Research Article, Jrgm Vol: 13 Issue: 2
EFFICACY AND SAFETY OF STEM CELLS IN STANDALONE LUMBAR INTERBODY FUSION FOR RECURRENT DISC HERNIATIONS
Alvaro Dowling, MD1* & Kai-Uwe Lewandrowski, MD2
1Orthopaedic Spine Surgeon, Director of Endoscopic Spine Clinic, Santiago, Chile. Visiting Professor, Department of Orthopaedic Surgery, USP, Ribeirão Preto, Brazil.
2Division of Personalized Pain Therapy Research & Education, Center for Advanced Spine Care of Southern Arizona, Tucson, AZ, USA
*Corresponding Author: Dowling A
Orthopaedic Spine Surgeon, Director of Endoscopic Spine Clinic, Santiago, Chile. Visiting Professor, Department of Orthopaedic Surgery, USP, Ribeirão Preto, Brazil
E-mail: adowling@dws.cl
Received: 14-Feb-2024, Manuscript No. JRGM-24-127552;
Editor assigned: 16-Feb-2024, PreQC No. JRGM-24-127552 (PQ);
Reviewed: 01-Mar-2024, QC No. JRGM-24-127552;
Revised: 04-Mar-2024, Manuscript No. JRGM-24-127552 (R);
Published: 11-Mar-2024, DOI:10.4172/2325-9620.1000295
Citation: Dowling A & Lewandrowski KU (2024) Efficacy and Safety of Stem Cells in Standalone Lumbar Interbody Fusion for Recurrent Disc Herniation’s. J Regen Med 13:2.
Copyright: © 2024 Dowling A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
1. Abstract
Objective: This study aimed to evaluate the therapeutic effectiveness and safety of allogeneic stem cell injections in the intervertebral discs of patients with recurrent disc herniations.
Methods: An observational cohort study involved 75 patients with a history of recurrent lumbar disc herniations. During their revision endoscopic transforaminal discectomy surgery, participants received allogeneic cortico-cancellous bone graft infused with allogeneic Mesenchymal Stem Cells (MSCs) placed into the affected hollow intervertebral vacuum discs to achieve un-instrumented spinal fusion aimed at improving clinical outcomes by lowering the risk of recurrent disc herniation at the index level. The primary outcome measure was the interbody fusion grading. Secondary outcomes included: 1) pain intensity (measured by the Visual Analog Scale), 2) functional disability evaluated using the Oswestry Disability Index., and 3) quality of life assessed by the modified Macnab criteria questionnaire. Assessments were made at baseline, 3, 6, 12, and 24 months post-treatment.
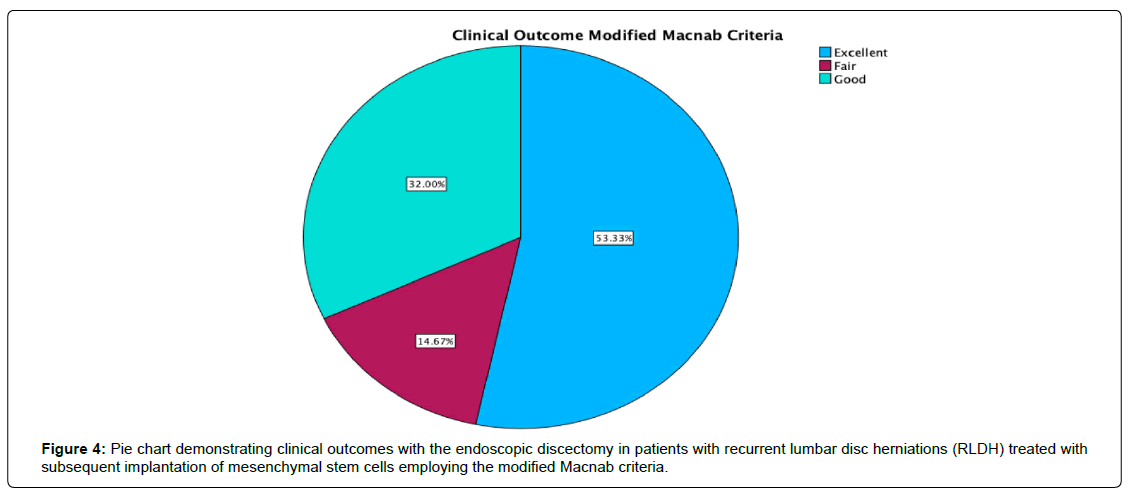
Results: At the 24-month follow-up, 40 patients (53.3%) reported excellent, 24 (32.0%) indicated good, and 11 (14.7%) declared fair functional Macnab outcomes. VAS Leg pain was substantially reduced from a preoperative mean of 8.86 ± 1.086 to 3.00 ± 4.423, signifying a mean decrease of 5.27 ± 2.15 (p < 0.001). The ODI decreased from 55.88 ± 17.12 to 16.40 ± 8.17. Of the 75 patients undergoing interbody fusion, 37 (48.7%) achieved complete fusion (Grade I) with evidence of remodeling and trabecular bone in the fusion space. Twenty-four patients (31.6%) had a Grade II fusion, indicating an intact graft that was not fully remodeled or incorporated. Grade III fusion, characterized by an intact graft with potential lucency at both ends, was observed in 6 patients (7.9%). In comparison, three patients (3.9%) experienced a Grade IV fusion, which was absent due to graft collapse or resorption. Incidental durotomies, nerve root injuries, wound complications, and postoperative instability was absent. No significant adverse events related to stem cell therapy were reported.
Conclusion: Lumbar interbody fusion with allogenic mesenchymal stem cell enriched corticocancellous bone graft in patients with recurrent disc herniations is a safe and effective treatment, leading to reduced pain, improved function, and quality of life. These findings suggest that stem cell-enriched standalone bone grafts could be a viable alternative to conventional lumbar spinal fusion treatments, offering a new avenue for managing patients with this challenging condition without spinal implants. Further research is needed to understand the long-term implications and mechanisms underlying the regenerative potential of stem cell therapy in spinal disorders.
Keywords: Safety and Efficacy of Stem Cell Therapy, Intervertebral Disc Regeneration, Recurrent Disc Herniation, Autologous Stem, Spinal Regenerative Medicine, Clinical Trial Outcomes, Disc Degeneration Treatment, Minimally Invasive Procedures, Functional Disability Reduction
Keywords: Safety and Efficacy of Stem Cell Therapy, Intervertebral Disc Regeneration, Recurrent Disc Herniation, Autologous Stem, Spinal Regenerative Medicine, Clinical Trial Outcomes, Disc Degeneration Treatment, Minimally Invasive Procedures, Functional Disability Reduction
Introduction
Recurrent Lumbar Disc Herniation (RLDH) represents a potentially severe complication affecting 5% to 18% of patients, varying with the follow-up duration. Characterized by significant pain and motor deficits, RLDH often proves refractory to conservative management, necessitating surgical intervention. Revision discectomy, while a standard procedure, typically yields inferior outcomes compared to primary surgery. A particular concern with revision discectomy is the risk of spinal instability due to extensive resection of intervertebral facet joints for nerve exposure, which can lead to more pain. Experience indicates that RLDH is associated with a vacuum disc with extensive inflammatory tissue suggesting a poor prognosis with increased risk of persistent pain. This risk often influences surgeons to consider instrumental fusions, even when they may not be strictly necessary.
A novel surgical approach, Full-Endoscopic Lumbar Discectomy (FELD), has emerged as a less invasive alternative to traditional revision discectomy. FELD can be performed via Trans Foraminal (TFED) or Inter Laminar (IFED) routes. Compared to conventional surgery, FELD offers several advantages, including preservation of dorsal musculature and spinal structures and reduced perioperative morbidity. Given these benefits, FELD has gained increasing acceptance. The technique can be applied through Inter Laminar (IL) or Trans Foraminal (TF) approaches, both providing effective clinical outcomes. However, the TF approach, allowing direct access to the herniated disc via the foramen without disturbing the ligamentum flavum or dural sac, is particularly advantageous for revision surgeries. This approach is preferable in cases with posterior scar tissue complications, offering a less invasive option and reducing the likelihood of revision failure.
Endoscopic spine surgery facilitates intradiscal access by employing the inside-out technique for interventions like allograft and mesenchymal stem cell insertion to promote interbody fusion. Another advantage lies in direct visualization of the endplate preparation. Recent attention has focused on Multipotent Mesenchymal Stem Cell (MSC) treatment delivered percutaneously as a novel approach for treating disco genic Lower Back Pain (LBP) and associated disc degeneration. MSC therapy, unlike traditional methods, supports cellular regeneration. Current evidence suggests MSCs’ unique ability to meet the tripartite goals of disc pathology treatment, alongside modulating immune responses and exerting anti-inflammatory effects on damaged tissues. Human Umbilical Cord Tissue-Derived Mesenchymal Stem Cells (HUC-MSCs) are particularly promising due to their ease of collection, potential for allogeneic application, and low immunogenicity, suggesting their suitability for localized immunosuppression.
Spinal fusion enhancement with MSC has been reported. A study by Stephen et al. 2021 reviewed 19 preclinical and 17 clinical studies examining MSC use in spinal fusion. These studies, though varied in design due to differing osteoconductive scaffolds, cells, and techniques, showed positive outcomes in animal models with appropriate scaffolds and osteogenic differentiation factors. Clinical studies, albeit with methodological and material variations, also indicated promising results. However, due to the heterogeneity of these studies, direct comparisons with autologous bone graft fusion rates remain challenging, underscoring the need for further research in this evolving field.
In this clinical trial, the authors explored the potential of intervertebral MSC delivery in conjunction with corticocancellous bone allograft via the endoscopic route as a minimally invasive intervention for patients suffering from RLDH to facilitate reliable interbody fusion to prevent re-herniation. The study’s rationale is rooted in the regenerative capacity of stem cells, which have shown promise in tissue engineering and regenerative medicine, offering new hope for reducing pain from degenerated intervertebral discs. The objective was to assess not only the efficacy of the endoscopic MSC delivery in reducing the size of disc herniation’s and alleviating symptoms but also to evaluate the safety profile of MSC in this clinical setting. By conducting a well-organized observational cohort study, the authors of this study aimed to provide robust, evidencebased insights into the feasibility of stem cell therapy as a viable alternative to conventional treatment modalities for recurrent disc herniations which in many cases includes instrumented spinal fusion – an aggressive option that is disliked by many patients. The author’s intent was to shift the focus from symptomatic surgical management to regenerative healing, thereby significantly impacting patient care and quality of life.
Materials and Methods
Study Group
A cohort of 75 patients experiencing severe claudication symptoms and sciatica-type low back and leg pain attributable to Recurrent Lumbar Disc Herniation (RLDH) were included in this study. Of the 75 patients, 56 had undergone traditional open micro discectomy surgery and the remaining 19 had a previous FELD. The mean time elapsed from the index operation to the revision intervention for RLDH was. The primary pain generator was identified preoperatively, [1-7] employing a peer-reviewed and published protocol.
Inclusion / Exclusion Criteria
The selection of patients for this study was conducted with rigorous adherence to well-defined criteria. These criteria included the failure of conservative management approaches, radiologically confirmed lumbar stenosis that correlated with the patient’s clinical symptoms and physical examination findings, and a history of recurrent lumbar disc herniation. The latter was specifically defined as a herniated disc reoccurring at the same level and side as previously treated by initial Full-Endoscopic Discectomy (FED) or open surgical intervention, necessitating further surgical management. The inclusion criteria for patients undergoing this procedure were:
1. Clinical presentation of symptoms such as lumbar radiculopathy, dysesthesias (sensory disturbances), and diminished motor function.
2. Diagnostic imaging evidence, including Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans, demonstrating severe central, foraminal, or lateral recess stenosis attributable to Recurrent Lumbar Disc Herniation (RLDH).
3. A history of unsuccessful non-operative treatments, encompassing physical therapy and transforaminal epidural steroid injections, persisting for at least 12 weeks.
4. An age range of 35 to 85 years.
Conversely, certain patients were deemed ineligible for the trans-facet endoscopic lumbar procedure based on the following exclusion criteria:
1. Segmental instability exceeding Grade I spondylolisthesis or translational motion surpassing 8 mm, as evident on preoperative extension-flexion radiographs.
2. The presence of an infection.
3. The existence of metastatic disease.
Endoscopic technique
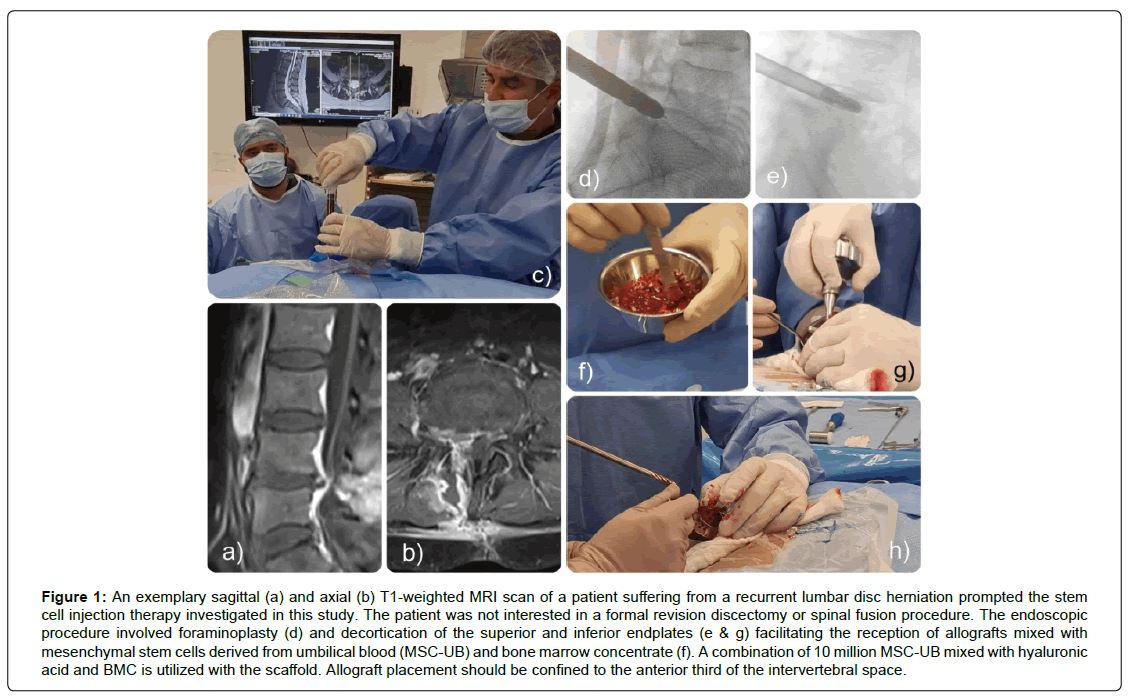
Each patient underwent a Full Endoscopic Discectomy and Transforaminal Foraminoplasty (FED-TF) procedure. Patients are positioned prone and sedated in accordance with Monitored Anesthesia Care (MAC) protocols. Prophylactic oral antibiotics, either cephalosporin or quinolone (in cases of cephalosporin allergy), were administered both before and after the procedure. The surgery commenced with a 1 cm skin incision, followed by the insertion of an endoscope 9 to 13 cm lateral to the midline. Careful resection of scar tissue was conducted. For optimal visualization of the anatomical structures and thorough removal of recurrent disc herniation, an extensive foraminoplasty was executed, extending medially to the edges of the dural sac, both superiorly and inferiorly (Figure 1).
Figure 1: An exemplary sagittal (a) and axial (b) T1-weighted MRI scan of a patient suffering from a recurrent lumbar disc herniation prompted the stem cell injection therapy investigated in this study. The patient was not interested in a formal revision discectomy or spinal fusion procedure. The endoscopic procedure involved foraminoplasty (d) and decortication of the superior and inferior endplates (e & g) facilitating the reception of allografts mixed with mesenchymal stem cells derived from umbilical blood (MSC-UB) and bone marrow concentrate (f). A combination of 10 million MSC-UB mixed with hyaluronic acid and BMC is utilized with the scaffold. Allograft placement should be confined to the anterior third of the intervertebral space.
Subsequently, the integrity and viability of the intervertebral disc were assessed via direct videoendoscopic visualization. Frequently, these discs exhibit significant degenerative changes, characterized by the presence of multiple avascular fragments. These fragments necessitate complete removal via endoscopic techniques. Throughout the procedure, the superior and inferior endplates of adjacent vertebrae are readily identifiable. When indicated, a drilling technique is employed to decorticate the endplates until a bleeding surface is achieved, facilitating the reception of allografts. These allografts are composed Of Mesenchymal Stem Cells Derived from Umbilical Blood (MSC-UB) and Bone Marrow Concentrate (BMC), harvested from the iliac crest. In the authors’ experience, preoperative disc height of 7 mm or less is predictive of vacuum disc and low propensity of postoperative allograft displacement. Allograft placement should be confined to the anterior third of the intervertebral space, ensuring meticulous inspection to avoid graft migration into the foramen or lateral recess. A combination of 10 million MSC-UB mixed with hyaluronic acid and BMC is utilized as a scaffold. Patients receiving this alternative treatment were excluded from the study (Figure 1).
Stem cell harvest & expansion
Allogeneic Mesenchymal Stem Cells (MSCs) were harvested from the mononuclear cell fraction of umbilical cords (UC) with informed maternal consent immediately post-delivery. These cells, specifically Wharton’s Jelly-derived MSCs (WJ-MSCs), genetically mirror the newborn and were procured under stringent aseptic conditions. Umbilical cord segments, approximately 10 cm long, were promptly immersed in a preservative medium with antibiotics and stored at 4°C for transport to the laboratory.
In the lab, the Wharton’s Jelly was meticulously sectioned into 1-2 mm fragments and placed in gelatin-coated 100 mm dishes. Each dish contained 2 grams of WJ tissue, submerged in low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM-LG), enriched with Fetal Bovine Serum (FBS), penicillin, and streptomycin. On the fifth day, half of the medium was refreshed, and by the eighth day, all tissue remnants were removed, and the medium was fully replaced. Cultivation of WJMSCs was conducted at 37°C in a humidified 5% CO2 atmosphere, with media changes ensuring 80% confluence. Cell detachment for passage utilized 0.05% Trypsin-EDTA, with subsequent PBS washing and centrifugation. Seeding density was maintained at 1 × 104 cells/ cm2.
Cell expansion was limited to five passages to preserve pluripotency and prevent functional degradation. The entire process, including procurement, expansion, cryopreservation, and storage, adhered to Current Good Manufacturing Practice (CGMP) standards at an AABB-accredited facility (Vidacel, Vitacura, Chile). Quality control involved Trypan blue exclusion for cell viability, flow cytometry for mesenchymal markers CD44, CD90, CD105, and CD34, and comprehensive testing for infectious diseases, sterility, endotoxins, and mycoplasma. Upon satisfactory evaluation, cells were cryopreserved, stored in sterile containers, and dispatched to clinical sites as “ready for use” preparations for injection into targeted discs.
Interbody Fusion Assessment
The interbody fusion was graded using the Bridwell classification: Grade I, segment fused with remodeling and trabeculae present; Grade II, graft intact, not fully remodeled and incorporated but no lucency present; Grade III, graft intact, potential lucency present at top and bottom of graft; and Grade IV, fusion absent with collapse or resorption of the graft [8].
Statistical Analysis
The primary clinical outcome measures to evaluate the efficacy of allogeneic Mesenchymal Stem Cells (MSCs) in ameliorating RLDH symptoms comprised the modified McNab criteria [9], the Visual Analog Scale (VAS) for low back pain intensity [10], and the Oswestry Disability Index (ODI) [11]. Patient assessments, both clinical and radiographic, were conducted at intervals of 3, 6, 12, and 24 months subsequent to treatment. Additionally, interviews were carried out to record any adverse events. Patient-reported outcome measures (PROMs) were aligned with clinical improvements as observed in post-injection MRI assessments using the Pfirrmann scoring system [12], specifically focused on the recuperating lumbar intervertebral discs, when postoperative imaging was available. The definitive criterion for treatment success was the absence of any further interventions at the treated disc level during the final followup. Analytical examination of demographic and outcome data was undertaken using descriptive statistical methods via SPSS™ version 27 software. The paired T-test was employed to determine the statistical significance of observed improvements.
Results
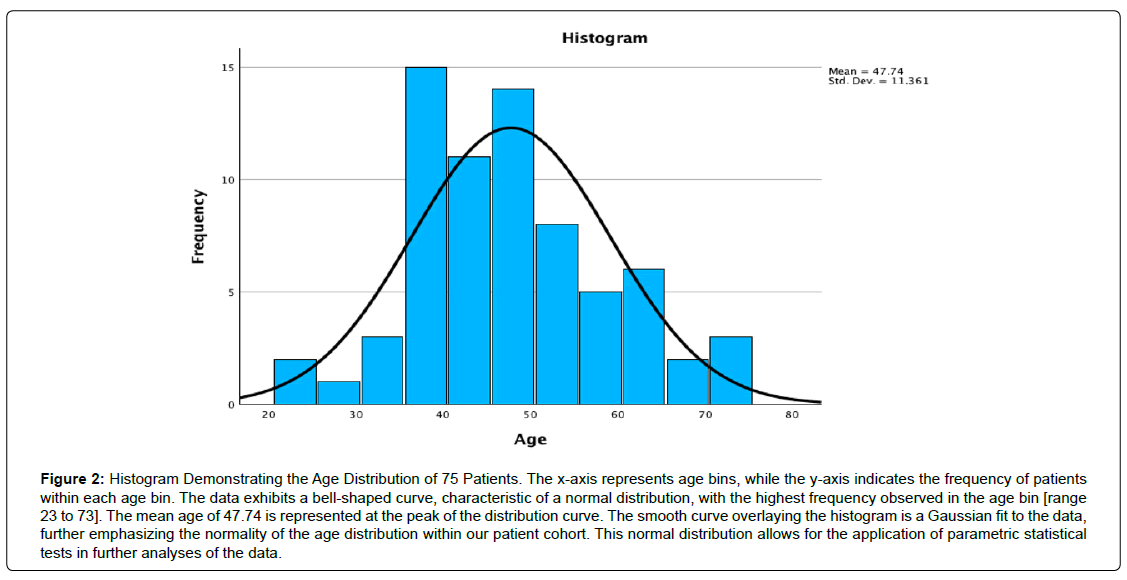
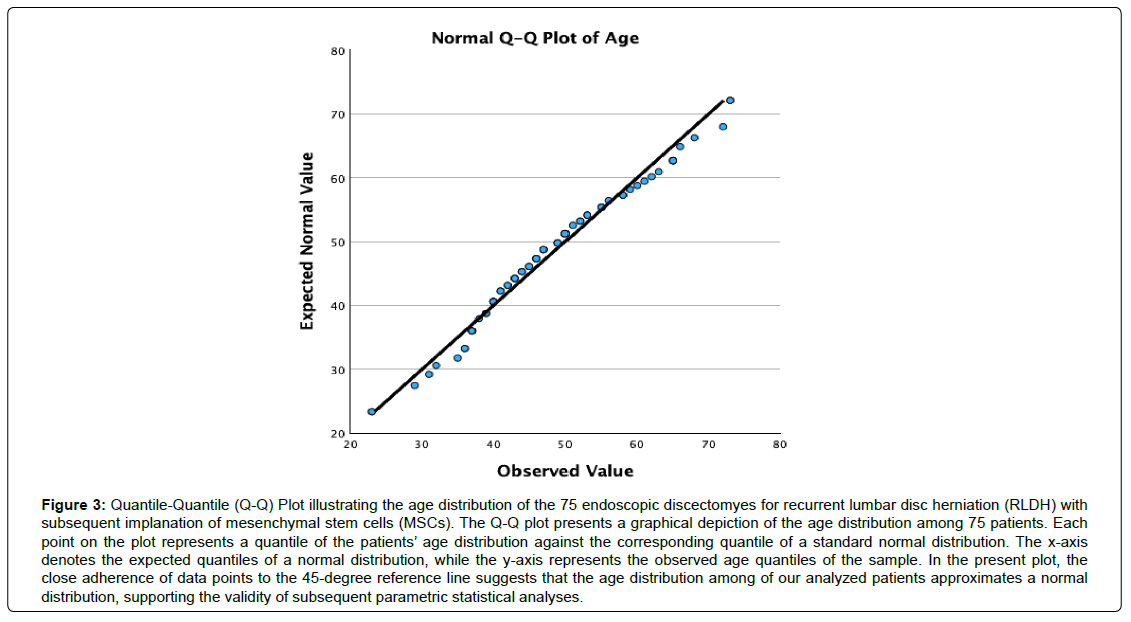
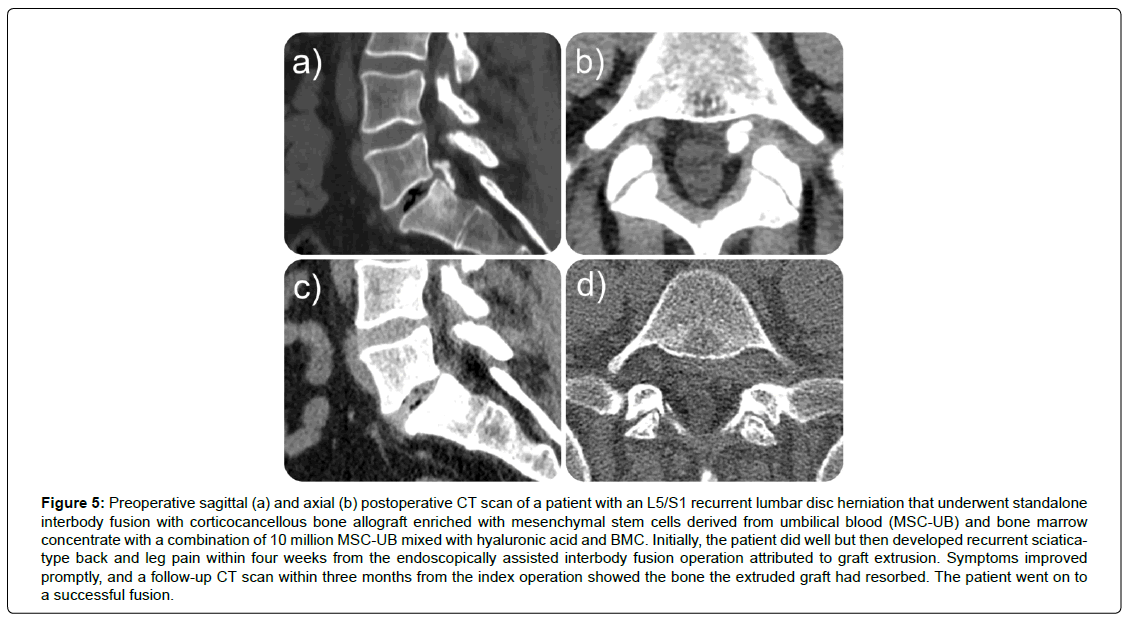
The investigation encompassed 75 participants, consisting of 27 females (36.0%) and 48 males (64.0%), exhibiting a normal age distribution (Figures 2, 3) and an average age of 47.74, spanning from 23 to 73 years. A mean postoperative follow-up period of 21.23 months was achieved, spanning 1 to 59 months (Table 1). At the final follow-up, 40 patients (53.3%) reported excellent, 24 (32.0%) indicated good, and 11 (14.7%) declared fair functional Macnab outcomes (Table 2, Figure 4). Leg pain, evaluated using the VAS score, demonstrated a substantial reduction from a preoperative mean of 8.86 ± 1.086 to 3.00 ± 4.423, signifying a mean decrease of 5.27 ± 2.15 (p < 0.001; (Table 3). The effect size measured as Cohen’s d was 2.212. The ODI decreased from 55.88 ± 17.12 to 16.40 ± 8.17 at final follow-up (Table 4). The effect size measured as Cohen’s d was 6.036. Therefore, the lumbar endoscopic MSC implantation benefit for patients suffering from RLDH was considered “large.” There were no instances of revision surgeries. Complete fusion of the interbody space was observed in 37 of the 75 patients (48.7%), who were successfully fused with remodeling and trabecular bone present in the interbody fusion space (Grade I). Grade II fusion with intact graft but not fully remodeled or incorporated graft in the interbody space was observed in 24 (31.6%) patients versus Grade III (graft intact, potential lucency present at top and bottom of graft in 6 (7.9%) and Grade IV (Fusion absent with collapse/resorption of graft) in 3 (3.9%) of patients, respectively (Table 5). Incidental durotomies, nerve root injuries, wound complications, and postoperative instability was absent. There was one patient who became symptomatic within the first two postoperative weeks with a new onset of sciatica back and leg pain after an initial interval of symptom resolution. His imaging workup with a lumbar CT scan showed extrusion of the stem cellenriched bone graft had occurred. The patient’s symptoms resolved spontaneously without additional surgery and supportive care measures (Figure 5).
Figure 2: Histogram Demonstrating the Age Distribution of 75 Patients. The x-axis represents age bins, while the y-axis indicates the frequency of patients within each age bin. The data exhibits a bell-shaped curve, characteristic of a normal distribution, with the highest frequency observed in the age bin [range 23 to 73]. The mean age of 47.74 is represented at the peak of the distribution curve. The smooth curve overlaying the histogram is a Gaussian fit to the data, further emphasizing the normality of the age distribution within our patient cohort. This normal distribution allows for the application of parametric statistical tests in further analyses of the data.
Figure 3: Quantile-Quantile (Q-Q) Plot illustrating the age distribution of the 75 endoscopic discectomyes for recurrent lumbar disc herniation (RLDH) with subsequent implanation of mesenchymal stem cells (MSCs). The Q-Q plot presents a graphical depiction of the age distribution among 75 patients. Each point on the plot represents a quantile of the patients’ age distribution against the corresponding quantile of a standard normal distribution. The x-axis denotes the expected quantiles of a normal distribution, while the y-axis represents the observed age quantiles of the sample. In the present plot, the close adherence of data points to the 45-degree reference line suggests that the age distribution among of our analyzed patients approximates a normal distribution, supporting the validity of subsequent parametric statistical analyses.
Figure 5: Preoperative sagittal (a) and axial (b) postoperative CT scan of a patient with an L5/S1 recurrent lumbar disc herniation that underwent standalone interbody fusion with corticocancellous bone allograft enriched with mesenchymal stem cells derived from umbilical blood (MSC-UB) and bone marrow concentrate with a combination of 10 million MSC-UB mixed with hyaluronic acid and BMC. Initially, the patient did well but then developed recurrent sciaticatype back and leg pain within four weeks from the endoscopically assisted interbody fusion operation attributed to graft extrusion. Symptoms improved promptly, and a follow-up CT scan within three months from the index operation showed the bone the extruded graft had resorbed. The patient went on to a successful fusion.
| Demographics & Follow up | N | Minimum | Maximum | Mean |
|---|---|---|---|---|
| Age [Years] | 70 | 23 | 73 | 47.74 |
| Postoperative Follow-up [Months] | 75 | 1 | 59 | 21.32 |
| Valid N (List Wise) | 70 | |||
| Gender | N | Percent | Valid Percent | Cumulative Percent |
| F | 27 | 36.0 | 36.0 | 36.0 |
| M | 48 | 64.0 | 64.0 | 100.0 |
| Total | 75 | 100.0 | 100.0 |
Table 1: Patient Demographic and Postoperative Follow-up Data
| Modified Macnab Outcome | Frequency | Percent | Valid Percent | Cumulative Percent |
|---|---|---|---|---|
| Excellent | 40 | 53.3 | 53.3 | 53.3 |
| Good | 24 | 32.0 | 32.0 | 85.3 |
| Fair | 11 | 14.7 | 14.7 | 100.0 |
| Total | 75 | 100.0 | 100.0 |
Table 2: Modified Macnab Outcome Criteria after Endoscopic Discectomy and MSC Implantation for RLDH
| Paired Samples Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Paired Differences | T | DF | Significance | ||||||
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | One-Sided p | Two-Sided p | ||||
| Lower | Upper | ||||||||
| Preop VAS Score - 1 Month Postop VAS Score | 4.318 | 2.338 | .498 | 3.282 | 5.355 | 8.664 | 21 | <.001 | <.001 |
| Preop VAS Score - 3 Months Postop VAS Score | 6.071 | 2.129 | .569 | 4.842 | 7.301 | 10.670 | 13 | <.001 | <.001 |
| Preop VAS Score - 6 Months Postop VAS Score | 4.875 | 1.808 | .639 | 3.364 | 6.386 | 7.628 | 7 | <.001 | <.001 |
| Preop VAS Score - 12 Months Postop VAS Score | 5.273 | 2.149 | .648 | 3.829 | 6.716 | 8.138 | 10 | <.001 | <.001 |
Table 3: Paired-Samples Tests VAS, Effect Size and Confidence Intervals
| Paired Samples Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Paired Differences | T | DF | Significance | ||||||
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | One-Sided p | Two-Sided p | ||||
| Lower | Upper | ||||||||
| Preop ODI - 1 Month Postop ODI | 31.555 | 16.008 | 3.773 | 23.594 | 39.516 | 8.363 | 17 | <.001 | <.001 |
| Preop ODI - 3 Months ODI | 35.692 | 18.381 | 5.098 | 24.584 | 46.800 | 7.001 | 12 | <.001 | <.001 |
| Preop ODI - 6 Months Postop ODI | 43.000 | 21.234 | 6.714 | 27.810 | 58.189 | 6.404 | 9 | <.001 | <.001 |
| Preop ODI - 12 Months Postop ODI | 42.272 | 24.980 | 7.531 | 25.490 | 59.054 | 5.613 | 10 | <.001 | <.001 |
| Preop ODI - 24 Months Postop ODI | 34.800 | 4.816 | 2.154 | 28.819 | 40.780 | 16.155 | 4 | <.001 | <.001 |
Table 4: Paired-Samples Tests ODI, Effect Size and Confidence Intervals
| Grade | Frequency | Percent | Valid Percent | Cumulative Percent |
|---|---|---|---|---|
| 5 | 7.9 | 7.9 | 7.9 | |
| I | 37 | 48.7 | 48.7 | 56.6 |
| II | 24 | 31.6 | 31.6 | 88.2 |
| III | 6 | 7.9 | 7.9 | 96.1 |
| IV | 3 | 3.9 | 3.9 | 100.0 |
| Total | 75 | 100.0 | 100.0 |
I Fused with remodeling and trabeculae present
II Graft intact, not fully remodeled and incorporated, but no lucency present
III Graft intact, potential lucency present at top and bottom of graft
IV Fusion absent with collapse/resorption of graft
Table 5: Bridwell Interbody Fusion Score [8]
Discussion
Numerous studies have demonstrated the effective use of Full- Endoscopic Lumbar Discectomy (FELD) in treating recurrent disc herniation [13-17]. Variants of this technique include FELD via the interlaminar (IL) [16] and transforaminal (TF) [18] approaches. Both methods have been noted for their safety and brevity of operative time, coupled with significant symptomatic improvement [19- 22]. Intraoperative direct visualization often confirms substantial decompression of the nerve roots. The transforaminal approach is particularly noted for safely separating scar tissue adhesions from the dura and lumbar disc [23, 24]. This aspect of the transforaminal technique is of relevance when operating patients with recurrent disc herniations after previous discectomy in whom scar tissue should be expected [25-28].
End-stage vacuum degenerative disease, often observed in patients with recurrent disc herniations, represents a severe and advanced form of spinal degeneration [1, 29, 30]. In this stage, intervertebral discs, which act as cushions between the vertebrae, undergo significant degenerative changes. These changes are characterized by the loss of disc hydration and height, leading to the formation of vacuum phenomena – visible as gas-filled spaces within the disc on radiographic imaging. This vacuum effect is indicative of severe disc degeneration and is often associated with chronic back pain and reduced spinal mobility [31]. Patients with recurrent disc herniations are particularly susceptible to this condition due to the repeated stress and injury to the disc material. The cumulative effect of these herniations accelerates the degenerative process, exacerbating the weakening and dehydration of the disc tissue. As a result, these patients may experience heightened pain, reduced flexibility, and an increased likelihood of spinal instability. The authors attempted to treat painful vacuum discs in patients undergoing surgery for recurrent disc herniations with interbody fusion with corticocancellous bone allograft enriched with mesenchymal stem cells derived from umbilical cord and bone marrow aspirate. This study introduces endoscopic visualization in recurrent disc herniation treatment, combined with preoperative MRI, tomography, and dynamic X-rays. Radiological analysis identified risk factors for recurrent herniation, such as lower disc height [32-39]. While lumbar lordosis and Cobb angles showed no significant differences, a larger lumbar lordosis at the L5/S1 level is suggested as a risk factor. The authors’ approach aims to prevent further herniation and alleviate back pain (Figure 6).
Figure 6: Preoperative sagittal (a) and axial (b) T2-weighted MRI scan of a patient with a L4/5 recurrent lumbar disc herniation within 6 months from the index operation who presented with excruciating left leg pain. The patient underwent decompression and placement of allografts mixed with mesenchymal stem cells derived from umbilical blood (MSC-UB) and bone marrow concentrate with a combination of 10 million MSC-UB mixed with hyaluronic acid and BMC is utilized with the scaffold. Symptoms resolved and the 6-months postoperative CT scan showed fusion of the treated motion segment.
Over 14 years, the authors have utilized biological products, including allografts, growth factors, and MSC stem cells, transitioning to allogenic U-MSC. MSCs contribute osteogenic and osteoinductive properties essential for successful spinal fusion. Ongoing research in regenerative medicine indicates that MSCs, synthetic materials, and BMP-2 proteins might become standard in spinal fusion surgeries [40-42]. The authors have previously demonstrated successful standalone interbody fusion in patients with painful end-stage degenerative disc disease in whom also vacuum disc were found [43]. The latter has been considered a risk factor for vertical and anterolateral instability 31 and a major cause for mechanical low back pain after successful endoscopic decompression surgeries for sciatica [44-47]. Decompression surgeries in the lumbar spine for soft tissue (herniated disc & ligamentum flavum hypertrophy) and bony (osteophytes) stenosis may produce iatrogenic instability which is one of the main indications for spinal instrumented spinal fusion surgery. While these fusion surgeries are largely successful at resolving sciatica and back pain symptoms [48] they bear the risk of producing more pain in later years due to adjacent segment disease, [49-53] implant loosening and failures [54-56]. Traditional biocompatible titanium and polyether-ether-ketone implants may also form a painful biofilm [57]. Therefore, the standalone endoscopic interbody fusion without posterior supplemental fixation is highly attractive [58-60]. It relies on the inherent rigidity of the degenerative lumbar spine and the more targeted decompression of the painful lumbar compressive pathology without the approach-related iatrogenic instability [61]. Spinal instability in the case of recurrent lumbar disc herniation (RLDH) is not well investigated but it is conceivable that the underlying instability of the diseased lumbar spinal motion segment is one of the main underlying reasons for the recurrent disc herniation to occur [62].
This study’s findings highlight the efficacy of lumbar endoscopic MSC implantation in patients with RLDH, reflected in the significant improvement in functional outcomes and pain reduction. The patient demographic, comprising a mix of 27 females and 48 males with a wide age range, indicates the procedure’s applicability across a diverse population. Notably, the absence of surgical complications such as incidental durotomies, nerve root injuries, wound complications, and postoperative instability underscores the safety of this surgical approach. The substantial decrease in leg pain, as evidenced by the VAS score reduction and the dramatic improvement in the ODI scores, underscores the procedure’s effectiveness in alleviating symptoms and enhancing patient quality of life. The significant effect sizes for both VAS and ODI scores further affirm the significant clinical impact of the intervention. The absence of revision surgeries within the follow-up period suggests a durable outcome from the procedure. As assessed by the Bridwell classification, Fusion rates reveal a successful fusion (Grade I) in nearly half of the participants, demonstrating the procedure’s potential to achieve biomechanical stability alongside symptomatic relief. Although a complete fusion was not observed in all patients, most exhibited some successful graft incorporation (Grades I and II combined), indicating a positive trend toward spinal stability. Therefore, the results of this investigation provide compelling evidence supporting lumbar endoscopic MSC implantation as an effective and safe treatment option for patients with RLDH. The significant improvements in pain and disability scores, a favorable fusion rate, and the absence of complications advocate for the procedure’s broader application. Further research with larger sample sizes and more extended follow-up periods will be essential to confirm these findings and to explore the long-term sustainability of the clinical benefits observed.
The exact mechanism of action is unknown. However, it is obvious from the authors’ investigation is that allogeneic Mesenchymal Umbilical Cord Stem Cells (MSCs) significantly enhance the process of standalone lumbar interbody fusion when used in conjunction with a corticocancellous bone allograft through a multifaceted cellular mechanism. These MSCs possess the inherent ability to differentiate into various cell types, including osteoblasts, which are crucial for new bone formation. When introduced into the interbody fusion site, these stem cells not only differentiate into boneforming cells but also secrete a range of growth factors and cytokines that promote angiogenesis, the formation of new blood vessels, and recruit additional progenitor cells to the site of the graft. This creates a favorable environment for bone healing and regeneration. The presence of the corticocancellous bone allograft provides a scaffold that supports and guides the new bone growth facilitated by the MSCs. Together; this synergistic action enhances the fusion process at a cellular level, leading to improved stability and integration of the graft with the host bone, ultimately resulting in a more robust and faster fusion. The latter is of utmost importance to patients with recurrent disc herniations as they have the most to loose with traditional repeat micro discectomy or fusion surgery.
Conclusion
This observational cohort study demonstrated the efficacy of an allogeneic corticocancellous bone graft enhanced with mesenchymal stem cells to reduce pain in patients with recurrent disc herniations in the lumbar intervertebral discs. The findings suggest an added benefit of stem cell therapy, specifically the implantation of mesenchymal stem cells (MSCs) during endoscopic decompression and direct visualization in patients with recurrent disc herniations. This combination procedure offers a promising alternative to traditional methods with a more reliable resolution of symptoms than fusion with an allogeneic cortico-cancellous bone graft alone. Patients receiving MSCs also showed enhanced quality of life compared to those who underwent traditional management methods, suggesting that MSC may contribute to the regeneration of bony tissue and the rapid formation of trabecular bone between the endplates of the affected motion segment. This regenerative capability is critical in addressing the underlying painful pathology of recurrent disc herniations, which one could argue occurs in earlier stages of the degenerative disc disease process. Furthermore, the safety profile of stem cell augmentation of allogeneic bone graft observed in this study underscores the viability of this treatment as a minimally invasive option, offering a lower risk of complications compared to instrumented fusions. Further research is necessary to better understand the mechanisms of tissue regeneration and symptom relief.
References
- Lewandrowski KU, Yeung A (2020) Lumbar Endoscopic Bony and Soft Tissue Decompression with the Hybridized Inside-Out Approach: A Review and Technical Note. Neurospine; 17:S34-S43.
- Yeung A, Lewandrowski KU (2020) Early and Staged Endoscopic Management of Common Pain Generators in the Spine. J Spine Surg; 6:S1-s5.
- Lewandrowski KU, Abraham I, Ramírez León JF, Telfeian AE, Lorio MP, et al. (2022) A Proposed Personalized Spine Care Protocol (SpineScreen) to Treat Visualized Pain Generators: An Illustrative Study Comparing Clinical Outcomes and Postoperative Reoperations between Targeted Endoscopic Lumbar Decompression Surgery, Minimally Invasive TLIF and Open Laminectomy. J Pers Med; 12(7):1065.
- Lewandrowski KU, Yeung A, Lorio MP, Yang H, Ramírez León JF, et al. (2023) Personalized Interventional Surgery of the Lumbar Spine: A Perspective on Minimally Invasive and Neuroendoscopic Decompression for Spinal Stenosis. J Pers Med; 13(5):710.
- Lewandrowski KU (2018) Successful Outcome after Outpatient Transforaminal Decompression for Lumbar Foraminal and Lateral Recess Stenosis: The Positive Predictive Value of Diagnostic Epidural Steroid Injection. Clin Neurol Neurosurg; 173:38-45.
- Yeung AT, Yeung CA (2003) Advances in Endoscopic Disc and Spine Surgery: Foraminal Approach. Surg Technol Int; 11:255-263.
- Yeung A, Roberts A, Zhu L, Qi L, Zhang J, et al. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine; 16:52-62.
- Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K (1995) Anterior Fresh Frozen Structural Allografts in the Thoracic and Lumbar Spine. Do They Work If Combined With Posterior Fusion And Instrumentation In Adult Patients With Kyphosis Or Anterior Column Defects? Spine: 20(12):1410-1418.’
- Macnab I. The Surgery of Lumbar Disc Degeneration. Surg Annu; 8:447-480.
- Reed CC, Wolf WA, Cotton CC, Dellon ES (2017) A Visual Analogue Scale and a Likert Scale are Simple and Responsive Tools for Assessing Dysphagia in Eosinophilic Oesophagitis. Aliment Pharmacol Ther; 45:1443-1448.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine; 25(22): 2940-2953.
- Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine; 26(17): 1873-1878.
- Ruetten S, Komp M, Merk H, Godolias G (2009) Recurrent Lumbar Disc Herniation After Conventional Discectomy: A Prospective, Randomized Study Comparing Full-Endoscopic Interlaminar And Transforaminal Versus Microsurgical Revision. J Spinal Disord Tech; 22:122-129.
- Smith JS, Ogden AT, Shafizadeh S, Fessler RG (2010) Clinical Outcomes after Microendoscopic Discectomy for Recurrent Lumbar Disc Herniation. J Spinal Disord Tech; 23(1): 30-34.
- Teli M, Lovi A, Brayda-Bruno M, Zagra A, Corriero A, et al. (2010) Higher Risk of Dural Tears And Recurrent Herniation With Lumbar Micro-Endoscopic Discectomy. Eur Spine J; 19:443-450.
- Chumnanvej S, Kesornsak W, Sarnvivad P, Kuansongthum V (2011) Full Endoscopic Lumbar Discectomy Via Interlaminar Approach: 2-Year Results in Ramathibodi Hospital. J Med Assoc Thai;94:1465-1470.
- Li Z, Jiancheng Z, Song Y, Kong Q, Wang X, et al. (2015) Effectiveness Of Percutaneous Endoscopic Transforaminal Discectomy For Recurrent Lumbar Disc Herniation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhik; 29(1): 43-47.
- Hoogland T, van den Brekel-Dijkstra K, Schubert M, Miklitz B (2008) Endoscopic Transforaminal Discectomy For Recurrent Lumbar Disc Herniation: A Prospective, Cohort Evaluation Of 262 Consecutive Cases. Spine; 33(9): 973-978.
- Tao XZ, Jing L, Li JH (2018) Therapeutic Effect Of Transforaminal Endoscopic Spine System In The Treatment Of Prolapse Of Lumbar Intervertebral Disc. Eur Rev Med Pharmacol Sci; 22:103-10.
- Wang Y, Zhang W, Lian L, et al. Transforaminal Endoscopic Discectomy for Treatment of Central Disc Herniation: Surgical Techniques and Clinical Outcome. Pain Physician 2018;21:E113-E23.
- Wu F, Kong W, Liao W, Ao J, Ye S, et al. (2018) Percutaneous Total Endoscopic Resection of Partial Articular Processes for Treatment of Lateral Crypt Stenosis and Lumbar Spinal Stenosis: Technical Report and Efficacy Analysis. Biomed Res Int; 2018.
- Elkheshin SE, Soliman AY (2020) Endoscopic Interlaminar Lumbar Discectomy: How To Decrease The Learning Curve. Surg Neurol Int; 11.
- Yeung A, Gore S (2014) Endoscopic Foraminal Decompression For Failed Back Surgery Syndrome Under Local Anesthesia. Int J Spine Surg; 8.
- Yao H, Xu YC, Chen BY, Hou G, Zhao HQ (2015) Surgical Revision Of Lumbar Vertebrae Using Transforaminal Endoscopic Spine System. Zhongguo Gu Shang; 28:712-716.
- Yeung A, Wei S-H (2020) Surgical Outcome Of Workman’s Comp Patients Undergoing Endoscopic Foraminal Decompression For Lumbar Herniated Disc. J Spine Surg; 6(Suppl 1): S116.
- Lewandrowski KU, Ransom NA, Yeung A (2020) Return to Work and Recovery Time Analysis after Outpatient Endoscopic Lumbar Transforaminal Decompression Surgery. J Spine Surg; 6(Suppl 1): S100.
- Telfeian AE, Shen J, Ali R, Oyelese A, Fridley J, Gokaslan ZL (2020) Incidence and Implications of Incidental Durotomy in Transforaminal Endoscopic Spine Surgery: Case Series. World Neurosurg; 134: e951-e955.
- Hagan MJ, Telfeian AE, Sastry R, Ali R, Lewandrowski KU, et al.(2022) Awake Transforaminal Endoscopic Lumbar Facet Cyst Resection: Technical Note and Case Series. J Neurosurg Spine;1-8.
- Lewandrowski KU, Leon JFR, Yeung A (2019) Use of "Inside-Out" Technique for Direct Visualization of a Vacuum Vertically Unstable Intervertebral Disc During Routine Lumbar Endoscopic Transforaminal Decompression-A Correlative Study of Clinical Outcomes and the Prognostic Value of Lumbar Radiographs. Int J Spine Surg; 13(5): 399-414.
- Dowling Á, Bárcenas JGH, Lewandrowski KU (2020) Transforaminal Endoscopic Decompression And Uninstrumented Allograft Lumbar Interbody Fusion: A Feasibility Study In Patients With End-Stage Vacuum Degenerative Disc Disease. Clin Neurol Neurosurg; 196:106002.
- Lewandrowski KU, Zhang X, León JF, de Carvalho PS, Hellinger S, et al. Lumbar Vacuum Disc, Vertical Instability, Standalone Endoscopic Interbody Fusion, And Other Treatments: An Opinion Based Survey Among Minimally Invasive Spinal Surgeons. J Spine Surg; 6:S165-S78.
- Chang SB, Lee SH, Ahn Y, Kim JM (2006) Risk Factor For Unsatisfactory Outcome After Lumbar Foraminal And Far Lateral Microdecompression. Spine; 31(10): 1163-1167.
- Moliterno JA, Knopman J, Parikh K, Cohan JN, Huang QD, et al. (2010) Results And Risk Factors For Recurrence Following Single-Level Tubular Lumbar Microdiscectomy. J Neurosurg Spine; 12:680-686.
- Shin BJ (2014) Risk Factors For Recurrent Lumbar Disc Herniations. Asian Spine J; 8:211-215.
- Huang W, Han Z, Liu J, Yu L, Yu X (2016) Risk Factors for Recurrent Lumbar Disc Herniation: A Systematic Review and Meta-Analysis. Medicine; 95.
- Yaman M, Kazanci A, Yaman N, Bas F, Ayberk G (2017) Factors That Influence Recurrent Lumbar Disc Herniation. Hong Kong Med J; 23(3):258-263.
- Yao Y, Liu H, Zhang H, Wang H, Zhang C, et al. (2017) Risk Factors for Recurrent Herniation After Percutaneous Endoscopic Lumbar Discectomy. World Neurosurg; 100:1-6.
- Shin EH, Cho KJ, Kim YT, Park MH (2019) Risk Factors For Recurrent Lumbar Disc Herniation After Discectomy. Int Orthop; 43:963-967.
- Yu C, Zhan X, Liu C, Liao S, Xu J, et al. (2020) Risk Factors for Recurrent L5-S1 Disc Herniation After Percutaneous Endoscopic Transforaminal Discectomy: A Retrospective Study. Med Sci Monit; 26: e919888-1.
- Duarte RM, Varanda P, Reis RL, Duarte AR, Correia-Pinto J (2017) Biomaterials and Bioactive Agents in Spinal Fusion. Tissue Eng Part B Rev; 23:540-551.
- Tateiwa D, Kaito T (2023) Advances in Bone Regeneration With Growth Factors For Spinal Fusion: A Literature Review. North American Spine Society Journal (NASSJ); 13:100193.
- Glaeser JD, Salehi K, Kanim LE, Ju DG, Hyuk Yang J, et al. (2020) Electrospun, Synthetic Bone Void Filler Promotes Human Msc Function and Bmp-2 Mediated Spinal Fusion. J Biomater Appl; 35(4-5): 532-543.
- Dowling A, Barcenas JGH, Lewandrowski KU (2020) Transforaminal Endoscopic Decompression And Uninstrumented Allograft Lumbar Interbody Fusion: A Feasibility Study In Patients With End-Stage Vacuum Degenerative Disc Disease. Clin Neurol Neurosurg; 196:106002.
- Martínez CR, Lewandrowski KU, Ortíz JG, Cuéllar GO, León JF (2020) Transforaminal Endoscopic Discectomy Combined With an Interspinous Process Distraction System for Spinal Stenosis. Int J Spine Surg; 14(s3): S4-S12.
- Lewandrowski KU, Abraham I, León JF, Cantú-Leal R, Longoria RC, et al. (2022) A Differential Clinical Benefit Examination of Full Lumbar Endoscopy vs Interspinous Process Spacers in the Treatment of Spinal Stenosis: An Effect Size Meta-Analysis of Clinical Outcomes. Int J Spine Surg; 16(1): 102-123.
- Lewandrowski KU, Ransom NA (2020). Five-Year Clinical Outcomes With Endoscopic Transforaminal Outside-In Foraminoplasty Techniques For Symptomatic Degenerative Conditions Of The Lumbar Spine. J Spine Surg; 6:S54-S65.
- Yeung A, Lewandrowski KU (2020) Five-Year Clinical Outcomes With Endoscopic Transforaminal Foraminoplasty For Symptomatic Degenerative Conditions Of The Lumbar Spine: A Comparative Study Of Inside-Out Versus Outside-In Techniques. J Spine Surg; 6:S66-S83.
- Kurra S, Lavelle WF, Silverstein MP, Savage JW, Orr RD (2018) Long-Term Outcomes Of Transforaminal Lumbar Interbody Fusion In Patients With Spinal Stenosis And Degenerative Scoliosis. Spine J; 18:1014-1021.
- Luque R, Echevarría M, Alcobía B, Urda A, Domínguez I, et al. (2020) Results And Complications Of Adjacent Segment Disease Treated By Minimally Invasive Lateral Intersomatic Arthrodesis. Acta Ortop Mex; 34:388-398.
- Sakti YM, Mafaza A, Lanodiyu ZA, Sakadewa GP, Magetsari R (2020) Management Of Distal Adjacent Segment Disease Due To Central Subsidence Of Plif Using Local Anesthetic Transforaminal Foraminotomy And Lumbar Discectomy. Int J Surg Case Rep; 77:269-275.
- Parish JM, Asher AM, Coric D (2021) Adjacent-Segment Disease Following Spinal Arthroplasty. Neurosurg Clin N Am; 32:505-510.
- Toivonen LA, Mäntymäki H, Häkkinen A, Kautiainen H, Neva MH (2022) Isthmic Spondylolisthesis is Associated with Less Revisions for Adjacent Segment Disease After Lumbar Spine Fusion Than Degenerative Spinal Conditions: A 10-Year Follow-Up Study. Spine; 47(4): 303.
- Zhang B, Hu Y, Kong Q, Feng P, Liu J, et al. (2023) Comparison of Oblique Lumbar Interbody Fusion Combined with Posterior Decompression (OLIF-PD) and Posterior Lumbar Interbody Fusion (PLIF) in the Treatment of Adjacent Segmental Disease(ASD). J Pers Med; 13(2):368.
- Marie-Hardy L, Pascal-Moussellard H, Barnaba A, Bonaccorsi R, Scemama C (2020) Screw Loosening in Posterior Spine Fusion: Prevalence and Risk Factors. Glob Spine J; 10:598-602.
- Veronesi F, Sartori M, Griffoni C, Valacco M, Tedesco G, et al. (2022) Complications in Spinal Fusion Surgery: A Systematic Review of Clinically Used Cages. J Clin Med; 11(21):6279.
- Koshimizu H, Nakashima H, Ohara T, Tauchi R, Kanemura T, et al. (2024) Implant-Related Complications After Spinal Fusion: A Multicenter Study. Glob Spine J; 14 (1):74-81.
- Kligman S, Ren Z, Chung CH, Perillo MA, Chang YC, et al. (2021) The Impact Of Dental Implant Surface Modifications On Osseointegration And Biofilm Formation. J Clin Med; 10(8):1641.
- Emstad E, Del Monaco DC, Fielding LC, Block JE (2015) The Varilift((R)) Interbody Fusion System: Expandable, Standalone Interbody Fusion. Med Devices (Auckl); 8:219-230.
- Lewandrowski KU, Ransom NA, León JF, Yeung A (2019) The Concept for A Standalone Lordotic Endoscopic Wedge Lumbar Interbody Fusion: The LEW-LIF. Neurospine; 16(1):82-95.
- León JF, Ardila ÁS, Ortíz JG, Martínez CR, Cuéllar GO, et al. (2020) Standalone Lordotic Endoscopic Wedge Lumbar Interbody Fusion (LEW-LIF) With a Threaded Cylindrical Peek Cage: Report of Two Cases. J Spine Surg; 6:S275-S84.
- Ntoukas V, Muller A (2010) Minimally Invasive Approach Versus Traditional Open Approach For One Level Posterior Lumbar Interbody Fusion. Minim Invasive Neurosurg; 53(01):21-24.
- Suk KS, Lee HM, Moon SH, Kim NH (2001) Recurrent Lumbar Disc Herniation: Results of Operative Management. Spine; 26:672-676.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi