Research Article, J Vet Sci Med Diagn Vol: 11 Issue: 7
Evaluation of Infectious Bovine Rhinotracheitis IBR, In Cattle and Buffalo in Albania
Arta Lugaj1* and Kristaq Berxholi2
1Department of Biology, Faculty of Natural Sciences, University of Tirana, Tirana, Albania
2Department of Veterinary Public Health, Agricultural University of Tirana, Tirana, Albania
*Corresponding Author: Arta Lugaj
Department of Biology,
University of
Tirana,
Tirana,
Albania;
E-mail: arta.lugaj@fshn.edu.al
Received date: 12 April, 2022, Manuscript No. JVSMD-22-60575; Editor assigned date: 15 April, 2022, PreQC No. JVSMD-22-60575 (PQ); Reviewed date: 29 April, 2022, QC No. JVSMD-22-60575; Revised date: 13 June, 2022, Manuscript No. JVSMD-22-60575 (R); Published date: 20 June, 2022, DOI: 10.4172/2325-9590.100033
Citation: Lugaj A, Berxholi K (2022) Evaluation of Infectious Bovine Rhinotracheitis IBR, In Cattle and Buffalo in Albania. J Vet Sci Med Diagn 11:7.
Abstract
Infectious Bovine Rhinotracheitis (IBR) is a highly contagious infectious disease, caused by Bovine Herpes virus 1(BHV-1). Like other herpes viruses, BHV-1 can establish latent infection in animals which become reservoirs of the virus in the herd. ELISA is considered the most rapid, reliable, inexpensive, and simple test and is particularly well suited to the analysis of a large number of samples. The objective of the present study is to estimate the seroprevalence of Bovine Herpes Virus-1 (BoHV-1) in Albania. This study was carried out in 2016 in 12 districts of Albania. Regarding the results of our current study, we can emphasize the presence of IBR infection in almost all regions that were taken into consideration to analyze the sera, collected from cattle and buffalo. These preliminary results indicate that the prevalence of IBR infection varies from 10% in Drenove-Korce to 96% in Terpan-Berat and the total prevalence is 51.3% with a 95% Confidence Interval (CI 95%) and established for the first time that BHV-1 is a subclinical prevalent virus in cattle and buffalo in Albania. The obtained results would contribute to the development and practical application of effective control and prevention measures in Albania. A quick and reliable test for the diagnosis of BHV-1 ELISA is required to be undertaken from the responsible authorities to solve this problem, which must be cheap, highly sensitive, and could be used on a large scale for screening and eradication programs.
Keywords: BHV-1; ELISA; IBR; Herpes viruses;
Seroprevalence
Introduction
Infectious Bovine Rhinotracheitis (IBR) is a highly contagious, infectious respiratory disease that is caused by Bovine Herpes Virus Type 1 (BHV1). It belongs to the genus Varicellovirus, family Herpesviridae, sub-family Alphaherpesvirinae. The BoHV-1 genome is made of a long double-stranded linear DNA molecule arranged as a class D genome with a total size 135.3 kilobase pairs (kbp) [1]. Bovine alphaherpesvirus 1 (BoHV-1) causes several clinical syndromes among cattle populations which may be observed in young and older cattle, after acute infection or after viral recrudescence, following periods of stress [2,3].
IBRV is widely distributed throughout the world, and adult animals are reservoirs of infection. Once these animals become infected for the first time, the virus is never completely removed as it stays behind in nerve cells in the brain as a latent infection just like other herpesviruses where BHV-1 can establish latent infection in animals which become reservoirs of the virus in the herd. But, in moments of stress, the process of virus multiplication can start again and can possibly be re-excreted, respectively from the nose and eyes and thus an infected animal can never be considered safe. There are several factors that affect the severity of the disease caused by BoHV-1, such as the virulence of the BoHV-1 strain, the host resistance factors, age of the animals and the possible concurrent bacterial infection [4-6]. The natural portal of BoHV-1 entry to the host is mainly through the mucous membrane of the upper respiratory or genital tracts. The preferred mode of transmission of BoHV-1 is mainly through direct nose-to-nose contact. Transmission occurs mainly by nasal secretions during and after the clinical signs of the disease [7]. BoHV-1 can be spread to hosts by viremia, gaining access to a wider range of tissues and organs and causing other clinical manifestations [8] for example abortion in pregnant cows [9,10] and fatal systemic infection in very young seronegative calves [11]. From an epidemiological point of view, BHV-1 is perpetuated in nature by interplay of short cycle of infection, latency, resistance to environmental factors and recrudescence under various stress conditions. Virus is quite resistant to environmental influence. Inactivation depends on factors such as temperature, pH, light, humidity, and kind of medium harboring the virus. BHV-1 infection is commonly diagnosed by detection of the host response to the virus (for example, antibodies in serum) or by direct detection of the agent. For the detection of BHV-1 infection, there are frequently used serological tests such as indirect Enzyme Linked Immuno Sorbent Assay (ELISA) that has been extensively used among the cattle population in different parts of the world, for the evaluation of seroepidemiological investigation of IBR antibodies [12]. Using ELISA test, antibodies can be detected within 2–3 weeks of infection in the serum samples. The dairy industry worldwide is most affected from Infectious Bovine Rhinotracheitis (IBR) in cattle disease that is responsible for the significant huge economic loss. The objective of the present study is to estimate the seroprevalence of Bovine Herpes Virus-1 (BoHV-1) in different regions of Albania. The obtained results would contribute to the development and practical application of effective control and prevention measures from the relevant Albanian authorities.
Materials and Methods
Sera from cattle and buffalo
This study was carried out in 2016 in 7 districts of Albania. We have collected a total of 360 serum samples from cattle and buffalo respectively in Berat, Vlore, Sarande, Korce, Elbasan, Tepelene, and Fushe-Kruje as shown in Figure 1.
Blood was obtained from the jugular vein of 339 cattle and 21 buffalo as following the normal procedures. After that, the blood was left to coagulate and the separated serum liquid was collected in screw capped vial plastics and transported directly to the laboratory of the Faculty of Veterinary Medicine, Agricultural University of Tirana. The sera were stored in the fridge adapter at -300℃ until controlled.
After that, in 2017 all blood samples were centrifuged at 5000 rpm and the serum samples were used for the detection of anti-BHV-1 antibodies in bovine serum and plasma using the serological test the indirect Enzyme Linked Immuno Sorbent Assay (ELISA) in infected bovine and buffalo with IBR (Infectious Bovine Rhinotracheitis) disease in Albania. A total of 360 serum samples from different parts of Albania were collected for the detection of antibodies against BHV-1 virus by using Indirect-ELISA kits as shown in Table 1.
| No. | Region/location (village) | Serum samples number | Animal species |
The year of sample |
Gender M-male/ |
Housing S-stable/ |
|---|---|---|---|---|---|---|
| CT-cattle/BF-buffalo | Collection | F-female | P-pasture | |||
| 1 | Dridhas-Korçë | 10 | CT | 2016 | F-female | P-pasture |
| 2 | Grekan-Elbasan | 15 | CT | 2016 | M-male | P-pasture |
| 3 | Lapardha-Berat | 21 | CT | 2016 | F-female | P-pasture |
| 4 | Nartë-Vlorë | 35 | CT | 2016 | F-female | P-pasture |
| 5 | Xarë-Sarandë | 34 | CT | 2016 | M-male | P-pasture |
| 6 | Tepelenë | 46 | CT | 2016 | F-female | P-pasture |
| 7 | Fushë-Krujë | 178 | CT | 2016 | F-female | P-pasture |
| 8 | Lushnje | 21 | BF | 2016 | F-female | P-pasture |
| Total number | 360 | CT | 2016 | F-female | P-pasture | |
Table 1. Collected serum samples.
Indirect ELISA
The indirect ELISA test was used for the detection of anti-BHV-1 antibodies in bovine serum and plasma. For this study, the indirect ELISA kits were obtained and imported by ID. Vet Firm, Innovative Diagnostics France which requires the following steps: All reagents are left at room temperature 21℃ (± 50℃). To each well of ELISA plate coated first with antigen BHV-1 is added 90 microliters of dilution solution, 10 microliters of negative reference serum, 10 microliters of positive reference serum, and 10 microliters of serum sample. After that ELISA plate is incubated for 45 minutes (± 4 minutes) at 37℃ (± 30℃). The solution is thrown away and the ELISA plates are washed three times with a washing buffer solution. Added 100 microliters of conjugate anti-ruminant IgG-HRP conjugate and after that is required to incubate the ELISA plate for 30 minutes at 37℃ (± 30℃). We throw away again the solution and washed the ELISA plates three times with a washing buffer. After that, we added 100 microliters of the substrate and incubated the ELISA plates for 15 minutes (± 2 minutes) at 21℃ (± 50℃). In the last process, we added 100 microliters of stop solution and read and record OD at 450 nm at ELISA reader. The calculation of values is based on the following formula:
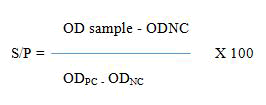
• PC=Positive Control
• NC=Negative Control
• OD=Opitcal Density
Evaluation of serum sample test
• Serum:
<50% =Negative
<60%=Doubts
Statistical analysis: The Confidence Interval (CI) of BoHV-1 seroprevalence and statistical significance of the differences between prevalence percentages were calculated at a 95% probability following the standard methods [13].
Results and Discussion
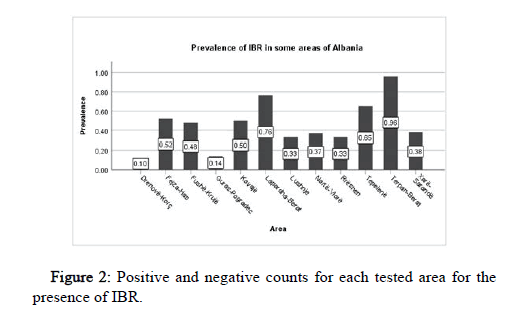
Regarding the results of our current study, we can emphasize the presence of IBR infection in almost all regions that were taken inconsiderate to analyze the sera, collected from cattle and buffalo (Drithas-Korce 10 positive samples from 10 in total, Elbasan-Grekan 0 positive samples from 15 in total, Lapardha-Berat 16 positive samples from 21 in total, Narte-Vlore 13 positive samples from 35 in total,Xare-Sarande 13 positive samples from 34 in total, Tepelene 30 positive samples from 46 in total and in Fushe Kruje 85 positive samples from 178 in total). The table below is shown the results for the detection of anti-BHV-1 antibodies in bovine and buffalo serum samples, from 12 regions of Albania including the results obtained from the first phase of the study that is published in ICRSET conference [14-16]. These preliminary results indicate that the prevalence of IBR infection varies from 10% in Drenove-Korce to 96% in Terpan-Berat and the total prevalence is 51.3% (281 positive serum samples from a total of 548) with a 95% confidence interval (CI 95%) (Table 2 and Figure 2).
| Area | Positive | Negative | Total | Prevalence | 95% - CI | |
|---|---|---|---|---|---|---|
| Terpan-Berat | 48 | 2 | 50 | 0.96 | 0.905 | 1.015 |
| Rrëshen | 14 | 28 | 42 | 0.333 | 0.086 | 0.58 |
| Drenovë-Korçë | 1 | 9 | 10 | 0.1 | -0.488 | 0.688 |
| Guras-Pogradec | 1 | 6 | 7 | 0.143 | -0.543 | 0.829 |
| Kavajë | 27 | 27 | 54 | 0.5 | 0.311 | 0.689 |
| Fejza-Has | 26 | 24 | 50 | 0.52 | 0.328 | 0.712 |
| Lapardha-Berat | 16 | 5 | 21 | 0.762 | 0.553 | 0.971 |
| Nartë-Vlorë | 13 | 22 | 35 | 0.371 | 0.109 | 0.634 |
| Xarë-Sarandë | 13 | 21 | 34 | 0.382 | 0.118 | 0.647 |
| Tepelenë | 30 | 16 | 46 | 0.652 | 0.482 | 0.823 |
| Lushnje | 7 | 14 | 21 | 0.333 | -0.016 | 0.683 |
| Fushë-Krujë | 85 | 93 | 178 | 0.478 | 0.371 | 0.584 |
| Total | 281 | 267 | 548 | 0.513 | 0.454 | 0.571 |
Table 2: The results were obtained from testing 545 serum samples by using an indirect ELISA assay.
As shown by the Chi-square test, there was a significant difference between areas for the % of positive cases (X2(10)= 70.893; p<0.0001).
Conclusion
In conclusion, the results of this study established for the first time that BHV-1 is a subclinical prevalent virus in cattle and buffalo in Albania. A quick and reliable test for the diagnosis of BHV-1 ELISA is required to be undertaken from the responsible authorities to solve this problem, which must be cheap, highly sensitive and could be used on a large scale for screening and eradication programs. Buffaloes are more resistant to the BHV-1 infection if compared with the cattle [8]. We can emphasize that the seroprevalence of BoHV-1 in cattle and buffalo in Albania tends to increase as it has occurred in other countries [4]. This study shows that there is a high BoHV-1 seroprevalence of BoHV-1 in Albania with a percentage of 51.3% in cattle and buffalo. It is also noted that this seroprevalence is due to the natural exposure of cattle to the virus. Cattle are the primary reservoir and infection is transmitted during initial clinical disease or from reactivation of latent infection with subsequent virus shedding. As soon as a cow with a clinical BoHVl infection is diagnosed, the whole herd may be vaccinated to protect the animals from disease, if there is a clinical outbreak of BoHV-1 in the close vicinity. Because of these results, urgent control and prevention measures must be put in place to reduce the prevalence of this disease, aiming at its eradication.
References
- Cowley BDJ, Clegg TA, Doherty ML, More SJ (2011) Aspects of bovine herpesvirus-1 infection in dairy and beef herds in Republic of Ireland. Acta Vet Scand 53:40.
- Nandi S, Kumar M, Manohar M, Chauhan S (2009) Bovine herpes virus infections in cattle. Anim Health Res Rev 19:85–98.
- Turin L, Russo S (2003) BHV-1 infection in cattle: An update. Vet Bull 73:15–21.
- Gibbs EPJ, Rweyemamu MM (1977) Bovine herpesviruses. Part I. Bovine herpesvirus. Vet Bull 47:317 343.
- Kaashoek MJ, Straver PH, Van RE, Quak J, van Oirschot JT (1996) Virulence, immunogenicity and reactivation of seven bovine herpesvirus 1.1 strains: clinical and virological aspects. Vet Rec 139:416–421.
- Schynts F, McVoy A, Meurens F, Detry B, Epstein AL, Thiry E (2003) The structures of bovine herpesvirus 1 virion and concatemeric DNA: implications for cleavage and packaging of herpesvirus genomes. Virol 314:326–335.
- Mars MH, de Jong MC, van Maanen C, Hage JJ, van Oirschot JT (2000) Airborne transmission of bovine herpesvirus 1 infections in calves under field conditions. Vet Microbiol 76:1–13.
- Shirvani E, Lotfi M, Kamalzadeh M, Bahriari M, Abdoshah M (2011) Dot-blot enzyme immunoassay for the detection of bovine herpes virus-1(BHV-1) antibodies. World Appl Sci J 15:781–784.
- Miller JM, Whetstone CA, Bello LJ, Lawrence WC (1991) Determination of ability of a thymidine kinase-negative deletion mutant of bovine herpesvirus-1 to cause abortion in cattle. Am J Vet Res 52:1038–1043.
- Wyler R, Engels M, Schwyzer M (1989) Infectious Bovine Rhinotracheitis/Vulvovaginitis (BHV1). In: Wittmann G (eds) Herpesvirus Diseases of Cattle, Horses, and Pigs. Developments in Veterinary Virology, vol 9. Springer, Boston, MA, 1-72.
[Crossref]
- Bryan LA, Fenton RA, Misra V, Haines DM (1994) Fatal, generalized bovine herpesvirus type-1 infection associated with a modified-live infectious bovine rhinotracheitis parainfluenza-3 vaccine administered to neonatal calves. Can Vet J 35:223–228.
- Jacevičius E, Šalomskas A, Milius J, Petkevičius S, Jacevičienė I, et al. (2010) Five year serological study of bovine herpesvirus type-1 in cattle in Lithuania. Bull Vet Inst Pulawy 54:289-292.
- Roshtkhari F, Mohammadi G, Mayameei A (2012) Serological evaluation of the relationship between viral pathogens (BHV-1, BVDV, BRSV, PI-3V, and Adeno3) and dairy calf pneumonia by indirect ELISA. Trop Anim Health Prod 44:1105–1110.
- Lugaj A, Cara L, Borakaj M, Berxholi K (2019) Evidences of Serological Studies for The Presence of Infectious Bovine Rhinotracheitis IBR, In Albania. 2nd International Conference on Research in Science, Engineering and Technology 22nd to 24th November 2019 Diamond Scientific Publication 22nd-24th of November in Paris, France.
- Mahmoud MA, Mahmoud NA, Allam AM (2009) Investigations on Infectious Bovine Rhinotracheitis in Egyptian Cattle and Buffaloes. Glob Vet 3:335-340.
- Majumder S, Ramakrishnan MA, Nandi S (2015) Infectious Bovine Rhinotracheitis: An Indian Perspective. Int J Curr Microbiol Appl Sci 4:844-858.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi