Case Report, Jceog Vol: 9 Issue: 4
Hepatocellular Carcinoma Post Viral Hepatitis B and C: Application of the Prognostic Score of Singapore
Dhouha Bacha1, Oussema Belkacem1, Sana ben Slama1, Bouraoui Saadia1, Wael Ferjaoui2*, Ghofrane Talbi2, Lasaad Gharbi2 and Limgala Prasanthi2
1Geisinger Community Medical Center, Scranton, USA.
2Wright Center for Graduate Medical Education, Scranton, USA.
*Corresponding Author: Wael Ferjaoui
Department of General surgery, Mongi Slim University Hospital, Faculty of Medicine of Tunis
Tel: + 216 52430099
E-mail: farjaouiwael4@gmail.com
Received: May 14, 2020 Accepted: July 22, 2020 Published: July 29, 2020
Citation: Bacha D, Belkacem O, Slama S, Saadia B, Ferjaoui W (2020) Hepatocellular Carcinoma Post Viral Hepatitis B and C: Application of the Prognostic Score of Singapore. J Clin Exp Oncol 9:4. doi: 10.37532/jceog.2020.9(4).245
Abstract
Surgical resection of hepatocellular carcinoma (HCC) is a major curative option in patients with adequate residual liver function. The risk of recurrence is extremely high. The Singapore liver cancer recurrence (SLICER) is a nomogram that evaluates the 3-year and 5-year recurrence-free survival (RFS) of patients operated for HCC. The objectives of our study were: To apply the SLICER prognostic score to a series of patients operated for a CHC To compare the results found with specific evolutionary data in our patients
Keywords: Hepatocellular carcinoma; Prognosis; Score; Viral hepatitis; liver.
Introduction
Hepatocellular carcinoma (HCC) is the 6th most common cancer in the world. It often develops on cirrhosis (75 to 80% of cases), more rarely on chronic non-cirrhotic liver disease and exceptionally on a healthy liver. It is usually associated with a poor prognosis and a high rate of mortality and morbidity [1]. Screening for HCC in patients with compensated cirrhosis by semi-annual abdominal ultrasound without an alpha-fetoprotein (α FP) is recommended and makes it possible to diagnose HCC in the curable stage in more than 70% of cases [2,3]. Currently, surgical resection of HCC remains a validated curative therapeutic option in patients with adequate residual liver function. After surgical resection, the risk of recurrence is extremely high with a rate at 5 years of up to 70% [4]. This fact highlights the need for an efficient prognostic score to predict this risk of recurrence. The Singapore liver cancer recurrence (SLICER) is a nomogram that combines 8 clinical pathological factors, making it possible to assess the risk of recurrence at 3 and 5 years of patients operated for HCC [5]. The validation of this score is necessary for its current practice.
The objectives of our work were:
✓ To apply the SLICER prognostic score on a series of patients operated on for HCC complicating viral hepatopathy B and C.
✓ To compare the results found with specific evolutionary data in our patients.
Patients and methods
This is a descriptive, cross-sectional and retrospective study which focused on 39 patients operated on for HCC complicating chronic viral hepatitis B and / or C, from January 2000 to January 2015, collected in the general surgery department at the Mongi Slim Hospital in Marsa.
Inclusion Criteria was hepatic resection for HCC post viral hepatitis B and / or C, confirmed histologically.
Exclusion Criteria were patients with fibro-lamellar carcinoma, patients who have had percutaneous alcoholization, radio frequency ablation, and chemoembolization, patients with other primary cancers associated with HCC, patients who have already had liver resection and patients with incomplete clinical records.
Data collected concerned clinical information of patients and pathological findings (macroscopic and microscopic features of tumors).
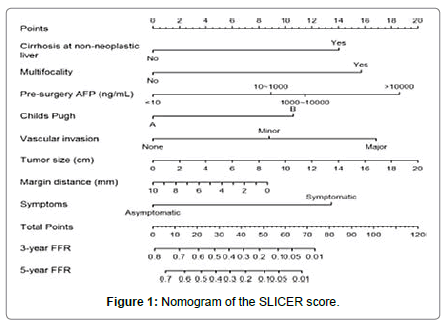
To determine the recurrence-free survival (RFS) rates at 3 and 5 years, we used the SLICER prognostic score, which is a nomogram with 8 clinical and biological and histological factors Figure 1 [6]. These factors were cirrhosis, Multifocality, pre-operative AFP level (ng / ml according to 4 intervals (<10, between 10 and 1000, between 1000 and 10000 and > 10000), Child Pugh score (A or B), vascular invasion (presence or absence and minor or major if present), tumor size (in 2 cm steps, from 0 to 20 cm), resection margins (in 2 mm increments, from 0 to 10 mm), symptoms (presence or absence).
Databases from Pub med, Cochrane Library, HINARI (Health InterNetwork Access to Research Initiative) and Ovid Medline were search using the following keywords: hepatocellular carcinoma, prognosis, score and viral hepatitis. Data were analyzed by the statistics program IBM SPSS version 25. The 95% confidence level is used in our work. The significance level is set at 0.05 or 5%. The comparison of two means was carried out using the Student test. Overall survival (OS) and recurrence-free survival (RFS) were determined by Kaplan- Meier method. The study of prognostic factors of survival was carried out in univariate analysis by comparing the survival curves using the Log rank test. The multivariate analysis made it possible to calculate adjusted relative risks, measuring the specific role of each factor on OS and RFS.
Result
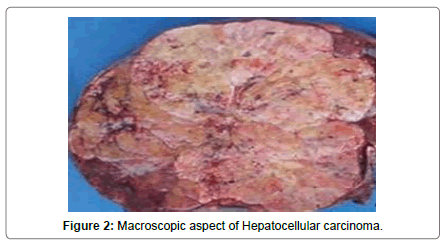
During the study period, 39 patients with 23 men and 16 women were included in the database. The median age was 63.5 (range 48- 81) years. Viral hepatitis B was found in 16 cases (with cirrhosis in 15 cases), viral hepatitis C was found in 19 patients (with cirrhosis in 14 cases) and 4 cases of cirrhosis post hepatitis B and C. The discovery of HCC followed nonspecific symptoms (abdominal pain, hepatomegaly, jaundice) is found in the majority of cases (n = 20). On physical examination, tenderness on palpation of the right hypochondrium was noted in 21 patients and collateral venous circulation in 9 patients. In the majority of cases, the level of AFP was between 10 and 1000 (n = 24). Bilirubin levels were normal in all patients. Child Pugh’s score was “A” in 36 patients and “B7” in 3 patients. The modified BCLC (Barcelona Clinic Liver Cancer) stage was very early (0) in 36 patients. On imaging, liver dysmorphia was noted in 35 patients. No portal thrombosis, satellite nodules, splenomegaly or ascites were noted. Computed tomography scan of the chest did not show lung metastasis. A liver biopsy was performed in 10 patients with atypical radiological signs of HCC. Macroscopic examination showed that the tumor was multifocal in 6 patients with 3 nodules in 3 patients, 2 nodules in 2 patients and 4 nodules in 1 patient. The average of tumor size was 4.5 cm with extremes ranging from 1 to 10 cm. 10 patients had a tumor > 5 cm and 29 patients had a tumor ≤ 5 cm. The HCC nodules were encapsulated in all cases Figure 2.
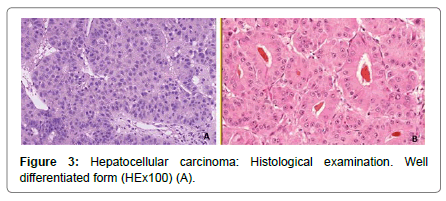
The capsule was complete in 20 cases, incomplete in 9 cases and ruptured in 10 cases. Millimeter satellite nodules were present in 5 cases (not seen on imaging). According to the WHO 2010 classification of HCC, it was moderately differentiated in 26 cases, well differentiated in 10 cases and poorly differentiated in 3 cases Figure 3.
Micro-vascular invasion was observed in 15 patients. The resection limits were invaded in patients. For the others, the margins varied between 2 and 30 mm. According to the 2017 pTNM classification of HCC, the patients were distributed as follows: pT1a (9 patients), pT1b (15 patients), pT2 (12 patients) and pT3 (3 patients). The duration of follow-up was 45 months in all cases.
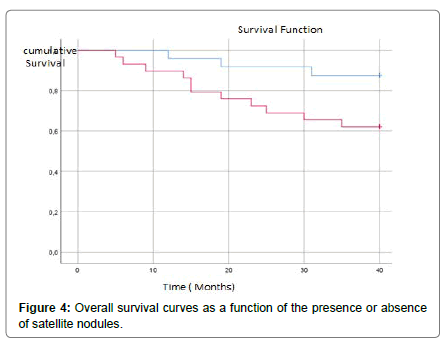
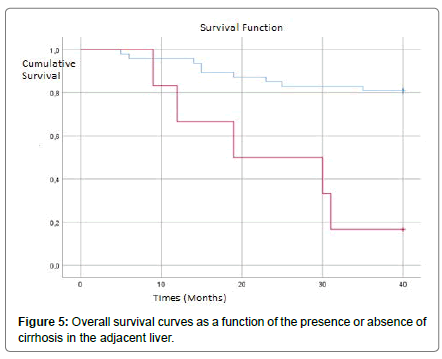
In our series, the uni-variat analysis showed that the presence of vascular invasion Figure 4, satellite nodules Figure 5 and cirrhosis in the liver parenchyma adjacent significantly worsened OS Figure 6. For the other factors (the presence of multiple nodules, the size, the presence or absence of a capsule, the resection margins and the differentiation of CHC), the OS is reduced but not significantly Table 1.
| Variables | Average survival time (months) | P |
|---|---|---|
| Number of nodules | ||
| 1 >1 |
34,2 29,5 |
0,92 |
| Size of nodules | ||
| <5 cm >5 cm |
35,5 30,7 |
0,51 |
| Vascular invasion | ||
| Yes No |
31,5 39,5 |
0,03 |
| Capsule | ||
| Complete Incomplete or broken |
35,6 33,1 |
0,78 |
| Satellite nodules | ||
| Oui Non |
21 36,1 |
0,01 |
| Resection Margin | ||
| Positive Negative |
32,8 39,8 |
0,53 |
| Tumor differentiation | ||
| Well/Moderately diff Poorly diff |
36 30,7 |
0,11 |
| Cirrhosis (adjacent liver parenchyma) | ||
| Yes No |
35,6 23,5 |
0,001 |
Table 1: Results of the uni-variat analysis of overall survival in the 39 patients.
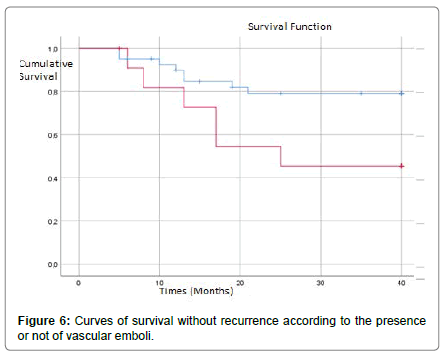
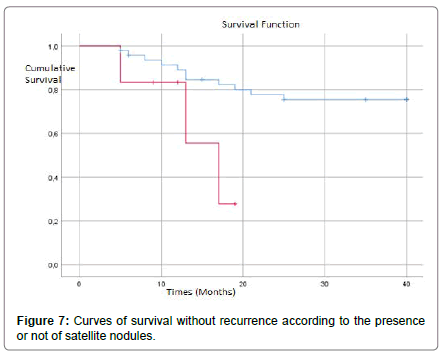
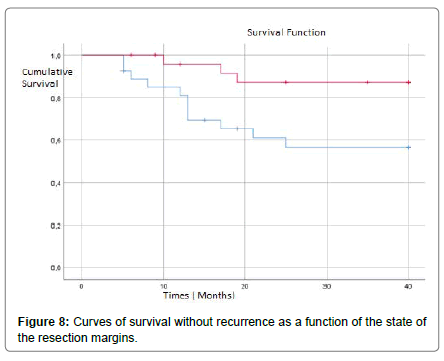
In multivariate analysis, the presence of vascular invasion, satellite nodules and cirrhosis of the adjacent liver were independent factors for OS Table 2. In our series, uni-variat analysis had shown that the presence of vascular invasion Figure 7, satellite nodules Figure 8, positive margins Figure 9 and cirrhosis in the adjacent liver Figure 10 significantly deteriorated RFS. For the other factors (the multiple character of the nodules, their size, the presence or absence of a capsule and the differentiation of HCC), the RFS was reduced but not significantly Table 3.
| Variables | p | OR | IC à 95% |
|---|---|---|---|
| Vascular invasion | 0,03 | 1,26 | 1,4-7,8 |
| satellite nodules | 0,04 | 2,14 | 0,7-15,3 |
| Cirrhosis | 0,001 | 6,31 | 0,6-59,6 |
Table 2: Results of the multivariate analysis of overall survival in the 39 patients.
| Variables | Average survival time (Months) | P |
|---|---|---|
| Number of nodules | ||
| 1 >1 |
36,2 29 |
0,5 |
| Size of nodules | ||
| <5 cm >5 cm |
39 32,2 |
0,57 |
| Vascular invasion | ||
| Yes No |
26,2 37 |
0,03 |
| Capsule | ||
| Complete Incomplete ou broken |
37,7 34,4 |
0,71 |
| satellite Nodules | ||
| Yes No |
14,4 33,5 |
0,01 |
| Resection margin | ||
| Positive Negative |
28,2 36,8 |
0,02 |
| tumor Differentiation | ||
| Well/Moderately diff Poorly diff |
32,7 30 |
0,4 |
| Cirrhosis (foie adjacent) | ||
| Yes No |
30,7 40 |
0,05 |
Table 3: Results of the uni-variate analysis of recurrence-free survival in the 39 patients.
In multivariate analysis, vascular invasion, the presence of satellite nodules as well as the presence of cirrhosis in the adjacent liver represented independent risk factors for the occurrence of recurrences Table 4.
| Variables | p | OR | IC à 95% |
|---|---|---|---|
| Vascular invasion | 0,029 | 9,62 | 1,36-28,8 |
| Satellite nodules | 0,045 | 5,08 | 1,14-14,85 |
| Cirrhosis | 0,001 | 1,97 | 1,22-7,4 |
Table 4: Results of the multivariate analysis of SRH in the 39 patients.
In all patients, the 8 factors of the SLICER score were determined. The total points varied between 18 and 56. The average RFS at 3 years (theoretical) was 28.5% with extremes ranging from 7.5% (9 patients) to 62% (19 patients). The mean RFS at 5 years (theoretical) was 20.6% with extremes ranging from 6% (10 patients) to 55% (19 patients). The comparison of theoretical and effective RFS rates had shown a significant difference both at 3 years (p = 0.03) and at 5 years (p = 0.04) Table 4.
| Theoretical rates (SLICER) | Effective rates | p | |
|---|---|---|---|
| RFS à 3 ans | 28,5% | 45,6% | 0,03 |
| RFS à 5 ans | 20,6% | 38,4% | 0,04 |
Table 5: Comparison of theoretical RFS rates (SLICER) and effective rates.
Discussion
After surgical resection for HCC, the recurrence remains the main risk and may affect the prognosis. Several predictive recurrence scores have been proposed in the literature. None of these scores has proven effective. In our series, we tested the SLICER nomogram on 39 patients. HCC accounts for over 85% of primary liver cancers [6]. Globally, it represents the sixth cause of cancer and the fourth cause of death from cancer with more than 800,000 deaths in 2015 [7]. More than 80% of cases complicate cirrhosis [6], the other cases occurring mainly on chronic hepatitis, essentially with virus B. In Tunisia, the incidence of HCC has been estimated at 1.86 new cases / 100,000 inhabitants in men and 1.12 / 100,000 inhabitants in women according to data from the North Tunisia Cancer Registry, making Tunisia a low incidence area [8]. The incidence of HCC is significantly higher in men and increases with age. In our series, the average age of the patients was 63.5 years with extremes ranging from 48 to 81 years. In accordance with the literature, we observed a male redominance with a sex ratio of 1.43. HCC is often discovered during screening in patients with chronic liver disease. Patients often present with abdominal pain, weight loss, a mass in the right hypochondrium. Fever may occur [9]. The AFP and radiological examinations with the LI-RADS system (Liver Imaging Reporting and Data System) are often used to make the diagnosis of HCC. The computed tomography scan and MRI are the two reference exams [6]. In atypical cases, liver biopsy of the tumor and the adjacent liver are necessary. Surgical resection is one of the curative treatments for CHC. Therapeutic indications follow different algorithms modified BCLC, MELD score, Milan criteria, α Foeto-Protein score.
For HCC in cirrhosis, resection is discussed in patients with preserved hepatic function (Child-Pugh A) and without signs of portal hypertension [10]. Ideally, this resection should be anatomical with margins of 2 cm [11]. In our series, the margins were invaded in 4 patients. For the others, the margins varied between 2 and 30 mm. The volume of the remaining liver should represent at least 40% of the total liver volume. The pathological study confirms the diagnosis and determines the histo-prognostic factors. It also makes it possible to determine the condition of liver parenchyma adjacent, in terms of grade and stage of liver disease according to the METAVIR score. Several of these data are the basis for achieving prognostic scores for recurrent HCC, such as the SLICER score which is used in our study. When the optimal conditions for resection of CHC are present, the post-operative mortality is <5% and the rates of OS and RFS at 5 years are respectively between 44 and 50% and between 21 and 70% [12]. In our series, the duration of follow-up was 45 months for all patients. The average rate of OS was lower than that of the literature (42.8% at 3 years vs 44 to 50% at 5 years). The rate of RFS was however in agreement with the data in the literature (38.4%) [13]. The high rate of recurrence justifies close follow-up after resection [13]. This follow-up is clinical and biological (liver function tests and AFP) every 3 months the first year and then every 6 months. It is also performed by a chest CT scan every 6 months for 2 years and liver imaging [13]. Recurrences complicate 70% of HCC after a period of 5 years, associating local recurrences (true recurrences secondary to therapeutic failure) and the appearance of de novo HCC [13]. According to Tabrizian, tumor recurrences developed in 356 patients, or 54% of the study population, after an average delay of 22 months after resection [14].
This rate is higher than that observed in our series, where a local recurrence was noted in 14 patients (36%) occurring after an average delay of 17 months. These recurrences are precocious (within 2 years following the curative resection of CHC) which are different from late recurrences. According to Hong, early recurrences significantly decrease OS compared to late recurrences (P <0.001) [15]. Indeed, early recurrences result from metastases of the same primary tumor while late recurrences result from de novo tumors occurring later.
Several risk factors of recurrence of HCC have been reported. These factors are biological, radiological and anatomo-pathological. In our series, the presence of micro-vascular invasion, satellite nodules and adjacent liver cirrhosis were independent factors for OS and RFS. This is concordant with the results of Nagasui, who showed that microvascular invasion and satellite nodules significantly altered RFS, in the same way as the poorly differentiated character of HCC [16]. According to several studies, micro-vascular invasion represents an independent risk factor, which affects the mortality rate and the risk of recurrence [16].
According to Kim’s series, having interested 430 cases of HCC on cirrhosis, the tumor size > 5 cm, the presence of vascular micro-invasion and the neutrophil / lymphocyte polynuclear ratio, are risk factors for early recurrences (2 years) [17]. According to Shehta, preoperative thrombocytopenia is a predictor of recurrence in cirrhotic patients. According to Jeng, cirrhosis, the grade of differentiation of HCC, the absence of a tumor capsule or its incomplete presence, vascular invasion, satellite nodules, post-operative positivity of AFP mRNA are associated with significantly high recurrence rate [16]. According to Zhang, data from pre-operative CT (large tumor size, positive radiogenomic venous invasion, absence of capsule) are non-invasive risk factors for SSR in patients with HCC on HBV after curative resection [18]. Few publications have shown that the level of AFP, tumor differentiation or the presence of vascular microinvasions do not influence survival [10].
Based on multiple prognostic factors, predictive scores have been proposed to stratify patients in order to optimize management. Among these scores, the “Cancer of the LiverItalian Program” (CLIP), the “ChineseUniversityPrognostic Index”, the “International Hepato-Pancreato-Biliary Association staging system”, the “Japan Integrated Staging score”, the “Okuda score” and the PRIPS score (post resection independent predictive score) which was validated with good sensitivity and specificity [18]. The 3 main specific scores to predict the risk of recurrence of resected HCC are The American MSKCC “Memorial Sloan-Kettering cancer center” score proposed in 2008 [19], The Chinoix SSCLIP score which is a modified version of the CLIP (Cancer of the Liver Italian Program) score proposed in 2015 [19] and the SLICER score which is studied in our study, proposed in 2015 [5]. The 3 independent histo-prognostic factors for SG and SSR in our series (vascular micro-invasion, satellite nodules and cirrhosis of the adjacent liver parenchyma) are part of the constitutive parameters of the SLICER score, confirming their important prognostic value. We chose the SLICER score because it was designed thanks to a more robust methodology (univariate analysis by Cox regression), which it involves both characteristics of the tumor and patients characteristics and because it has been validated on a larger cohort of patients (n = 405) [5].
The use of nomogram SLICER is more complex than a simple score, since the evaluation approach is done in 2 stages (the score then the probability). This use could be facilitated by the use of applications on a telephone or on a computer [5]. The other drawback concerns the lack of precision of certain parameters, such as the definition of a minor or major vascular invasion in the SLICER score or the multifocality. Moreover, it is unable to differentiate between early and late recurrence, with different prognosis [20]. The etiology of HCC is not also taken into account by this prognostic score, whereas it is an important prognostic parameter. Applying the SLICER score in our series, we noted that it significantly underestimated the RFS rates (at 3 and 5 years). This could be explained by three hypotheses: The absence, in the SLICER score, of important prognostic factors such as age, differentiation of CHC, the state of the capsule if it is present and the expression of stem / progenitor markers such as CK19 -The studied sample is small and the results should be validated on a larger cohort with a longer follow-up and the possible errors in the use of this nomogram in certain cases. Our results cannot be compared with those of the literature since there is no study to date using the SLICER score to assess RFS rates.
Conclusion
SLICER score was not validated in our series since it underestimates the RFS. In order to be applied in daily practice, it must be tested and validated on a larger cohort of patients with different demographic and clinical characteristics and for a longer period of time.
References
- Trinchet JC (2009) Hepatocellular carcinoma: increasing incidence and optimized management. Gastroenterol Clin Biol,33 :830-839.
- Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, et al. (2011) Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology, 54 :1987-1997.
- European Association for the Study of the Liver (2018). EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol, 69:182-236.
- Llovet JM, Burroughs A, Bruix J (2003). Hepatocellular carcinoma. Lancet 362:1907-1917.
- Ang SF, Li H, Ong Y-H, Choo SP, Ngeow J, et al. (2015) The Singapour Liver Cancer Recurrence (SLICER) Score for relapse prediction in patients with surgically resected hepatocellular carcinoma. PLoS ONE 10: 18-58.
- Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology, 127: 35-50.
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. (2018) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol, 4:1553-1568.
- Said Y, Debbeche R, Ben Ali Z, Bouzid K, Trabelsi S, et al. (2012) Epidemiological, clinical and therapeutic features of hepatocellular carcinoma in cirrhotic patients. Tunis Med, 90:468-472.
- Clark T, Maximin S, Meier J, Pokharel S, Bhargava P,et.al. (2015) Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr Probl Diagn Radiol, 44:479-486.
- Bruix J, Sherman M (2011) American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology, 53: 1020–1022.
- Shi M, Guo R-P, Lin X-J, Zhang Y-Q, Chen M-S, et al. (2007) Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg, 245:36-43.
- Bruix J, Sherman M (2005) Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology, 42:1208-1236.
- Llovet JM, Schwartz M, Mazzaferro V (2005) Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis, 25: 181-200.
- Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S, et.al. (2015) Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg, 261: 947-955.
- Hong YM, Cho M, Yoon KT, Chu CW, Yang KH, et al. (2017) Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol 39 :10-24.
- Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, et al. (1993) Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 105:488-494.
- Kim W-J, Lim T-W, Park P-J, Choi S-B, Kim W-B, et.al. (2019) Prognostic markers affecting the early recurrence of hepatocellular carcinoma with liver cirrhosis after curative resection. Int J Biol Markers, 34:123-131.
- Zheng J, Chou JF, Gönen M, Vachharajani N, Chapman WC, et.al. (2017) Prediction of Hepatocellular Carcinoma Recurrence Beyond Milan Criteria After Resection: Validation of a Clinical Risk Score in an International Cohort. Ann Surg, 266: 693-701.
- Cho CS, Gonen M, Shia J, Kattan MW, Klimstra DS, et al. (2008) A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg, 206:281-291.
- Chan H, Chow H (2017) A review of prognostic scores after liver resection in hepatocellular carcinoma: the MSKCC, SLICER and SSCLIP scores. Jpn J Clin Oncol, 47:287-293.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi