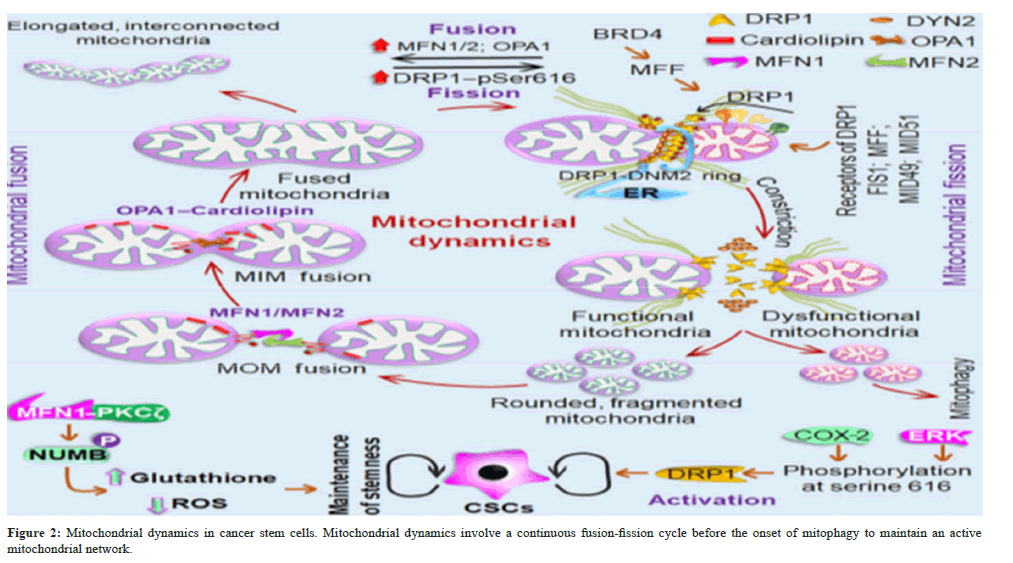
The mechanisms of mitochondrial fusion and how they affect CSCs in order to preserve the integrity of the mitochondrial genome, sustain mitochondrial homogeneity (i.e., structural and functional), and preserve the balance between energy production and cellular mass, mitochondrial fusion is required [35]. Three essential GTPases, namely the Optic Atrophy Protein 1 (OPA1), which acts on the Mitochondrial Inner Membrane (MIM) to coordinate the fusion event, and two Mitofusins, Mitofusin 1 (MFN1) and Mitofusin 2 (MFN2), which act on the Mitochondrial Outer Membrane (MOM), are primarily responsible for controlling the entire fusion event [36,37]. Furthermore, through the C-terminal heptad repeats, neighboring mitochondria may be pulled together and start to mix their lipid bilayers resulting in the formation of an intermolecular anti-parallel coiled coil and MOM fusion of MFN1 and MFN2 [38]. Fusion of MIM is largely controlled by OPA1 [39]. OPA1 couples MOM fusion with MIM fusion by forming intermembrane protein complexes with MFNs [40]. Lipid bilayers so combine in a coordinated manner to form a single, fused mitochondrial matrix that is encased in the MIM.
The primary protein that keeps the mitochondria in their proper form is called Mitofilin, or Mic60. The outer membranes of mitochondria lacking both MFN1 and MFN2 did not fuse together. Similar to this, in the absence of OPA1, cells are unable to complete mitochondrial fusion; instead, only MOM fusion can occur, which can be resolved by mitochondrial fission [41]. Because of the increased number of mitochondrial cristae and enlarged mitochondria resulting from mitochondrial fusion, complex dimerization of ATP synthase, component V of the Electron Transport Chain (ETC), indicates increased ATP production [33]. The ability to consume more oxygen and mitochondrial fusion control the stemness of cancer. Neural Stem Cells (NSCs) exhibited increased mitochondrial fragmentation in the absence of essential fusion proteins such MFN1/2, which resulted in a decrease in stemness and greater differentiation. Additionally, acute RNA interference (RNAi)-mediated silencing of OPA1 demonstrated comparable outcomes, such as a sharp decline in the development of primary and secondary neutrospheres [42]. Because it promotes GSH production to mediate EMT-associated stemness, mitosis is also crucial in deciding the destiny of stem cells. Additionally, the regulation of the miR200c–Par coactivator 1α (PGC1α)–MFN1 pathway, which is activated by MFN1 activation forming a complex with Protein Kinase-Cζ (PKCζ) and boosting NUMB phosphorylation for asymmetric cell division, is how EMT triggers mitochondrial fusion [43]. Therefore, specific suppression of the MFN1–PKC axis could be beneficial in eliminating EMT-related tumour stem cell populations. Even though enough findings are not yet proven connecting mitochondrial fusion with CSC behavior, the studies mentioned above on normal stem cells and its association with EMT enlighten the possibilities of mitochondrial fusion in CSCs. (Figure 2).
Three essential GTPases control the mitochondrial fusion, such as two mitofusins MFN1 and MFN2, that act on the MOM, and OPA1, on the MIM, along with a mitochondrial membrane lipid known as cardiolipin. This protein lipid interaction coordinates the fusion event, leading to elongated, interconnected mitochondria with mixed mitochondrial content. However, mitochondrial fission involves separating a damaged mitochondrial part from the active mitochondrial network through the coordinated action of two GTPases Dynamin-Related Protein1 (DRP1)-DYN2. DRP1 recruitment to the MOM through different receptor proteins (FIS1, MFF, MID49, and MID51), where GTP-dependent oligomerization of DRP1, causing the spiral constriction around the MOM. This event relies on both the action of MFF and actin filaments followed by the recruitment of Dynamin-2 (DYN2) to the MOM to complete membrane division. Mitochondrial fusion also regulates the stem cell fate through MFN1, which forms a complex with PKCζ promoting NUMB phosphorylation leads to asymmetric cell division to mediate EMT-associated stemness. Similarly, DRP1 also positively regulates cancer stemness when it is activated by Cyclooxygenase 2 (COX- 2) and Extracellular Signal-Regulated Kinases (ERK) dependent phosphorylation.
The mechanism of mitochondrial fission and how it affects CSCs, according to Bao et al., and Sun et al., there is an increase in cell migration, autophagy, tumor-associated macrophage infiltration, and the advancement of several cancer types due to mitochondrial fission [44,45]. Furthermore, maintaining healthy mitochondrial populations during the mitochondrial segregation of asymmetric stem cell division is similarly important for CSCs. First, the contraction of the mitochondrial tubule is caused by mitochondrial association with the Endoplasmic Reticulum (ER) [46]. This contraction is then followed by the recruitment of the large GTPase Dynamin-Related Protein 1 (DRP1) to MOM by the cytosolic protein through association with four single-pass MOM proteins, namely Mitochondrial Fission Factor (MFF), FIS1, MID49, and MID51, which function as DRP1’s receptor protein [47-51]. These protein receptors are required for DRP1-dependent fission since DRP1 lacks a certain lipid-binding domain. The process that drives mitochondrial fission, spiral constriction around the MOM, is brought about by GTP-dependent oligomerization following recruitment [52]. Actin filaments and MFF are necessary for the oligomerization process on mitochondria to begin in the ER [53]. To finish membrane division, Dynamin 2 (DYN2), another dynamin GTPase, is brought to the MOM [54]. It is also known that in cells lacking both DRP1 and DYN2, human FIS1 controls mitochondrial fission.
Human Mitochondrial Fission 1 protein (hFIS1) primarily blocks the fusion machinery by inhibiting MFN1, MFN2, and OPA1’s GTPase activity when they interact molecularly [55]. According to Kong et al., ATP availability and intracellular calcium levels are also necessary for mitochondrial fission [56]. Fission events are initiated by the calcium-dependent phosphatase calcineurin by dephosphorylating cytosolic DRP1, which results in its translocation into the mitochondria [57]. In CSCs of human prostate cancer, mitochondrial plasticity has been discovered to represent a novel anti-cancer target [58,59]. One of the Bromodomain and Extra- Terminal Domain (BET) proteins, Bromodomain-containing protein 4 (BRD4), upregulates MFF (DRP1 receptor) in prostate CSCs, hence initiating mitochondrial biogenesis and metabolic plasticity. Additionally, BRD4 inhibition, both genetically and chemically, stops MFF’s transcription activity, which inhibits mitochondrial fission and accelerates the wear and tear of prostate CSCs [58]. Another discovery is that, depending on the kind of cell, the mitochondrial fission protein DRP1 undergoes two site-specific phosphorylation’s.
In Brain Tumor-Initiating Cells (BTICs), DRP1 is phosphorylated at in bulk tumor cells, it exhibited phosphorylation at Serine 373, indicating its inactivation [60]. This indicates that Serine 616 leads to activation. Through site-specific phosphorylation at Serine 616, COX-2, an important regulator of cancer stemness, activates DRP1 [61,62]. Furthermore, it has been observed that pharmacological (using Mdivi-1) and genetic (using siDNM1L) inhibition of DRP1 has suppressed cancer stemness but had no effect on mitochondrial COX-2 activity, indicating that mitochondrial COX-2 is an upstream regulator of the DRP1-dependent mitochondrial fission in Nasopharyngeal Carcinoma (NPC) [63]. Another study found that in T-cell acute lymphoblastic leukemia, Mesenchymal Stem Cell (MSC)- triggered drug resistance is favored by regulating mitochondrial fission, which is facilitated by ERK1-mediated activation of DRP1. T-cell Acute Lymphoblastic Leukemia (T-ALL) cells and shields them from medications used in chemotherapy [64]. In addition to increasing drug sensitivity towards camptothecin in breast CSCs, specific inhibition of clusterin (a molecular chaperone) altered mitochondrial dynamics and caused necrosis [65,66]. Consequently, a more effective target for the targeted destruction of CSCs would be mitochondrial fission in CSCs (Figure 2).
A mechanism to remove aged or dysfunctional mitochondria in CSCs is called mitophagy. Mitophagy is a type of selective autophagy in which autophagic machinery eliminates damaged or malfunctioning mitochondria from cells [67,68]. Depending on the protein involved, mitophagy is classified into two subtypes: Receptor-mediated mitophagy and PTEN Induced Kinase 1 (PINK1)- Parkin-dependent mitophagy [69,70]. Regular mitochondrial activity causes PINK1 to undergo mitochondrial translocation. Once inside MIM, it is cleaved by Presenilin Associated Rhomboid-Like (PARL) protein, a serine protease [71]. However, depolarization or damage to the mitochondria prevents PINK1 from being cleaved, which leads to its accumulation on MOM [72]. Next, PINK1 enlists E3 ubiquitin.
Parkin ligase, causing it to be phosphorylated and activated. This, in turn, leads to the ubiquitination of many target mitochondrial proteins, which enables the mitophagic activation process [73].
However, in receptor-mediated mitophagy, different proteins with the LC3 Interacting region (LIR motif) present on the MOM, such as p53-Upregulated Modulator of Apoptosis (PUMA) autophagy, Activating Molecule Beclin 1-Regulator Autophagy (AMBRA1), B-Cell Lymphoma BCL-2-like 13 (Bcl2L13), BCL-2 and adenovirus E1B 19-kDa interacting protein 3, BCL2 Interacting Protein 3 (BNIP3), BNIP3-like (BNIP3L), FK506-Binding Protein 8 (FKBP8), FUN14 Domain-Containing 1 (FUNDC1), Nipsnap Homologue 1 (NIPSNAP1), NDP52, NBR1, Optoneurin (OPTN), p62, Tax1- Binding Protein 1 (TAX1BP1), and PUMA present on MOM; few proteins, such as Prohibitin-2 (PHB2) and lipid, such as cardiolipin present on MIM of depolarized mitochondria, interact with ATG8 family members (LC3s). Gamma-aminobutyric Acid Receptorassociated Protein (GABARAPs), as well as Abdrakhmanov et al., Panda et al., Villa et al., Yamano et al., enlisting mitophagosomes surrounding injured mitochondria [74-77]. Since successful mitochondrial fission is a necessary step for mitophagy because it separates the damaged mitochondrial portion from the active mitochondrial network, mitochondrial dynamics and mitophagy are interwoven [78,79].
In this regard, the mammalian mitophagy receptor FUNDC1 interacts with DNM1L/DRP1 and OPA1 to coordinate mitochondrial dynamics and mitophagy, as well as with LC3 to promote mitophagy [80,81]. PINK1, an additional mitophagy regulator, disrupts the antifission apparatus to validate the segregation of impaired mitochondria. DRP1 and PINK1 interact indirectly, increasing DRP1 activity [82]. Avoid It has recently been discovered that the Mitochondrial Fission Protein FIS1 and the protein Syntaxin 17 (STX17) interact. When FIS1 is not present, STX17 accumulates abnormally on mitochondria, which encourages mitophagy and self-oligomerization [83]. Furthermore, Leukemic Stem Cells (LSCs) have a regulated mitophagy status due to the action of FIS1 and AMP-Activated Protein Kinase (AMPK). Pei et al., reported that genetic suppression of both FIS1 and AMPK causes reduced mitophagy, which in turn causes cell cycle arrest and loss of stemness in LSCs. By destroying mitochondrial phosphorylated p53, which is recognized for its NANOG-suppressing action, mitochondriaphagy also modifies cancer stemness [84,85].
Furthermore, pharmacological inhibition of mitophagy increases the amount of phosphorylated p53 in the mitochondria. This p53 subsequently translocates to the nucleus and binds to NANOG to start the suppression of that protein. the hepatic CSCs were drastically eliminated by its promoter region [85,86]. In oesophageal squamous cell carcinoma, mitophagy-mediated regulation of oxidative stress promotes CD44+ cell proliferation, which is suppressed and directed towards cell death in the absence of Parkin-dependent mitophagy [87]. Through altering glycolytic metabolism, BNIP3L-dependent mitophagy mediated by the Hepatitis B virus X protein (HBx) increases the stemness of liver CSCs. The authors of this work measure several genes linked to cancer stemness, such as ATP-Binding Cassette Subfamily G Member 2 (ABCG2), B Cell-Specific Moloney Murine Leukemia Virus Integration Site (BMI1), Kruppel- Like Factor 4 (KLF4), NANOG, and Octamer-Binding Transcription Factor (OCT4), in order to determine cancer stemness. Notably, these genes are upregulated at the mRNA and protein levels by HBx therapy [88].
According to a different study, pancreatic CSC stemness is severely impacted by deregulation of mitophagy [89]. Among them is ISGylation its promoter region, leading to the hepatic CSCs’ severe elimination [85,86].The proliferation of CD44+ cells in oesophageal squamous cell carcinoma is facilitated by mitophagy-mediated control of oxidative stress; in the absence of Parkin-dependent mitophagy, this growth was hindered and directed towards cell death [87]. The BNIP3L-dependent mitophagy induced by the Hepatitis B Virus X protein (HBx) improves the stemness of liver CSCs by regulating glycolytic metabolism. The researchers measured several cancer stemness-related genes, such as ABCG2, BMI1, KLF4, NANOG, and OCT4, in order to determine cancer stemness. Interestingly, these genes are upregulated at the mRNA and protein levels by HBx therapy [90]. According to a different study Alcala et al., dysregulation of mitophagy has a deleterious impact on pancreatic CSCs’ stemness. One kind of isogylation is post-translational modification in which a lysine residue of cytoplasmic and nuclear target proteins is covalently attached to a ubiquitin-like protein, also referred to as IFN-Stimulated Gene 15 (ISG15) [89,91,92]. In addition, CSCs demonstrated increased ISG15 activity and ISGylation of proteins to maintain their metabolic flexibility. As a result of decreased mitophagy and impaired self-renewal potential as well as in vivo tumorigenicity of pancreatic CSCs, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-based suppression of ISG15 impedes mitochondrial ISGylation [89]. All things considered, mitophagy is a crucial component of cancer stemness and may be the focus of anti-CSC cancer therapy.
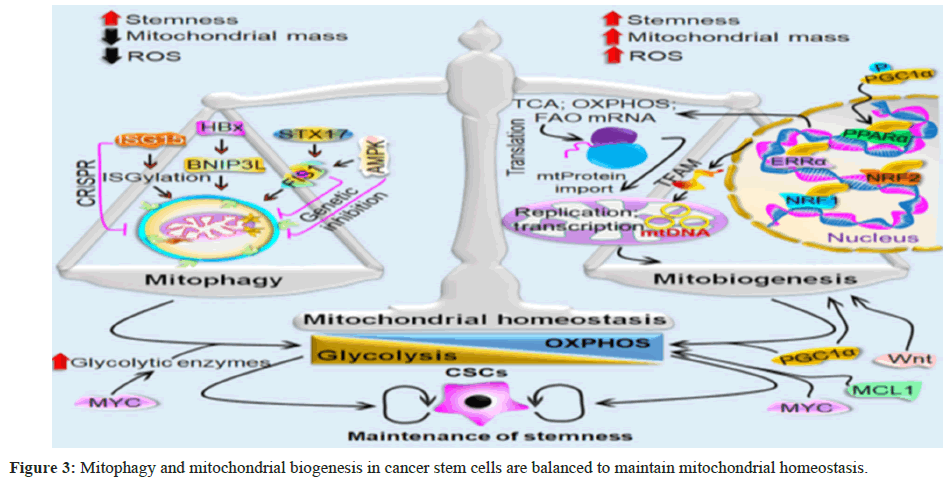
Mitochondrial homeostasis in CSCs requires mitochondrial biogenesis, an event that is balanced with mitophagy
Both the production of new, functional mitochondria and the removal of damaged mitochondria by mitophagy are necessary for maintaining mitochondrial homeostasis. via the synthesis of mitochondria [10]. According to mounting data, these two opposing cellular processes are required for CSCs to meet their bioenergetic requirements. Most notably, mitochondrial dynamics-which keeps an active mitochondrial network under various metabolic and external stress conditions-connects these activities [3]. More significantly, improved adaptation allows CSCs to develop, survive, and retain their tumorigenicity more effectively under various physiological and metabolic stressors thanks in large part to mitochondrial homeostasis.
The role of mitochondrial biogenesis in CSCs Nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) work together as a multifactorial regulatory system during mitochondrial biogenesis to coordinate several transcription factors and create new, fully functional mitochondria. Nuclear Respiratory Factor 1 (NRF1), Peroxisome Proliferator-Activated Receptor-Gamma Coactivator (PGC1α/1β), PGC1-Related Coactivator (PRC), and other transcription factors are involved in controlling this event. Estrogen-Related Receptor-α (ERRα) and Nuclear Respiratory Factor 2 (NRF2) [93]. PGC1α is primarily found in the cytoplasm and is regarded as a nodal transcription factor among these transcription factors. PGC1α changes post-translationally in response to physiological stressors that cells encounter, such as starvation and calorie restriction. As an illustration, PGC1α phosphorylation facilitates its nuclear translocation which in turn activates multiple downstream transcription factors, including NRF1/NRF2, to initiate mitochondrial biogenesis [94-96].
Once triggered, Peroxisome Proliferator-Activated Receptors (PPARs) stimulate the transcription of genes involved in Fatty Acid Oxidation (FAO) while NRFs increase the transcription of OXPHOS-related genes [97]. Following its translocation to mitochondria, mtDNA is replicated and transcriptionally regulated by TFAM, another essential transcription factor [98]. Following its entry into the mitochondria, TFAM combines with the mtDNA to create a stable protein–DNA complex known as a mitochondrial nucleoid, which is necessary for packaging the mtDNA and shielding it from proteolytic degradation [99]. Enhanced mitochondrial biogenesis and mitochondrial protein translation, which result in an increase in mitochondrial mass, are uncommon and characteristic aspects of CSCs [100,101]. The anchorage-independent growth and chemoresistance characteristics of CSCs are made possible by mitochondrial biogenesis, demonstrating the dependence of CSCs on mitochondrial function [102-104].
Chemoresistance characteristics of CSCs have been shown to correlate their increased mitochondrial mass with their capacity for metastatic spread and insensitivity to DNA damage [100]. The cellular plasticity of pancreatic CSCs is determined by PGC1α in conjunction with Myelo-Cytomatosis (MYC). MYC promotes the Warburg-like glycolytic phenotype by upregulating key glycolytic enzymes and reducing PGC1α activity through transcription inhibition. Therefore, increased PGC1α-mediated mitochondrial biogenesis is supported by lower MYC activity [105]. WNT signaling, another crucial stemness signal, also plays a role in controlling mitochondrial biogenesis in CSCs [106,107]. Consequently, it is believed that mitochondrial biogenesis is a crucial mitochondrial process required for the existence of and growth of CSCs in the Tumour Micro-Environment (TME) (Figure 3).
Mitophagy is a type of selective autophagy in which CSCs’ defective mitochondria are eliminated by recruiting mitophagosomes and then lysosome-based destruction. Additionally, FIS1 and its upstream regulator AMPK alter the status of mitophagy in LSCs. Genetic suppression of FIS1 and AMPK causes cell cycle arrest, loss of stemness, and reduced mitophagy. ISGylation is another way that ISG15, another mitophagy regulator, controls mitophagy-dependent metabolic switching. ISG15 is inhibited by CRISPR-based methods, which prevent mitochondrial ISGylation. This causes a greater build-up of damaged mitochondria, which lowers CSCs’ OXPHOS activity. By altering glycolytic metabolism, HBx-induced BNIP3-like (BNIP3L)-dependent mitophagy also increases the cancer stemness. The multifactorial regulating mechanism of mitochondrial biogenesis entails cooperatively producing new mitochondria are Peroxisome Proliferator-activated Receptor γ (PPARγ) coactivator 1α/1β (PGC1α/1β), PGC1-related Coactivator (PRC), NRF1, NRF2, and ERRα. PGC1α is phosphorylated during physiological stress, which causes it to translocate to the nucleus and co-activate these important downstream transcription factors.
NRFs increase the transcription of OXPHOS-related genes once they are activated, while PPARs encourage the transcription of FAO-related genes. Once translocated to mitochondria, TFAM, another essential transcription factor, controls both transcription and replication of mtDNA. In CSCs, cellular plasticity is determined by PGC1α and MYC. While OXPHOS is dependent on CSCs, PGC1α- mediated mitochondrial biogenesis supports the glycolytic reliance by boosting key glycolytic enzymes. In CSCs, MYC, MCL1, and WNT signaling additionally promotes the amount of mitochondrial OXPHOS. The role of metabolic homeostasis in controlling the metabolism of CSCs According to a number of investigations, CSCs preferentially use OXPHOS over glycolytic metabolism to produce ATP and maintain stem-like characteristics. Furthermore, compared to non-CSCs, CSCs have a higher level of OXPHOS reliance [108]. Glioma CSCs, for instance, produced less lactate and used less glucose, but sustained greater ATP levels than its differentiated counterpart through the activation of OXPHOS [109,110]. A tiny subpopulation of quiescent tumor cells, sometimes referred to as surviving cells with CSC characteristics that cause tumor recurrence, exhibits a reliance on OXPHOS for life.
According to Valerie et al these cells exhibit increased sensitivity to OXPHOS inhibitors that prevent tumor recurrence. In addition, compared to non-lung CSCs exhibiting distinct characteristics such increased mitochondrial membrane potential and decreased mtDNA, ATP level, and oxygen consumption, lung CSCs demonstrated a preference for OXPHOS over glucose metabolism for energy production. OXPHOS reliance was also demonstrated by LSCs and breast CSCs to meet their energy needs, highlighting the significance of mitochondrial respiration [111,112]. Key enzymes linked to the mitochondrial OXPHOS and FAO pathway in ovarian cancer were also more active in CSCs [13]. Furthermore, compared to their non- CSC counterpart, CSCs isolated from the spheroid culture in ovarian and cervical carcinomas had modified tricarboxylic acid cycle-driven metabolism for energy production [113]. In CSCs, Fork head box protein M1 (FoxM1)-driven Peroxiredoxin 3 (Prx3) activity and FAO-mediated NADH production work together to form a more potent anti-oxidant defense system, even in the face of increased mitochondrial activity [114].
The degree of mitochondrial reliance in CSCs varies depending on the environment. In pancreatic cancer, for instance, a tiny CD133+ CSC population revealed a reduced mitochondrial mass with greater metabolic adaptability and developed metformin resistance. Interestingly, the phenotypic that shown resistance to OXPHOS inhibition indicated an improved glycolytic program. In contrast, a sizable population of CD133+ CSCs within the same CSC compartment displayed an enlarged mitochondrial mass [105]. What’s more, a number of driving factors that are either directly or indirectly linked to mitochondrial homeostasis also influence this kind of metabolic flipping, indicating how reliant these mitochondriacentric processes are on CSCs. LeBleu et al., Lee et al., Yajima et al., and others have reported increased activity of PGC1α, the major modulator of mitochondrial biogenesis, which is connected to higher OXPHOS phenotypes and appears to be tied with chemoresistance in CSCs [115-117].
In a different investigation, the combined activity of MCL1 and MYC maintains the chemotherapy-resistant phenotype. According to Lee et al., CSCs control the activity of mitochondrial OXPHOS in breast cancer [117]. The metabolic phenotype and adaptability of CSCs in pancreatic cancer are largely determined by the intracellular balance between MYC and PGC1α [106]. Changes in cellular metabolism impact mitochondrial dynamics, which in turn modify the state of cellular metabolism to promote tumorigenesis and the development of acquired resistance to treatment [3,118]. By modifying ROS signaling to start an NRF2-dependent retrograde signaling cascade, mitochondrial dynamics also significantly influence the destiny of stem cells [42]. More significantly, mitochondrial dynamics demonstrated the relationship between the availability of nutrients and the need for energy, with mitochondrial fusion increasing ATP production and its inhibiting causes mtDNA depletion, ROS generation, and OXPHOS impairment [119].
In a different study, the Nestin–Cyclin-Dependent Kinase-5 (CDK5)–DRP1 axis uses metabolic switching to control the stem-like characteristics of brain stem or progenitor cells. Additionally, by blocking DRP1-mediated mitochondrial fission, nestin inhibition led to mitochondrial elongation and greater basal and maximal oxygen consumption, which indicated an increase in mitochondrial respiration [120]. According to Naik et al., mitophagy is also essential for controlling metabolic switch CSCs [121]. It was discovered that the mitophagy regulator ISG15 regulates the amount of OXPHOS in pancreatic CSCs. ISG15 is inhibited by CRISPR-based methods, which prevent mitochondrial ISGylation and increase the accumulation of injured mitochondria, which lowers OXPHOS activity [89]. In light of these discoveries, comprehending the metabolic characteristics of CSCs as well as in order to reduce CSC-associated tumor relapses, it is imperative to create novel therapeutic approaches that are more effective than existing treatment plans, which requires maintaining correct mitochondrial dynamics in CSCs (Figure 3).
Medical manipulation of mitochondria-centric processes in CSCs: A therapeutic approach for CSC selective eradication
Nowadays, there is a great deal of interest in the development of CSC-based anti-cancer therapy techniques since these cells play a crucial role in regulating the development of chemoresistance and tumor relapse. This area is made more fascinating by the importance of mitochondria-associated cellular processes that sustain highly active mitochondrial networks in CSCs and by their role as a critical regulator of CSC phenotypes [122-124]. In order to target mitochondria-associated processes like biogenesis, dynamics (fusion– fission), and mitophagy, a deeper understanding of mitochondrial pharmacology would be efficient for cancer therapy that targets CSCs Targeted drug delivery using pharmacological methods in CSCs The majority of CSCs are found in the tumor niche, where they are shielded from traditional treatments that usually aim to eradicate the tumor cells’ rapid proliferation [125]. By boosting ATP-dependent drug efflux and controlling many mitochondria-associated processes as mitophagy and mitochondrial biogenesis, CSCs exhibit such a resistance mechanism against the majority of chemotherapeutic medicines [10,126].
Combination treatments currently employ targeted drug delivery systems based on nanotechnology, which enable anti-CSC medications to selectively kill CSCs while also reaching their therapeutic target. Liposomes, polymeric micelles, Polymeric Nanoparticles (NPs), or nanogels are a few examples, serve as carriers of nanomedicines intended to specifically target cancer cells [127]. Targeting liposomes with mitochondria demonstrated efficacious therapeutic potential in targeting CSCs. Dequalinium–PEG2000–DSPE-containing liposomes, measuring 98 nm, were prepared using quinacrine (mepacrine) and daunorubicin for this method. According to Zhang et al., these produced liposomes with the mitochondria-specific chemical Dequalinium (DQA) had a lasting circulatory impact. They could also aggregate specifically within the mitochondria and trigger the pro-apoptotic Bcl-2-Associated X protein (BAX) protein to cause CD44+/CD24− breast CSCs to undergo apoptosis [128]. Another method involves the frequent usage of Triphenyl Phosphonium (TPP) in delocalized lipophilic cations, which are mostly attached to the surfaces of Nanoparticles (NPs) or covalently bonded to nanocarriers in order to target mitochondria. Compared to normal cells, the mitochondria of tumor cells exhibit a greater degree of accumulation of these compounds because of the cancer cells with significant negative mitochondrial membrane potentials [129].
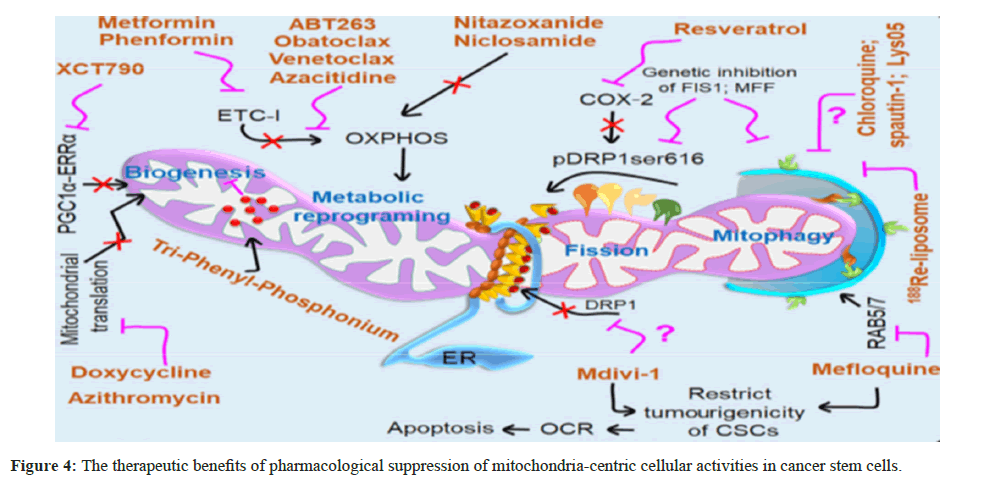
Chloroquine (CQ) mediated nanocarrier-mediated autophagy inhibition greatly increased the effectiveness of chemotherapeutic agents (doxorubicin and docetaxel) against breast CSCs. The nanocarriers greatly prolonged the half-life of CQ’s circulation and increased the drug’s accumulation within the tumor tissues, which resulted in enhanced tumor regression. CSC-specific selective suppression of mitochondrial dynamics determining the functional role of mitochondrial dynamics in human malignancies is crucial since it will provide new information on associated therapies. By preventing mitochondrial fission, CSCs become less capable of self-renewal and eventually run out of energy, which opens up a new target for anti-cancer therapies [130,131]. Additionally, the role of immune cells like T cells in the cancer microenvironment are dependent on the regulation of mitochondrial fission and fusion, indicating that focusing on mitochondrial dynamics may be a viable way to enhance anti-tumor immunity. The development of cancer is facilitated by mitochondrial fission, which also contributes to medication resistance, metastasis, and cell proliferation.
It is still debatable today whether Mdivi-1 is the direct inhibitor of DRP1-dependent mitochondrial fission. Prior research by Cassidy-Stone et al., revealed that Mdivi-1 directly associates with DRP1 to decrease its GTPase activity and self-assembly around mitochondria, hence inhibiting DRP1-dependent fission. But it also reverses electron transfer-mediated ROS generation and reversibly suppresses mitochondrial complex I-dependent O2 consumption [132]. According to Peiris-Pages et al., Mdivi-1 is also known to limit the tumorigenic potential of CSCs and is helpful in getting rid of CSCs [130]. Additionally, genetic and Pharmacological inhibition of DRP1-mediated mitochondrial fission resulted in a decrease in the rate at which cells consumed oxygen, causing metabolic stress. This, in turn, led to cell death in BTICs through apoptosis and suppressed the cell’s ability to proliferate, indicating that DRP1 functions as a regulatory switch that controls the population of CSCs [60].
In a different investigation, resveratrol-a naturally occurring phytochemical that can likewise inhibit cancer stemness and enhance chemosensitization to 5-Fluorouracil (5-FU) in nasopharyngeal cancer via decrease of the COX-2/p-Drp1Ser616 axis in the mitochondria [63]. A better therapeutic option against CSC-associated chemoresistance and disease relapse would be to selectively inhibit COX-2 and develop more specific inhibitors against DRP1-mediated mitochondrial fission without affecting alternative pathways. Mitophagy and mitochondrial biogenesis in CSCs are selectively inhibited Oral and colorectal CSCs have chemoresistance against cisplatin and doxorubicin, respectively, due to mitophagy-driven stemness [133,134]. Oral CSCs and colon CSCs undergo apoptosis as a result of genetic and pharmacological suppression of mitophagy, which increases cisplatin and doxorubicin sensitivity. Moreover, proteins implicated in the endolysosomal through modifying the mitophagy route, a pathway like that of the Ras-related protein Rab-5/7 (RAB5/7) similarly favorably regulates the stemness and longevity of CSC. Furthermore, the disruption of colon CSCs’ tumorigenic activity can be effectively achieved by inhibiting RAB5/7 with mefloquine hydrochloride in conjunction with chemotherapeutic medicines as irinotecan or oxaliplatin [135].
In order to control CSC-associated drug resistance, a recently developed nanomedicine called 188Re-liposome is used to selectively inhibit mitophagy in the tumor microenvironment. This approach has been shown to significantly reduce tumourgenicity in two xenograft model systems [136]. Even though a number of studies indicate that various pharmacological inhibitors, such as CQ or its derivatives (spautin-1 and Lys05) can effectively target lysosomal activity in a variety of cancer types, this may not always be the case beneficial to the population of CSCs that rely on mitophagy for growth and survival. Consequently, creating additional mitophagy inhibitors with powerful inhibition of mitophagy provides an effective means of sensitizing CSCs to traditional anti-cancer treatment in an alternative cancer paradigm. In a similar vein, CSCs showed significant mitochondrial biogenesis, which is linked to CSC-associated treatment resistance and results in development of cancer and disease recurrence. As a result, substances that target mitochondrial biogenesis have a direct inhibitory effect on the growth and maintenance of CSCs, aiding in the treatment of neoplastic diseases [100].
For instance, TPP derivatives (dodecyl-TPP [d-TPP]) are tiny, bioactive, nontoxic compounds that selectively reduce mitochondrial function, which suppresses cancer cells as well as CSCs [137]. CSC survival is decreased by pharmacological suppression of the PGC1α– ERRα axis with XCT790, which specifically decreases mitochondrial biogenesis [9]. Because doxycycline can target CSCs, it is also used for clinical care of breast cancer at an early stage [138]. Doxycycline is known to decrease mitochondrial protein translation. Five distinct Food and Drug Administration (FDA)-approved antibiotics were repurposed in another study: Azithromycin, doxycycline, tigecycline, pyrvinium pamoate, together with chloramphenicol to get rid of CSCs. According to Lamb et al., these antibiotics prevent the formation of tumorospheres in 12 distinct cancer cell lines from various cancer types, including breast, DCIS, glioblastoma, lung, melanoma, ovarian, prostate, and pancreatic cancer (Figure 4) [107].
Tri Phenyl Phosphonium (TPP) accumulates in the mitochondria of Cancer Cells and Stem Cells (CSCs) and preferentially decreases their activity. By selectively inhibiting mitochondrial biogenesis, XCT790 preferentially inhibits the PPARγ coactivator 1α (PGC1α)– Estrogen-related Receptor-α (ERRα) axis, reducing CSC survival. CSCs are also targeted by the antibiotic doxycycline, which is known to impede the translation of mitochondrial proteins. Metformin and its chemical counterpart phenformin demonstrated strong anti-tumor effect, lowering tumor development and more effective against CSCs, sensitizing CSCs to conventional cancer therapy. Metformin efficiently reduces OXPHOS activity through inhibition of Electron Transport Chain (ETC) I. CQ, spautin-1, Lys05, and 188Re-liposome are examples of autophagy and/or mitophagy inhibitors that are utilized to manage CSC-associated drug resistance. Mercedoquin Combining hydrochloride with chemotherapeutic medicines like oxaliplatin or irinotecan causes a disruption in the tumorigenicity of colon CSCs by preferentially inhibiting the Ras-related protein Rab- 5/7 (RAB5/7)-dependent mitophagy.
Through its direct interaction with DRP1, which reduces its GTPase activity and self-assembly around mitochondria, Mdivi-1 suppresses mitochondrial fission and limits the capacity of CSCs to cause tumors. Bcl-2 inhibitors, such obatoclax and ABT263 specifically destroy quiescent LSCs by quickly impairing OXPHOS and lowering ATP levels. Using azacitidine, a hypomethylating drug, and venetoclax, another BCL-2 inhibitor, LSCs are specifically eliminated by inhibiting amino acid metabolism, which lowers OXPHOS activity and causes cell death.
Additionally, it interferes with the TCA cycle by increasing succinate levels and decreasing α-ketoglutarate, which inhibit OXPHOS activity. selective suppression of metabolic switching centered on the mitochondria in CSCs The ability to adapt to various energy pathways may be the cause of CSCs’ increased “cellular fitness,” which helps them survive amid therapeutic stress. As an illustration, CSCs like LSCs utilized the nearby Gonadal Adipose Tissue (GAT) as a survival niche to obtain energy, supporting their metabolism and enabling them to withstand the stress of chemotherapy [139]. For improved adaptation both during and after anti-cancer treatments, such metabolic plasticity changed the metabolic dependency of CSCs from glycolysis to OXPHOS [140-143].
To enhance their survival during metabolic stress, CSCs also exhibit an upregulation in sarcoplasmic reticulum/ER calcium ATPase that is reliant on Calcium/Calmodulin-dependent Protein Kinase 2α (CaMK2α) [144]. Currently, a number of pharmaceutical inhibitors that target this metabolic flexibility are used against CSCs. For instance, metformin, a well-known anti-diabetic medication that efficiently inhibits ETC I to decrease OXPHOS activity, demonstrated a strong anti-tumor effect that decreased tumor growth and was more effective against CSCs, making them more susceptible to conventional cancer therapy [145-147]. Similar to this, phenformin, another inhibitor of mitochondrial complex I, also suppresses the ability of Glioblastoma stem cells (GSCs) to self-renew. This is seen in situations where mesenchymal phenotypes (like fibronectin and YKL40) and markers linked to stemness (like OCT4, SOX2, and CD44) are reduced through modulation of the H19/let-7/HMGA2 pathway. Furthermore, both in vitro and in vivo, phenformin alone or in combination with temozolomide demonstrated an improved anti-tumor activity on GSCs. the circumstances [148]. By modifying cellular energy (ATP synthesis) and metabolic state (OXPHOS activity), a number of other drugs also demonstrated anti-tumourigenic characteristics in cancer cells, such as decreased survival efficiency and metastatic potential [108,149,150]. In chronic myeloid leukemia, therapy-resistant CSCs are similarly eliminated by selective suppression of mitochondrial OXPHOS [151].
Drugs that block mitochondrial OXPHOS in pancreatic cancer specifically target the metabolism of CSCs while leaving non-CSCs intact [105]. Through altering cellular OXPHOS status, the FDA-approved anti-helminthics niclosamide and nitazoxanide shown selective activity against CSCs [152-154] (Figure 4). Creation of low-MW inhibitors that are similar to by reactivating cell death signaling in CSCs, the action of BH3-only proteins-now known as BH3 mimetics-improves the effectiveness of anti-CSC-targeted cancer therapy [155]. Acute Myeloid leukemia (AML) is brought on and maintained by Lymphoblast-Stimulating Cells (LSCs), which subsequently results in worse clinical outcomes when the malignancy recurs. For instance, Bcl-2 inhibitors like ABT263 (navitoclax) and obatoclax specifically destroy quiescent LSCs by quickly impairing OXPHOS and lowering ATP levels. Following them, there was a decrease in cellular Glutathione (GSH) activity and an increase in mitochondrial Reactive Oxygen Species (mROS) buildup, which ultimately results in apoptotic cell death [111].
Amino acid metabolism powers OXPHOS, which is essential for LSCs isolated from AML patients to meet their energy needs. But LSCs separated from AML patients who have relapsed exhibit a more complicated metabolic profile and a reliance on fatty acid metabolism to meet their energy needs. By inhibiting amino acid metabolism, which lowers OXPHOS activity and causes cell death, the combination of the BCL-2 inhibitor venetoclax and the hypomethylating drug azacitidine specifically kills LSCs. Conversely, relapsed patient-derived LSCs exhibit reduced sensitivity to venetoclax and azacitidine therapy [156]. Another study found that treating venetoclax and azacitidine together disturbs the TCA cycle by raising succinate levels and lowering α-ketoglutarate. This effectively eliminates LSCs by suppressing OXPHOS and blocking energy metabolism.
Bcl-2 inhibitors have had outstanding results in treating many haematological malignancies, including chronic lymphocytic leukemia, mantle cell Figure 4 shows an overview of T-cell lymphoma, myeloma, blastic plasmacytoid dendritic cell neoplasm, acute lymphoblastic leukaemia, and T-cell prolymphocytic leukaemia [157]. Two pharmaceutical molecules were used in a recent study to investigate the functional role of mitochondrial dynamics mediated OXPHOS regulation in preserving the stemness of liver CSC. 2-Deoxy-D-Glucose (2-DG) stimulates LCSC stemness by up-regulating OXPHOS level, and Mdivi-1 attenuates stemness by preventing mitochondrial fission-driven OXPHOS activity [158]. Therefore, it might be helpful to combat CSC-associated behaviors by specifically inhibiting such a metabolic shift in CSCs using various pharmacological agents.
Final summary and next directions
The normal functioning of cells, the growth of cancer cells, and the survival and stemness of CSCs-the cause of treatment resistance and tumor recurrence-depend on mitophagy and mitochondrial dynamics. In order to maintain mitochondrial homeostasis and improve survival under various physiological and metabolic stressors, CSCs take advantage of mitochondria-associated cellular activities such as mitophagy, biogenesis, and dynamics. Furthermore, metabolic switching-that is, moving from glycolysis to OXPHOS or vice versa-illustrated the complexity of CSCs, and it is important to note that mitochondrial mechanisms are crucial in regulating CSC-associated functions in both CSC phenotypes. Thus, it would be possible to effectively destroy CSCs and provide therapeutic benefit by selectively targeting important variables that promote CSC-associated mitochondrial rewiring in order to pharmacologically manipulate such mitochondria-associated survival mechanisms. Thus far, numerous facets of Extensive research has been conducted on mitophagy and mitochondrial homeostasis in CSCs, as well as their modification by various inhibitory mechanisms for targeted cancer therapy.
There are still some unanswered problems, such as (i) what are the main variables controlling the interactions between these dependent pathways when non-malignant CSCs turn into malignant CSCs? (ii) How can CSCs regulate excessive activation that results in cell death in the TME and take use of these mitochondria-centric activities exclusively for their own survival? (iii) Targeting specific OXPHOS components in CSCs would be intriguing to observe, as would the alternative pathways that are altered in these circumstances, despite the fact that selective suppression of OXPHOS decreases CSC survival. According to Kuramoto et al., verteporfin shields against OXPHOS in this situation causes GSCs to selectively undergo cell death. Additionally, they stated that GSCs’ OXPHOS activity was higher than that of their differentiated counterpart and that this activity is necessary for their survival [159]. It’s interesting to note that GSCs expressed more of the mtDNA-coded complexes III and IV components than differentiated GSCs did extending the therapeutic windows that are particular to CSCs. Furthermore, the combination strategy of various chemotherapeutic medications and inhibitors that target mitochondrial rewiring may hinder CSCs’ survival mechanism and offer patients with aggressive cancer viable treatment alternatives.
References
- Schon EA (2008) A toolkit for the cell's powerhouse. Nat Biotechnol 26(3):294-296.
- Chan DC (2006) Mitochondria: Dynamic organelles in disease, aging, and development. Cell 125:1241-1252.
- Trotta AP, Chipuk JE (2017) Mitochondrial dynamics as regulators of cancer biology. Cell Mol Life Sci 74:1999-2017.
- Desai SP, Bhatia SN, Toner M, Irimia D (2013) Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys J 104(9):2077-2088.
- Ayob AZ, Ramasamy TS (2018) Cancer stem cells as key drivers of tumour progression. J Biomed Sci 25:20.
- Batlle E, Clevers H (2017) Cancer stem cells revisited. Nat Med 23:1124-1134.
- Li J, Huang Q, Long X, Guo X, Sun X et al. (2017) Mitochondrial elongation-mediated glucose metabolism reprogramming is essential for tumour cell survival during energy stress. Oncogene 36(34):4901-4912.
- Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Patra S et al. (2019) The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin Cancer Biol 66:45-58.
- De Luca A, Fiorillo M, Peiris-Pagès M, Ozsvari B, Smith DL et al. (2015) Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget 6(17):14777.
- Praharaj PP, Panigrahi DP, Bhol CS, Patra S, Mishra SR et al. (2021) Mitochondrial rewiring through mitophagy and mitochondrial biogenesis in cancer stem cells: A potential target for anti-CSC cancer therapy. Cancer Lett 498:217-228.
- Shen YA, Wang CY, Hsieh YT, Chen YJ, Wei YH (2015) Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 14(1):86-98.
- Ye XQ, Li Q, Wang GH, Sun FF, Huang GJ, et al. (2011) Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. International Journal of Cancer 129:820-831.
- Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M et al. (2014) Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose-deprivation. Oncotarget 5(12):4305-4319.
- Najafi M, Farhood B, Mortezaee K (2019) Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol 234(6):8381-8395.
- Akbarzadeh M, Maroufi NF, Tazehkand AP, Akbarzadeh M, Bastani S et al. (2019) Current approaches in identification and isolation of cancer stem cells. J Cell Physiol 234(9):14759-14772. [Crossref]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L et al. (2007) Identification of pancreatic cancer stem cells. Cancer Res 67(3):1030-1037.
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW et al. (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell 1(3):313-323.
- Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W et al. (2011) ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PloS one 6(6):e20636.
- Li C, Wu JJ, Hynes M, Dosch J, Sarkar B et al. (2011) c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 141(6):2218-2227.
- Seino T, Kawasaki S, Shimokawa M, Tamagawa H, Toshimitsu K et al. (2018) Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell stem cell 22(3):454-467.
- Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A et al. (2011) Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell stem cell 9(5):433-446.
- Matsubara S, Ding Q, Miyazaki Y, Kuwahata T, Tsukasa K et al. (2013) mTOR plays critical roles in pancreatic cancer stem cells through specific and stemness-related functions. Sci Rep. 3(1):3230.
[Crossref][Google Scholar][PubMed]
- Wang YH, Li F, Luo B, Wang XH, Sun HC et al. (2009) A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 56(5):371-378.
- Hong SP, Wen J, Bang S, Park S, Song SY (2009) CD44‐positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer 125(10):2323-2331.
- Xia M, Zhang Y, Jin K, Lu Z, Zeng Z et al. (2019) Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell Biosci 9(1):1-9.
- Butow RA, Avadhani NG (2004) Mitochondrial signaling: The retrograde response. Mol Cell 14:1-15.
- Srinivasan S, Guha M, Kashina A, Avadhani NG (2017) Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg 1858(8):602-614.
- Guha M, Srinivasan S, Ruthel G, Kashina AK, Carstens RP et al. (2014) Mitochondrial retrograde signaling induces epithelial–mesenchymal transition and generates breast cancer stem cells. Oncogene 33(45):5238-5250.
- Wen YA, Xiong X, Scott T, Li AT, Wang C, et al (2019) The mitochondrial retrograde signaling regulates Wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ 26(10):1955-69.
- Salnikov AV, Liu L, Platen M, Gladkich J, Salnikova O, et al (2012) Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS One 7(9):e46391
- Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79-99.
- Chen H, Chan DC (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab 26:39-48.
- Gomes LC, Benedetto GD, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13(5):589-598.
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J (2011) Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108(25):10190-5.
- Miettinen TP, Björklund M (2017) Mitochondrial function and cell size: An allometric relationship. Trends Cell Biol 27(6):393-402.
- Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity J Cell Sci 117(26):6535-6546.
- Mishra P, Carelli V, Manfredi G, Chan DC (2014) Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab 19(4):630-41.
- Martens S, McMahon HT (2008) Mechanisms of membrane fusion: Disparate players and common principles. Nat Rev Mol Cell Biol 9(7):543-56.
- Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25(13):2966-2977.
- Cipolat S, de Brito OM, Zilio BD, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A 101:15927-15932.
- Chen H, Chan DC (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab 26:39-48.
- Khacho M, Clark A, Svoboda DS, Azzi J, MacLaurin JG et al. (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19(2):232-247.
- Wu MJ, Chen YS, Kim MR, Chang CC, Gampala S, et al (2019) Epithelial-mesenchymal transition directs stem cell polarity via regulation of mitofusin. Cell Metab 29(4):993-1002.
- Bao D, Zhao J, Zhou X, Yang Q, Chen Y, et al (2019) Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 38(25):5007-20.
- Sun X, Cao H, Zhan L, Yin C, Wang G, et al (2018) Mitochondrial fission promotes cell migration by Ca2+/CaMKII/ERK/FAK pathway in hepatocellular carcinoma. Liver Int 38(7):1263-72.
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J et al. (2011) ER tubules mark sites of mitochondrial division. Science. 334(6054):358-362.
- Lima AR, Santos L, Correia M, Soares P, Sobrinho-Simões M, et al (2018) Dynamin-related protein 1 at the crossroads of cancer. Genes 9(2):115.
- Kraus F, Ryan MT (2017) The constriction and scission machineries involved in mitochondrial fission. J CellSci 130(18):2953-2960.
- Losón OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24(5):659-67.
- Ma J, Zhai Y, Chen M, Zhang K, Chen Q, et al (2019) New interfaces on MiD51 for Drp1 recruitment and regulation. PLoS One 14(1):e0211459.
- Richter V, Singh AP, Kvansakul M, Ryan MT, Osellame LD (2015) Splitting up the powerhouse: Structural insights into the mechanism of mitochondrial fission. Cell Mol Life Sci 72:3695-707.
- Bui HT, Shaw JM (2013) Dynamin assembly strategies and adaptor proteins in mitochondrial fission. Curr Biol 23:R891-R899.
- Ji WK, Chakrabarti R, Fan X, Schoenfeld L, Strack S et al. (2017) Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J Cell Biol 216(12):4123-4139.
- Lee JE, Westrate LM, Wu H, Page C, Voeltz GK (2016) Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 540(7631):139-143.
- Yu R, Jin SB, Lendahl U, Nistér M, Zhao J (2019) Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J 38(8):e99748.
- Kong D, Xu L, Yu Y, Zhu W, Andrews DW et al. (2005) Regulation of Ca 2+-induced permeability transition by Bcl-2 is antagonized by Drp1 and hFis1. Mol Cell Biochem 272:187-199.
- Cereghetti GM, Costa V, Scorrano L (2010) Inhibition of Drp1-dependent mitochondrial fragmentation and apoptosis by a polypeptide antagonist of calcineurin. Cell Death Differ 17:1785-1794.
- Civenni G, Carbone GM, Catapano CV (2019) Mitochondrial fission promotes self-renewal and tumorigenic potential in prostate cancer. Mol Cell Oncol 6(5):e1644598.
- Passaniti A, Hussain A (2019) Novel approaches targeting mitochondrial fission to deplete stem-like tumor cells in prostate cancer and improve treatment outcomes. Ann Transl Med 7(8):S335
- Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, et al (2015) Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18(4):501-10.
- Ooki A, Del Carmen Rodriguez Pena M, Marchionni L, Dinalankara W, Begum A, et al (2018) YAP1 and COX2 coordinately regulate urothelial cancer stem-like cells. Cancer Res 78(1):168-81.
- Tian J, Hachim MY, Hachim IY, Dai M, Lo C, et al (2017) Cyclooxygenase-2 regulates TGFβ-induced cancer stemness in triple-negative breast cancer. Sci Rep 7(1):40258.
- Zhou TJ, Zhang SL, He CY, Zhuang QY, Han PY, et al (2017) Downregulation of mitochondrial cyclooxygenase-2 inhibits the stemness of nasopharyngeal carcinoma by decreasing the activity of dynamin-related protein 1. Theranostics 7(5):1389.
- Cai J, Wang J, Huang Y, Wu H, Xia T, et al. (2016) ERK/Drp1-dependent mitochondrial fission is involved in the MSCinduced drug resistance of T-cell acute lymphoblastic leukemia cells. Cell Death Dis 7:e2459.
- Arumugam P, Samson A, Ki J, Song JM (2017) Knockdown of clusterin alters mitochondrial dynamics, facilitates necrosis in camptothecin-induced cancer stem cells. Cell Biol Toxicol 33:307-321.
- Praharaj PP, Patra S, Panigrahi DP, Patra SK, Bhutia SK (2020) Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim Biophys Acta Rev Cancer 1875:188500.
- Praharaj PP, Naik PP, Panigrahi DP, Bhol CS, Mahapatra KK, et al. (2019) Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: Its implication in cancer therapeutics. Cell Mol Life Sci 76:1641-1652.
- Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12:9-14.
- Liu L, Sakakibara K, Chen Q, Okamoto K (2014) Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res 24:787-795.
- Whitworth AJ, Pallanck LJ (2009) The PINK1/Parkin pathway: A mitochondrial quality control system? J Bioenerg Biomembr 41:499-503.
- Meissner C, Lorenz H, Weihofen A, Selkoe DJ, Lemberg MK (2011) The mitochondrial intra membrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117:856-867.
- Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R et al. (2012) Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13(4):378-385.
- Harper JW, Ordureau A, Heo JM (2018) Building and decoding ubiquitin chains for mitophagy. Nat Rev Mol Cell Biol 19(2):93-108.
- Abdrakhmanov A, Gogvadze V, Zhivotovsky, B (2020) To eat or to die: Deciphering selective forms of autophagy. Trends Biochem Sci 45:347-364.
- Panda PK, Naik PP, Meher BR, Das DN, Mukhopadhyay S, et al. (2018) PUMA dependent mitophagy by Abrus agglutinin contributes to apoptosis through ceramide generation. Biochim Biophys Acta Mol Cell Res 1865:480-495.
- Villa E, Marchetti S, Ricci JE (2018) No Parkin zone: Mitophagy without Parkin. Trends Cell Biol 28:882-895.
- Yamano K, Matsuda N, Tanaka K (2016) The ubiquitin signal and autophagy: An orchestrated dance leading to mitochondrial degradation. EMBO Rep 17:300-316.
- Burman JL, Pickles S, Wang C (2017) Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J Cell Biol 216:3231-3247.
- Hamacher-Brady A, Brady NR (2016) Mitophagy programs: Mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 73:775-795.
- Liu L, Feng D, Chen G, Chen M, Zheng Q, et al. (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14:177-185.
- Wu W, Li W, Chen H, Jiang L, Zhu R, et al. (2016) FUNDC1 is a novel Mitochondrial-Associated-Membrane (MAM) protein required for hypoxia-induced mitochondrial fission and mitophagy. Autophagy 12:1675-1676.
- Pryde KR, Smith HL, Chau KY, Schapira AH (2016) PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy. J Cell Biol 213:163-171.
- Xian H, Yang Q, Xiao L, Shen HM, Liou YC (2019) STX17 dynamically regulated by Fis1 induces mitophagy via hierarchical macro autophagic mechanism. Nat Commun 10:2059.
- Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, et al. (2018) AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell 23:86-100.e6.
- Liu K, Lee J, Kim JY, Wang L, Tian Yet al. (2017) Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell 68:281-292.e5.
- Lee J, Liu K, Stiles B, Ou JJJ (2018) Mitophagy and hepatic cancer stem cells. Autophagy 14:715-716.
- Whelan KA, Chandramouleeswaran PM, Tanaka K, Natsuizaka M, Guha M, et al. (2017) Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene 36:4843-4858.
- Chen YY, Wang WH, Che L, Lan Y, Zhang LY, et al. (2020) BNIP3Ldependent mitophagy promotes HBx-induced cancer stemness of hepatocellular carcinoma cells via glycolysis metabolism reprogramming. Cancers (Basel) 12:655.
- Alcalá S, Sancho P, Martinelli P, Navarro D, Pedrero C, et al. (2020) ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nat Commun 11:2682.
- Chen YY, Wang WH, Che L, Lan Y, Zhang LY, et al. (2020) BNIP3Ldependent mitophagy promotes HBx-induced cancer stemness of hepatocellular carcinoma cells via glycolysis metabolism reprogramming. Cancers (Basel) 12:655.
- Sainz B Jr, Martín B, Tatari M, Heeschen C, Guerra S (2014) ISG15 is a critical micro environmental factor for pancreatic cancer stem cells. Cancer Research 74:7309-7320.
- Beltri CV, Guerra S, Madrid FS (2017) ISGylation-A key to lock the cell gates for preventing the spread of threats. J Cell Sci 130:2961-2969.
- Popov LD (2020) Mitochondrial biogenesis: An update. J Cell Mol Med 24:4892-4899.
- Chang JS, Huypens P, Zhang Y, Black C, Kralli A, et al. (2010) Regulation of NT-PGC-1α subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1. J Biol Chem 285:18039-18050.
- Luo C, Widlund HR, Puigserver P (2016) PGC-1 coactivators: Shepherding the mitochondrial biogenesis of tumors. Trends Cancer 2:619-631.
- Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, et al. (2011) Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 286:10605-10617.
- Scarpulla RC (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88(2):611-638.
- Picca A, Lezza AM (2015) Regulation of mitochondrial biogenesis through TFAM–mitochondrial DNA interactions: Useful insights from aging and calorie restriction studies. Mitochondrion 25:67-75.
- Kukat C, Davies KM, Wurm CA, Spahr H, Bonekamp NA et al. (2015) Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc Natl Acad Sci 112(36):11288-11293.
- Farnie G, Sotgia F, Lisanti MP (2015) High mitochondrial mass identifies a sub-population of stem-like cancer cells that are chemo-resistant. Oncotarget 16(31):30472.
- Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G et al. (2013) PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer cell. 23(3):287-301.
- De Luca A, Fiorillo M, Peiris-Pagès M, Ozsvari B, Smith DL et al. (2015) Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget 6(17):14777.
- Lamb R, Harrison H, Hulit J, Smith DL, Lisanti MP et al. (2014) Mitochondria as new therapeutic targets for eradicating cancer stem cells: Quantitative proteomics and functional validation via MCT1/2 inhibition. Oncotarget 5(22):11029.
- Vlashi E, Lagadec C, Vergnes L, Reue K, Frohnen P et al. (2014) Metabolic differences in breast cancer stem cells and differentiated progeny. Breast Cancer Res Treat. 146:525-554.
- Sancho P, Burgos-Ramos E, Tavera A, Kheir TB, Jagust P et al. (2015) MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22(4):590-605.
- Bernkopf DB, Jalal K, Brückner M, Knaup KX, Gentzel M, et al. (2018) Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J Cell Biol 217:1383-1394.
- Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A et al. (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 6(7):4569-4584.
- Gao C, Shen Y, Jin F, Miao Y, Qiu X (2016). Cancer stem cells in small cell lung cancer cell line H446: Higher dependency on oxidative phosphorylation and mitochondrial substrate-level phosphorylation than non-stem cancer cells. PLoS One 11:e0154576.
- Janiszewska M, Suva ML, Riggi N, Houtkooper RH, Auwerx J et al. (2012) Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev 26(17):1926-1944.
- Vlashi E, Lagadec C, Vergnes L, Matsutani T, Masui K et al. (2011) Metabolic state of glioma stem cells and nontumorigenic cells. Proc Natl Acad Sci U S A 108(38):16062-16067.
- Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ et al. (2013) BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell stem cell 12(3):329-341.
- Vlashi E, Lagadec C, Vergnes L, Reue K, Frohnen P et al. (2014) Metabolic differences in breast cancer stem cells and differentiated progeny. Breast Cancer Res Treat. 146:525-554.
- Sato M, Kawana K, Adachi K, Fujimoto A, Yoshida M, et al. (2016) Spheroid cancer stem cells display reprogrammed metabolism and obtain energy by actively running the tricarboxylic acid (TCA) cycle. Oncotarget 7(22):33297-33305.
- Choi HJ, Jhe YL, Kim J, Lim JY, Lee JE, et al. (2020) FoxM1-dependent and fatty acid oxidationmediated ROS modulation is a cell-intrinsic drug resistance mechanism in cancer stem-like cells. Redox Biol 36:101589.
- LeBleu VS, O Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K et al. (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16(10):992-1003.
- Lee KM, Giltnane JM, Balko JM, Schwarz LJ, Guerrero-Zotano AL et al. (2017) MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metab 26(4):633-647.
- Yajima T, Ochiai H, Uchiyama T, Takano N, Shibahara T et al. (2009) Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol. 35(2):273-280.
- Senft D, Ze’ev AR (2016) Regulators of mitochondrial dynamics in cancer. Curr Opin Cell Biol 9:43-52.
- Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17(4):491-506.
- Wang J, Huang Y, Cai J, Ke Q, Xiao J et al. (2018) A nestin–cyclin-dependent kinase 5–dynamin-related protein 1 axis regulates neural stem/progenitor cell stemness via a metabolic shift. Stem Cells 36(4):589-601.
- Naik PP, Birbrair A, Bhutia SK (2019) Mitophagy-driven metabolic switch reprograms stem cell fate. Cell Mol Life Sci 76:27-43.
- Jeon JH, Kim DK, Shin Y, Kim HY, Song B et al. (2016) Migration and invasion of drug-resistant lung adenocarcinoma cells are dependent on mitochondrial activity. Exp Mol Med. 48(12):e277.
- Sancho P, Barneda D, Heeschen C (2016) Hallmarks of cancer stem cell metabolism. Br J Cancer 114(12):1305-1312.
- Seo SK, Kim JH, Choi HN, Choe TB, Hong SI, et al. (2014) Knock down of TWIST1 enhances arsenic trioxide- and ionizing radiation-induced cell death in lung cancer cells by promoting mitochondrial dysfunction. Biochem Biophys Res Commun 449:490-495.
- Plaks V, Kong N, Werb Z (2015) The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell stem cell 16(3):225-238.
- Aleksakhina SN, Kashyap A, Imyanitov EN (2019) Mechanisms of acquired tumor drug resistance. Biochim Biophys Acta Rev Cancer 1872(2):188310.
- Dalpiaz A, Paganetto G, Botti G, Pavan B (2020) Cancer stem cells and nanomedicine: New opportunities to combat multidrug resistance? Drug Discov Today 25(9):1651-1667.
- Zhang L, Yao HJ, Yu Y, Zhang Y, Li RJ, et al. (2012) Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials 33:565-582.
- Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O et al. (2017) Mitochondria-targeted triphenylphosphonium-based compounds: Syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev 117(15):10043-10120.
[Crossref][Google Scholar][Pubmed]
- Peiris-Pagès M, Bonuccelli G, Sotgia F, Lisanti MP (2018) Mitochondrial fission as a driver of stemness in tumor cells: mDIVI1 inhibits mitochondrial function, cell migration and cancer stem cell (CSC) signalling. Oncotarget 9(17):13254-13275.
- Skoda J, Borankova K, Jansson PJ, Huang ML, Veselska R et al. (2019) Pharmacological targeting of mitochondria in cancer stem cells: An ancient organelle at the crossroad of novel anti-cancer therapies. Pharmacol Res 139:298-313.
- Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L et al. (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell. 40(6):583-594.
- Naik PP, Mukhopadhyay S, Panda PK, Sinha N, Das CK et al. (2018) Autophagy regulates cisplatin‐induced stemness and chemoresistance via the upregulation of CD 44, ABCB 1 and ADAM 17 in oral squamous cell carcinoma. Cell Prolif 51(1):e12411.
- Yan C, Luo L, Guo CY, Goto S, Urata Y et al. (2017) Doxorubicin-induced mitophagy contributes to drug resistance in cancer stem cells from HCT8 human colorectal cancer cells. Cancer Lett 388:34-42.
- Takeda M, Koseki J, Takahashi H, Miyoshi N, Nishida N et al. (2019) Disruption of endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res. 79(7):1426-1437.
- Chang CM, Lan KL, Huang WS, Lee YJ, Lee TW et al. (2017) 188Re-liposome can induce mitochondrial autophagy and reverse drug resistance for ovarian cancer: from bench evidence to preliminary clinical proof-of-concept. Int J Mol Sci 18(5):903.
- De Francesco EM, Ózsvári B, Sotgia F, Lisanti MP (2019) Dodecyl-TPP targets mitochondria and potently eradicates cancer stem cells (CSCs): Synergy with FDA-approved drugs and natural compounds (vitamin C and berberine). Front Oncol 9:615.
- Scatena C, Roncella M, Di Paolo A, Aretini P, Menicagli M et al. (2018) Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (CSCs) in early breast cancer patients: A clinical pilot study. Front Oncol. 8:452.
- Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M et al. (2016) Leukemic stem cells evade chemotherapy by metabolic adaptation to an adipose tissue niche. Cell stem cell 19(1):23-37.
- Araujo EP, Carvalheira JB, Velloso LA (2006) Disruption of metabolic pathways-Perspectives for the treatment of cancer. Curr Cancer Drug Targets 6:77-87.
- Kim SY (2019) Targeting cancer energy metabolism: A potential systemic cure for cancer. Arch Pharm Res 42:140-149.
- Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: Cancer's Achilles' heel. Cancer cell. 13(6):472-482.
- Obre E, Rossignol R (2015) Emerging concepts in bioenergetics and cancer research: Metabolic flexibility, coupling, symbiosis, switch, oxidative tumors, metabolic remodeling, signaling and bioenergetic therapy. Int J Biochem Cell Biol 59:167-181.
- Park KC, Kim SW, Jeon JY, Jo AR, Choi HJ, et al. (2018) Survival of cancer stem-like cells under metabolic stress via CaMK2α-mediated upregulation of sarco/endoplasmicreticulum calcium ATPase expression. Clin Cancer Res 24:1677-1690.
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K (2009) Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69(19):7507-7511.
- Lonardo E, Cioffi M, Sancho P, Ripoll YS, Trabulo S, et al. (2013) Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One 8:e76518.
- Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB et al. (2014) Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. elife 3:e02242.
- Jiang W, Finniss S, Cazacu S, Xiang C, Brodie Z et al. (2016) Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget 7(35):56456-56470.
- Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, et al. (2018) An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med 24:1036-1046.
- Zhou H, Zhang B, Zheng J, Yu M, Zhou T et al. (2014) The inhibition of migration and invasion of cancer cells by graphene via the impairment of mitochondrial respiration. Biomaterials 35(5):1597-607.
- Kuntz EM, Baquero P, Michie AM, Dunn K, Tardito S et al. (2017) Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells Nature medicine 23(10):1234-1240.
- Senkowski W, Zhang X, Olofsson MH, Isacson R, Höglund U et al. (2015) Three-dimensional cell culture-based screening identifies the anthelmintic drug nitazoxanide as a candidate for treatment of colorectal cancer. Mol Cancer Ther 14(6):1504-1516.
- Wang YC, Chao TK, Chang CC, Yo YT, Yu MH et al. (2013) Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PloS one. 8(9):e74538.
- Yo YT, Lin YW, Wang YC, Balch C, Huang RL et al. (2012) Growth inhibition of ovarian tumor–initiating cells by niclosamide. Mol Cancer Ther 11(8):1703-1712.
- Cerella C, Dicato M, Diederich M (2020) BH3 mimetics in AML therapy: Death and beyond? Trends Pharmacol Sci 41:793-814.
- Jones CL, Stevens BM, D'Alessandro A, Reisz JA, Culp-Hill R et al. (2018) Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell 34(5):724-740.
- Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW et al. BH3-mimetic drugs: blazing the trail for new cancer medicines. Cancer cell. 2018 Dec 10;34(6):879-91.
- Liu G, Luo Q, Li H, Liu Q, Ju Y, et al (2020) Increased oxidative phosphorylation is required for stemness maintenance in liver cancer stem cells from hepatocellular carcinoma cell line HCCLM3 cells. Int J Mol Sci 21(15):5276.
- Kuramoto K, Yamamoto M, Suzuki S, Sanomachi T, Togashi K et al. (2020) Verteporfin inhibits oxidative phosphorylation and induces cell death specifically in glioma stem cells. FEBS J 287(10):2023-236.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi