Research Article, J Vet Sci Med Diagn Vol: 12 Issue: 2
Epidemiology of Gastro-Intestinal Parasites of Cattle Managed Under Extensive Production System in Arbaminch Zuria Wereda of Gamo Zone, Southern Ethiopia
Desalegn Dosa*, Misganaw Mulugeta and Tekile Alaro
Department of Veterinary Epidemiology, Sodo Regional Veterinary Laboratory, Wolaita Sodo, Ethiopia
*Corresponding Author: Desalegn Dosa
Department of Veterinary Epidemiology, Sodo Regional Veterinary Laboratory, Wolaita Sodo, Ethiopia
E-mail: d.drdesvet12@gmail.com
Received date: 20 September, 2022, Manuscript No. JVSMD-22-75251; Editor assigned date: 23 September, 2022, PreQC No. JVSMD-22-75251 (PQ); Reviewed date: 07 October, 2022, QC No. JVSMD-22-75251; Revised date: 03 February, 2023, Manuscript No. JVSMD-22-75251 (R); Published date: 10 February, 2023, DOI: 10.4172/2325-9590.100037
Citation: Dosa D, Mulugeta M, Alaro T (2023) Epidemiology of Gastro-Intestinal Parasites of Cattle Managed Under Extensive Production System in Arbaminch Zuria Wereda of Gamo Zone, Southern Ethiopia. J Vet Sci Med Diagn 12:2
Abstract
In order to determine the prevalence of GIT parasites and related risk factors, a cross-sectional study was carried out in the Gamo Zone, Arbamich Zuriya Woreda, SNNPR, Ethiopia during the years of 2012 and 2013 E.C. The sedimentation and floatation technique was used to conduct a qualitative feces examination. 594 cattle were examined and 333 (56.23%) of them tested positive for at least one egg or more of Strongyle type, Fasciola spp., Emeria, Neoascaris, mixed type, Strongyloides spp., and Paramphistomum spp. parasites, respectively (42.5%), (16.16%), (9.8%), (9.58%), (6.58%), (5.68%) and (3.58%). According to this finding, Strongyle type eggs were more common than those of other helminths while Paramphistomum eggs were less common. Age and physical condition were significantly different risk factors (p=0.05, P-value =0.001, AOR=3.96, 95% CI=2.75-5.65).
Keywords: Cattle; Gastrointestinal parasites; Prevalence; Risk factor
Introduction
Ethiopia, a country in Eastern Africa, depends heavily on agriculture. In every ecological region of the nation, animal production is carried out [1]. There are around 53.99 million cattle in Ethiopia [2]. However, due to a number of reasons including many prevailing socioeconomic norms and attitudes, conventional management techniques, restricted genetic potential and widespread illness, the contribution from this enormous cattle resource to the national revenue is disproportionately minimal. One of the causes of low productivity and production is disease [3]. Parasitic illnesses are regarded as a significant barrier to the health and productivity of livestock. Small and large scale farmers’ a like struggle with Digestive Tract (GIT) infections, but their effects are more severe in Ethiopia in particular, due to the presence of a variety of agro ecological elements favorable for a variety of host and parasite species and sub-Saharan Africa in general. Productivity losses are caused by these parasite infestations [4].
There are numerous types of Gastroin Testinal (GIT) parasites that can infect cattle globally. Nematodes like Strongyle species (Haemonchus, Ostartagia, Trichostrongylus and Cooperia) and as well as trematodes of economic significance, are among the most significant. Along with cestodes like Monezia species (Monezia benideni and Monezia expanza), Fasciola species (Fasciola hepatica and Fasciola gigantica) and Paramphistomum species (Paramphistomum cervei) could also be significant barriers to animal production [5]. The prevalence and severity of GI helminths are influenced by a number of linked risk factors. Age, gender, weather and husbandry or management practices are a few of them [6]. GI parasitism in ruminants often occurs throughout the year, with the rainy season having the highest occurrence rate [7]. The growth, development and survival of many parasites, including eggs, larvae, cysts and oocysts or their intermediate hosts, are mostly influenced by climatic elements including temperature and humidity. Especially in young animals, the projected mortality rate is around 10% and weight loss of 6 kg-12 kg per animal per year is possible [8]. According to a research done in and around Holleta, 82.8% of cattle had parasite diseases overall. Trematodes (Fasciola and Paraphistomum spp.), Strongyles (66.25%) and combined infections (Trematodes and Strongyles) (63.12%) were the most common helminths found, however others like Trichuris and Monezia 1.5%. Another investigation of Gastrointestinal (GI) parasites in ruminants in Western Oromia revealed that the prevalence of GIT parasites overall in cattle was 69.6%, with the strangles and Eimeria parasites having the highest prevalence rates [9]. These reports from several regions of the nation suggested that GIT parasites limit animal output. However, the current study region is lacking in such data. This study's aims were to: In order to determine the prevalence of GIT parasites and related risk factors in cattle in the Arbaminch Zuriya woreda Gamo Zone, Ethiopia.
Materials and Methods
Description of the study area
The study was conducted from 2012 to 2013 E.C in ten selected Kebeles or peasant associations namely Shele, Wozeka, Kola Shara, Lante, Chano Mile, Zigite Babole, Elgo, Kanchama, Zigite Merche and Kola Shele in Arbaminch Zuria district, Gamo Zone, South Nation Nationalities and People Regional state (SNNPRs). Arbaminch is located at a distance of 505 Kms South West of Addis Abeba and 208 Kms from the regional town, Hawassa. Geographically, found in Longitude: 37°C 34' 59.99" E and Latitude: 6°C 00' 0.00" N with altitude of 1200-3125 meters above sea level. The area has a subhumid climate with a moderately hot temperature. The vegetation is dominantly occupied by wooden grass land. The annual rain fall ranges from 750-930 millimeters with mean average temperature of 30°C. The town is situated in the well-known East African Rift valley and surrounded by Lake Chamo and Abaya as well as 'Nech-sar' national park. The main occupation of the rural population is mixed farming system whereby crop and livestock production are managed. Main crop is maize and vegetation that includes coffee, banana, papaya, mango, avocado, inset, apple and others. The livestock population in the Gamo Zone includes cattle 1,243,017, sheep 196,575, goats 543,385 and equine 58,664 (Figure 1).
Figure 1: Map of Arbaminch Zuriya district of study area.
Study population, sample size and sampling method
A study was conducted on 594 local cattle randomly selected from five sites in dry season and (Shele, Wozeka, Kola Shara, Lante and Chano Mile) and five sites in wet season area (Zigite Babole, Elgo, Kanchama, Zigite Merche and Kola Shele). A cross sectional survey was carried out by employing a simple random sampling method to select the sample animals. The sample size was determined using the simple random sampling formula given by Thrusfield.
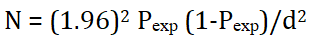
Where, N=required sample size pexp=expected prevalence (50%), d=desired absolute precision (0.05). Accordingly, 384 samples were needed, even though, 593 cattle were sampled and examined.
Faecal sample collection: Plastic rectal gloves were used to collect feces samples per-rectum. Each sample included an edible pen label that included the animal number, collecting date, age and gender. The gathered samples were transferred to the lab in an icebox with the top securely fastened. Samples shall be held in four coolers and processed within 12 hours, unless there were circumstances where rapid processing was not practicable.
Faecal sample examination: Different qualitative and quantitative inspection techniques were used to examine feces for the presence of parasite eggs and the level of those eggs. The sedimentation and floatation technique was used to conduct a qualitative feces examination. The Sodium Chloride (NaCl) saturated solution utilized in this work as the floating medium was created in a lab. The above mentioned parasitological techniques were used to monitor the procedure described.
Data analysis
Stata 13 intercooled statistical software was used to edit and analyze the data after it was entered into a Microsoft excels spreadsheet [10]. For the purpose of calculating the prevalence of each parasite type, descriptive statistics were used. The presence of an association between the prevalence of parasites, gender, age and animal type was evaluated using pearson's chi-square. When the estimated P-value is less than 0.05 (P=0.05) at the 95% confidence level, a connection was considered statistically significant.
Results
From March 2012 E.C. to February 2013 E.C., we collected 594 samples of cattle feces from ten kebels in the Arbaminch Zuriya district for this study. We discovered cattle GI parasites in practically every kebel. 56.23% of cattle were affected overall. Strongyle-type parasites (42.5%) were used to identify the highest prevalent among seven different parasite species, which included 3 nematodes, 2 trematodes, 1 cestode and 1 protozoan. Fasciola spp. (16.16%) was the second most common GI infection overall. Emeria (9.8%), Neoascaris (9.58%), mixed type (6.58%), Strongyloides spp. (5.68%) and Paramphistomum spp. (3.58%), which was the least prevalent (Table 1).
148 (52.6%) of the 334 animals that tested positive were in the dry season and 186 (59.42%) were in the rainy season. The results of the fecal egg investigation did not differ significantly (p>0.05, P=0.098, CI=0.95-1.82) depending on the seasonal category, 148 (43%) of the eggs were from young and 186 (74.4%) were from adults (Table 2). The significant difference between age category and fecal egg results was detected (p=0.05, P=0.001, CI=2.69-5.49), 206 (56.13%) were males and 128 (56.4%) were females, with no significant difference in fecal egg discoveries between the genders (P>0.05, P=0.951, CI= 0.72-1.41). Additionally, 177 (53.5%) of the 334 cattle who tested positive for fecal egg detections had high body condition scores, whereas 157 (59.7%) had poor body conditions. Additionally, there was a significant difference between cattle with poor and good physical conditions while looking for fecal eggs (OR=1, CI= 0.55-1.07; p=0.05) (Table 3).
Table 1: Species of gastro-intestinal helminths parasites detected in Arbaminch Zuriya district.
Table 2: Univariable logistic regression results of season, gender, age and body condition variables.
Table 3: Multivariable logistic regression results of Season, age and body condition variables.
Discussion
In this study, the prevalence of gastrointestinal helminth parasites was found to be 56.23% (95% CI=1.09-1.51). This finding is higher than the 41.2% prevalence reported in a subset of Addis Abeba, Ethiopia, dairy farms. In addition, reported that in Tanzania, large and small scale dairy cattle, respectively, had an overall prevalence of 44.4% and 37.0%. The current result is in agreement with the findings of earlier research, which involved three municipalities in Colombia's Northeastern Mountain. However, the prevalence of gastrointestinal parasites revealed in the current study is lower than that reported for Holleta and its surrounds, which was 82.8%, for Assela and its surroundings, which was 81%. The disparity in occurrence between research areas may have been caused by variations in the management approach, topography, deworming procedures and climatic conditions that favor the survival of the parasite's infective stage and intermediate hosts [11-14]. The current study's findings showed that Strongyle (42.5%), Fasciola (16.16%), Emeria (9.8%), Neoascaris (9.56%), mixed type (6.58%), Strongylodes (5.68%), Monezia (3.9%) and Paraphostomum (3.58%) were the genera with the most common helminth eggs. Strongyle was determined to be the most common parasite in the current study (42.5%), followed by Fasciola spp. (16.16%). The guidelines for grazing management, anthelmintics, as well as economic conditions and the level of education of farmers, are additional factors influencing the incidence of GI parasites. Farmers allowed their cattle to graze close to the Abaya River, which led to comparatively high rates of infection with Fasciola spp. flukes.(16.16%). Because of this, Fasciola species are frequently found close to water sources. Strongyle (41%) is the most common, followed by Fasciola (36.5%) in this result, which is consistent with the findings of (2014). The results don't correspond with which was F. hepatica was the most common in cattle (22.3%), followed by Eimeria spp. (17.4%) and the Strongylida order's parasite genera (16.5%). The variation in helminth prevalence among helminth genera in each study location suggests that each study area's topography and climate contribute differently to the establishment of different parasites' intermediate hosts. Additionally, this research showed that there was no variation in infection based on the location of fecal eggs in relation to the time of year and the gender of the animals thought to be risk factors. This might be the result of a more or less consistent practice of grazing animals on the same pasture regardless of the animals' gender groups and the time of year the study was conducted. The presence of such high levels of infection in parasite eggs or oocytes, particularly during the dry season, may indicate that the research area has favorable climatic conditions all year long for the growth and survival of free-living stages. However, the relationship between age and bodily condition must show variety; with animals in the adult age ranges having a higher incidence of intestinal parasite infections are common revealed a similar observation, which is that the prevalence of GI parasites increases with age. Contrarily, as revealed by younger stages of both cattle and buffaloes are more sensitive to GI parasites than the older. Although the reasons for changes in parasite prevalence at various age groups are difficult to pinpoint, they may be related to the animals' immunological status, variations in the grazing area and management practices. The shift in the animals' physical state may be a sign that they have both mature and immature stages of a blood sucking parasite or a less aggressive form that can nevertheless do significant harm to the host.
Conclusions
In the current study area, GIT parasite prevalence in cattle was generally high. There were found to be seven different forms of GIT parasites, including the Strongyle and Fasciola species, Trichuris spp., Paramphistomum spp., and Eimeria spp., Strongylodes type and include Monezia species. Additionally, this study discovered the probable risk factors, including the animals' age and body condition, which differed statistically significantly and were linked to a high prevalence rate. The following suggestions are made in light of these findings:
• In order to achieve the anticipated economic gains, worm control efforts need be combined with a major improvement in nutrition and management.
• To avoid any misuse, the role of veterinarians in providing expert advice on preventive and control methods against gastrointestinal helminthes should be highlighted.
• Additionally, it is advised that more research be done to determine the level of anti-protozoan and anti-anthelminthic resistance in the region.
References
- Getahun KT, Siyoum T, Yohannes A, Eshete M (2017) Prevalence of gastrointestinal parasites in dry season on dairy cattle at holeta agricultural research center dairy farm, Ethiopia. J Vet Med Anim Health 9:356-360.
- Central Statistical Agency (CSA) (2017) Livestock and Livestock Characteristics, Agricultural sample Survey. Addis Ababa, Ethiopia. Statistical Bull 2:9-13.
- Holmes PH (1985) Pathogenesis of trichostrongylosis. Vet Parasistology 18:89-101.
[Crossref] [Google Scholar] [PubMed]
- Rafiullah TA, Sajid A, Shah SR, Ahmad S, Shahid M (2011) Prevalence of gastrointestinal tract parasites in cattle of khyber pakhtunkhwa. J Agri Biol Sci 6:9.
- Ijaz M, Khan MS, Avais M, Ashraf K, Ali MM, et al. (2009) Infection rate and chemotherapy of various helminthes in diarrhoeic sheep in and around Lahore. J Anim Plant Sci 19:13-16.
- Marskole P, Verma Y, Dixit AK, Swamy M (2016) Prevalence and burden of gastrointestinal parasites in cattle and buffaloes in Jabalpur, India. Vet World 9:1214-1217.
[Crossref] [Google Scholar] [PubMed]
- Jittapalapong S, Sangwaranond A, Nimsuphan B, Inpankaew T, Phasuk C, et al. (2011) Prevalence of gastrointestinal parasites of dairy cows in Thailand. Agric Nat Resour 45:40-45.
- Regassa F, Teshale S, Reta D, Yosef K (2006) Epedemiology of gastrointestinal parasite of ruminants in western Oromia, Ethiopia. Int J Appl Res Vet Med 4:51-56.
- Keyyu JD, Kassuku AA, Msaliilwa PL, Monrad J, Kyvsgaard NC (2006) Cross-sectional prevalence of helminth infections in cattle on traditional, small-scale and large-scale dairy farms in iringa District, Tanzania. Vet Res Commun 30:45-55.
[Crossref] [Google Scholar] [PubMed]
- Pinilla Leon JC, Delgado NU, Florez AA (2019) Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian northeastern mountain. Vet World 12:48-54.
[Crossref] [Google Scholar] [PubMed]
- Baihaqi ZA, Widiyono I, Nurcahyo W (2019) Prevalence of gastrointestinal worms in Wonosobo and thin-tailed sheep on the slope of Mount Sumbing, Central Java, Indonesia. Vet World 12:1866-1871.
[Crossref] [Google Scholar] [PubMed]
- Telila C, Abera B, Lemma D, Eticha E (2014) Prevalence of gastrointestinal parasitism of cattle in East Showa Zone, Oromia Regional State, Central Ethiopia. J Vet Med Anim Health 6:54-62.
- Nayana G, Dimuthu N, Deepika A, Lahiru U (2018) Prevalence of gastrointestinal parasitic infections and assessment of deworming program among cattle and buffaloes in gampaha District, Sri Lanka. Biomed Res Int 2018.
[Crossref] [Google Scholar] [PubMed]
- Regassa F, Sori T, Dhuguma R, Kiros Y (2006) Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Intern J Appli Res Vet Medi 4:51.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
