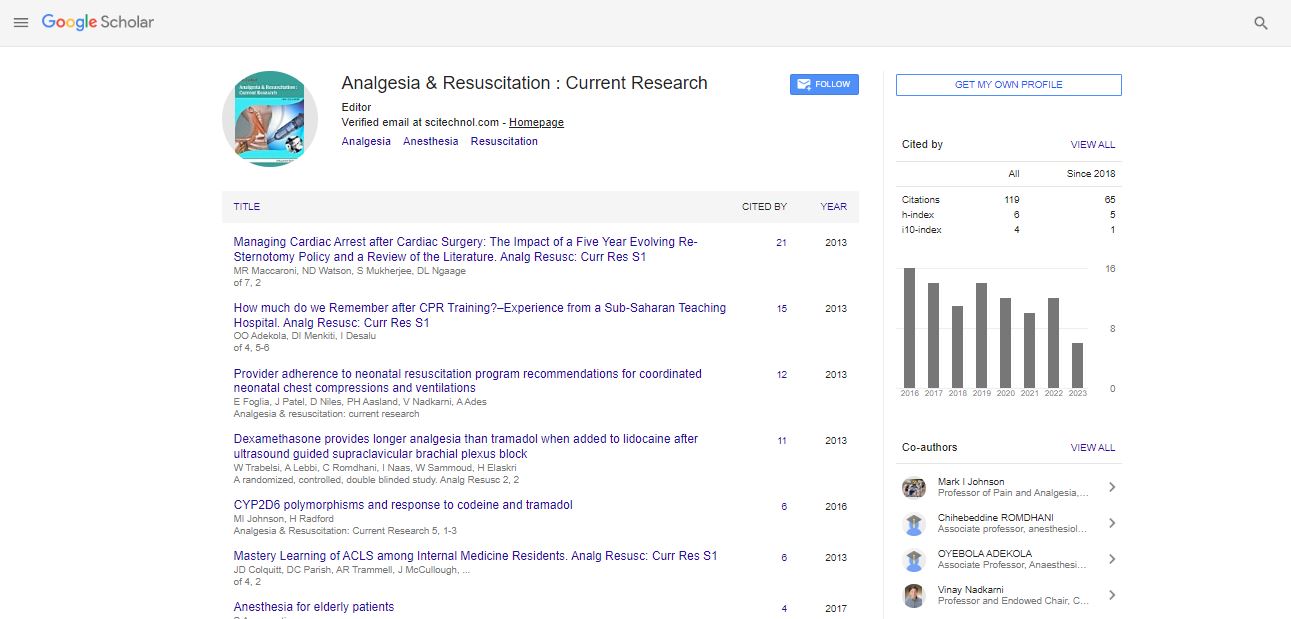
Editorial, Arcr Vol: 14 Issue: 1
Epigenetics of Heart Disease: Unraveling the Layers Beyond Genetics
Meenal Reddy*
Department of Pharmacology, NIMS, Hyderabad, India
- *Corresponding Author:
- Meenal Reddy
Department of Pharmacology, NIMS, Hyderabad, India
E-mail: meenal.r@nims.edu.in
Received: 01-March-2025, Manuscript No. arcr-25-169185; Editor assigned: 4-March-2025, Pre-QC No. arcr-25-169185 (PQ); Reviewed: 20-March-2025, QC No arcr-25-169185; Revised: 26-March-2025, Manuscript No. arcr-25- 169185 (R); Published: 30-March-2025, DOI: 10.4172/2324-903X.1000131
Citation: Meenal R (2025) Epigenetics of Heart Disease Unraveling the Layers Beyond Genetics. Analg Resusc: Curr Res 14:131
Introduction
Heart disease remains the leading cause of morbidity and mortality worldwide. While genetic predisposition plays a critical role in cardiovascular risk, it does not fully explain the complexity of heart disease development and progression [1]. Epigenetics—the study of heritable changes in gene expression that do not involve alterations to the DNA sequence—has emerged as a vital field in understanding cardiovascular pathology. Epigenetic modifications regulate how genes are turned on or off, influencing cellular function and phenotype. This article explores the key epigenetic mechanisms involved in heart disease and their potential clinical implications [2].
Epigenetic Mechanisms in Heart Disease
Epigenetics encompasses several molecular processes that modulate gene expression:
DNA Methylation: The addition of methyl groups to cytosine residues in DNA, particularly in gene promoter regions, typically suppresses gene transcription. Aberrant DNA methylation patterns have been linked to atherosclerosis, cardiac hypertrophy, and heart failure by altering the expression of genes involved in inflammation, fibrosis, and metabolism [3].
Histone Modifications: Histones are proteins around which DNA is wound, and their chemical modifications (such as acetylation, methylation, phosphorylation) affect chromatin structure and gene accessibility. For instance, histone acetylation usually promotes gene expression, while deacetylation silences genes. Dysregulation of histone-modifying enzymes contributes to pathological cardiac remodeling and vascular dysfunction [4].
Non-Coding RNAs: Beyond microRNAs, long non-coding RNAs (lncRNAs) and other RNA species participate in epigenetic regulation by interacting with chromatin-modifying complexes or influencing mRNA stability. These molecules are increasingly recognized as important players in cardiovascular disease.
Epigenetic changes are dynamic and can be influenced by environmental factors such as diet, smoking, stress, and physical activity. This interplay helps explain why individuals with similar genetic backgrounds may experience different cardiovascular outcomes [5].
Clinical Implications and Future Directions
Understanding epigenetic regulation in heart disease opens new avenues for diagnosis, prevention, and therapy. Epigenetic markers in blood or tissues may serve as early biomarkers of disease risk or progression. Moreover, drugs targeting epigenetic modifiers—such as histone deacetylase inhibitors—are being explored for their potential to reverse maladaptive cardiac remodeling.
Importantly, lifestyle interventions that modify epigenetic patterns offer non-pharmacological strategies to reduce cardiovascular risk. As research advances, integrating epigenetics into personalized medicine promises to improve patient outcomes by tailoring interventions based on epigenetic profiles.
Conclusion
Epigenetics provides a crucial layer of regulation beyond the genome, shaping cardiovascular health and disease. By influencing gene expression without changing DNA sequence, epigenetic mechanisms contribute to the onset and progression of heart disease in response to environmental and lifestyle factors. Continued research into these processes holds promise for innovative diagnostics and therapies, ultimately transforming cardiovascular medicine.
References
- Greco CM, Condorelli G (2015) Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nature Reviews Cardiology 12: 488â??497.
- Movassagh M, Choy MK (2015) Epigenetics in heart disease: Role of DNA methylation and histone modifications. Heart 101: 1402â??1411.
- Papait R., Serio S, Condorelli G (2013) Epigenetics of cardiac hypertrophy and failure. Basic Research in Cardiology 108: 361.
- Latif N, Sharma R, Madhavan S (2017) Epigenetics in cardiovascular disease: Basic concepts and clinical implications. Biomolecules 7: 76.
- Ferguson BS, McKinsey TA (2019) Epigenetics in heart failure: The promise of epigenetic therapies. Circulation Research 124: 1054â??1070.
PubMed, Google Scholar, CrossRef
PubMed, Google Scholar, CrossRef
PubMed, Google Scholar, CrossRef
PubMed, Google Scholar, CrossRef
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi