Briefreport, Int J Ophthalmic Pathol Vol: 12 Issue: 1
Efficacy of Dexamethasone Sustained Released Implant in Treatment of Diabetic Macular Oedema: A Study during the COVID-19 Pandemic
Hamoud A*, Zacharaki F, Rashad M, Awwad S, Herscu K, Rezai P and Habeb M
1Department of Ophthalmology, Ashford and St. Peter’s Hospitals, NHS Foundation Trust, London Road, Guildford, England, United Kingdom
*Corresponding Author: Hamoud A,
Department of Ophthalmology, Ashford and St. Peter’s Hospitals, NHS Foundation Trust, London Road, Guildford, GU4-7, England, United Kingdom
Tel: +447470185991
E-mail: aseelhamoud@yahoo.com
Received date: 04 March, 2023, Manuscript No. IOPJ-23-91495;
Editor assigned date: 08 March, 2023, PreQC No. IOPJ-23-91495 (PQ);
Reviewed date: 22 March, 2023, QC No. IOPJ-23-91495;
Revised date: 29 March, 2023, Manuscript No. IOPJ-23-91495 (R);
Published date: 05 April, 2023, DOI: 10.4172/2324-8599.12.1.1
Citation: Hamoud A, Zacharaki F, Rashad M, Awwad S, Herscu K, et al. (2023) Efficacy of Dexamethasone Sustained Released Implant in Treatment of Diabetic Macular Oedema: A Study during the COVID-19 Pandemic. Int J Ophthalmic Pathol 12:1.
Abstract
Aim: The aim of this study is to report the efficacy of dexamethasone intravitreal implant in patients with Diabetic Macular Oedema (DMO) in a real life setting, under limitations posed by the COVID-19 pandemic we performed a retrospective observational review twenty patients (25 eyes) diagnosed with DMO were included. Patients were diagnosed and treated between January 2020 and December 2021 at Ashford and St. Peter’s hospitals NHS foundation trust. All patients were treated with intravitreal implants releasing 0.7 mg of dexamethasone. The mean age was 72 years (59-85) 60%of patients were males and 40% were females. The mean Best- Corrected Visual Acuity (BCVA) changed from 0.528 LogMAR to 0.436 LogMAR after 3-6 months, (p-value was not significant). The CMT changed from 469 μm before treatment to 399 μm after 3-6 months (p-value=0.000202). Only two patients needed to be treated with topical anti-glaucoma medication to control Intraocular Pressure (IOP). Five patients had a cataract extraction surgery at the time of their dexamethasone injection.
Purpose: The purpose of this study is to document the efficacy and safety of using dexamethasone 700 μg intravitreal IV injections as treatment for Diabetic Macular Oedema (DMO) over a 6 months period in Ashford and St. Peter’s hospitals NHS foundation trust. Further to this we reported type of complications and management.
Keywords: Dexamethasone; Diabetic macular oedema; COVID-19; Best-Corrected Visual Acuity
Introduction
Diabetic Macular Oedema (DMO) is one of the leading causes of visual impairment in diabetic patients [1]. It affects about 20% of patients with Diabetic Retinopathy (DR), with 50% of them are losing are losing up to two or more lines of their vision due to their DMO when left untreated [2,3]. With the initiation of Vascular Endothelial Growth Factor (VEGF) the visual acuity and vision-related quality of life of people who have DMO have significantly improved. Nevertheless, many DMO patients still need frequent and multiple injections of anti-VEGF. Furthermore, up to half of the treated DMO patients do not achieve long-term resolution of their pathology. As Inflammation plays an important role in the pathophysiology of DMO, intravitreal steroids were the first class of intravitreal medications that were evaluated for the management of such cases of refractory DMO and are mediated through the expression of prostaglandins, leukotrienes and VEGF [1]. As a result, steroids can have a vital role in reducing intracellular and extracellular oedema through inhibition of these factors (Figure 1) [2].
Figure 1: DMO patients still need frequent and multiple injections of anti-VEGF.
Methodology
We performed a retrospective observational analysis of clinical notes of 20 consecutive patients (25 eyes) diagnosed with DMO. They were treated from January 2020 to December 2021 at Ashford and St. Peter’s hospitals NHS foundation trust. The eyes had DMO with increasing Central Macular Thickness (CMT) of 400 μm or more on Optical Coherent Tomography (OCT). Treatment with IV inserts releasing 0.7 μg of dexamethasone (ozurdex) was given to these patients. We collected data on demographics, measured the change of their vision, change of central macular thickness CMT, and Intraocular Pressure (IOP) over 3-6 months’ period. We documented adjuvant treatment given to these eyes over the same time; we recorded complications and secondary effects from the injections.
Results and Discussion
The mean age at baseline was 72 years (59-85), 60% of patients were males and 40% were females. The mean best corrected visual acuity BCVA changed from 0.528 LogMAR to 0.436 LogMAR after 3-6 months, (p-value was not significant). The CMT changed from 469 μm before treatment to 399 μm after 3-6 months (p-value=0.000202), IOP in most of the patients was fluctuating between 17-21 mm Hg, only two eyes have to be treated with topical anti glaucoma medication to control IOP. Thirteen of our patients (52%) are naive i.e., they didn’t have any type of injection before the IV dexamethasone, twelve (48%) did have anti-VEGF intravitreal injections before their treatment. Five of the eyes observed had a cataract extraction surgery with intra-ocular lens implant during the time of their dexamethasone injection.
The use of steroids is associated with possible adverse side effects. Raised IOP may occur in up to 50% of patients, as well as a 40% risk of cataract formation in phakic eyes [2]. However, a study by Mathis, et al. which followed up DMO patients treated with steroid implant concluded that these occurrences did not offset the visual gain nor did they pose a therapeutic and/or visual challenge in the long term. Regarding IOP elevation, a transient increase in IOP ≥ 25 mm Hg was observed in 14.1% of eyes; all cases were sufficiently controlled by topical IOP-lowering medications alone [4].'
Additionally, similar to anti-VEGF drugs such as ranibizumab, the therapeutic effect of intravitreal steroid is short-lived, and patients require frequent injections with higher cumulative risks of side effects. To help counteract this, sustained release corticosteroids have been developed with the aim of offering longer lasting effects thus reducing the burden of frequent clinical visits [2]. The dexamethasone intravitreal implant-Ozurdex-is a sustained-release biodegradable implants, containing dexamethasone that is injected into the vitreous and slowly releases 700 mg dexamethasone into the posterior segment [2]. Some studies that reported the outcomes of the dexamethasone implant for DMO in routine clinical practice found that the implant was safe and effective in improving visual acuity and reducing macular thickness from a few injections [5]. Furthermore, the intravitreal bevacizumab vs. intravitreal dexamethasone for DMO (BEVORDEX) study found that the proportion of eyes with vision improvement at 12 months was similar in the two groups from a mean number of injections of 2.7 in the dexamethasone and 8.6 in the bevacizumab group [5].
A study carried out by Bonfiglio et al. looking into dexamethasone for unresponsive DMO, demonstrated that in eyes with DMO poorly responding to ranibizumab, dexamethosone significantly improves BCVA one month after treatment (from baseline 51.5 ± 8.3 letters to 56.9 ± 8.8 letters, p=0.017) and reduces CMT significantly at the 1st and 3rd months (from 618 ± 94 to 417 ± 149 μm and 469 ± 128 μm, respectively, both p<0.001) (Figure 2) [6].
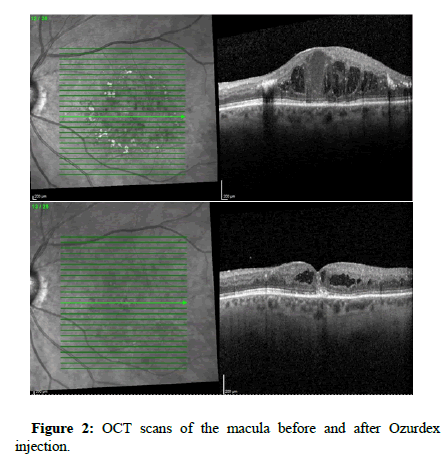
Figure 2: OCT scans of the macula before and after Ozurdex injection.
These results are consistent with the findings in our study as well of those of other studies. One month after dexamethasone, Cicinelli, et al. reported a mean visual gain of 5.3 letters (baseline 63.8 ± 23 letters) with a CMT reduction of 153 μm (baseline 376 ± 136.1 μm) and Yucel, et al. reported a significant BCVA increase (from 0.68 ± 0.27 to 0.56 ± 0.30 LogMAR) and CMT decrease (from 578.93 ± 17.95 μm to 282.10 ± 21.42 μm) (both p=0.001) [3,7]. Arikan, et al. showed no BCVA improvement at 1 month (from 0.85 ± 0.54 to 0.76 ± 0.5 LogMAR; p=0.08) but significant improvement at 3 months (0.69 ± 0.4 LogMAR; p=0.02) and decreased mean CMT (from 572.4 ± 123.1 μm to 264.2 ± 114.4 μm; p<0.05) 1 month after dexamethasone implant [8]. Furthermore, a recent metanalysis of 15 studies including a total of 3859 patients showed that patients treated with the dexamethasone implant for refractory DMO gained a mean of four lines (20 ETDRS letters); with a mean improvement of 0.471 LogMAR (Figure 3) [9].
Figure 3: Fundal fluorescence angiography of DMO.
Mathis, et al. observed improvement in visual acuity and significant CMT reduction after treatment with dexamethasone implant from the second month, with Bhandari, et al. reporting peak changes in the first 2 months [4,5].
In conclusion, treatment using dexamethasone implant for DMO is effective in the long term, keeping excellent long-term visual gain. The safety results are also favorable with a low occurrence of side effects such as ocular hypertension or cataract that remained manageable with topical medication only [4].
Conclusion
With the recent COVID-19 pandemic it was not easy for us to follow-up with our patients as we used to do. The advantage of the dexamethasone intravitreal insertion is that it has a relatively long duration of action that allows patients to get a smaller number of hospital visits with effective treatment of their visual symptoms. Although dexamethasone has no effect on ischaemic changes in the retina as it doesn’t prevent neovascularation, but it has a good role in controlling macular oedema. Side effects were minimal. Our study proved that dexamethasone sustained release is an effective medicine for treating DMO. Furthermore, it’s well tolerated by patients. More studies are needed to record long-term safety and effectivity of dexamethasone insert, but our data aims to contribute to that debate.
References
- Ramu J, Yang Y, Menon G, Bailey C, Narendran N, et al. (2015) A randomized clinical trial comparing fixed vs. pro-re-nata dosing of ozurdex in refractory diabetic macular oedema (OZDRY study). Eye 29: 1603–1612.
[Crossref] [Google scholar] [Indexed]
- Hay AA, Sivaprasad S, Subramanian A, Barbur JL (2018) Acuity and colour vision changes post intravitreal dexamethasone implant injection in patients with diabetic macular oedema. PLoS One 13: e0199693.
[Crossref] [Google scholar] [Indexed]
- Cicinelli MV, Cavalleri M, Querques L, Rabiolo A, Bandello F, et al. (2017) Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. Br J Ophthalmol 101: 1689–1693.
[Crossref] [Google scholar] [Indexed]
- Mathis T, Lereuil T, Abukashabah A, Voirin N, Sudhalkar A, et al. (2020) Long-term follow-up of diabetic macular oedema treated with dexamethasone implant: A real-life study. Acta Diabetol 57: 1413–1421.
[Crossref] [Google scholar] [Indexed]
- Bhandari S, Gabrielle PH, Nguyen V, Daien V, Viola F, et al. (2022) Dexamethasone implant for diabetic macular oedema: 1-year treatment outcomes from the fight retinal blindness! registry. Ophthalmol Ther 11: 797–810.
[Crossref] [Google scholar] [Indexed]
- Bonfiglio V, Reibaldi M, Pizzo A, Russo A, Macchi L, et al. (2019) Dexamethasone for unresponsive diabetic macular oedema: Optical coherence tomography biomarkers. Acta ophthalmol 97: e540–e544.
[Crossref] [Google scholar] [Indexed]
- Yucel OE, Can E Ozturk HE, Birinci H, Sullu Y, et al. (2017) Dexamethasone implant in chronic diabetic macular edema resistant to intravitreal ranibizumab treatment. Ophthalmic Res 57: 161–165.
[Crossref] [Google scholar] [Indexed]
- Arikan YM, Toklu Y, Mutlu M, Uysal BS, Çakmak HB. (2016) Efficacy of single-dose dexamethasone implantation in patients with persistent diabetic macular edema. Int Ophthalmol 36: 531–539.
[Crossref] [Google scholar] [Indexed]
- Khan Z, Kuriakose RK, Khan M, Chin EK, Almeida DRP. (2017) Efficacy of the intravitreal sustained-release dexamethasone implant for diabetic macular edema refractory to anti-vascular endothelial growth factor therapy: Meta-analysis and clinical implications. Ophthalmic Surg Lasers Imaging Retina 48: 160–166.
[Crossref] [Google scholar] [Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi