Research Article, J Vet Sci Med Diagn Vol: 7 Issue: 1
Histologic Analysis of Retrieved Synthetic Ligaments Implanted in Dogs for Treatment of Cranial Cruciate Ligament Disease
Matthew D Barnhart1*, David Getzy2 and David W Gardiner3
1MedVet Medical and Cancer Centers for Pets, Worthington, Ohio, USA
2IDEXX Reference Laboratories, Fort Collins, Colorado, USA
3Animal Reference Pathology, Salt Lake City, Utah, USA
*Corresponding Author : Matthew D. Barnhart, DVM, MS
Diplomate ACVS, MedVet Medical & Cancer Centers 300 E. Wilson Bridge Rd Worthington OH 43085, USA
E-mail: mbarnhart@medvetohio.com; Matthew.Barnhart@medvetforpets.com
Received: January 17, 2018 Accepted: February 06, 2018 Published: February 11, 2018
Citation: Barnhart MD, Getzy D, Gardiner DW (2018) Histologic Analysis of Retrieved Synthetic Ligaments Implanted in Dogs for Treatment of Cranial Cruciate Ligament Disease. J Vet Sci Med Diagn 7:1. doi: 10.4172/2325-9590.1000248
Abstract
Objective: To histologically analyze retrieved synthetic liagments implanted in canine stifles for the treatment of cranial cruciate ligament disease.
Animals: 6 client-owned dogs
Procedures: Synthetic ligaments (SL) were retrieved from 6 dogs. Five had experienced postoperative complications which necessitated their removal and 1 dog died of unrelated causes. The formalin fixed SLs were stained with hematoxylin and eosin, Masson’s trichrome, Alcian blue, Vimentin and Reticulin stains using standard histologic staining protocols and examined histologically via normal light microscopy. The histologic evaluation involved both a qualitative description and semiquantitative assessment of each dog’s cellular response to the implanted SL. Additionally, a semiquantitative scoring system was used for describing the distribution and amount of fibroblasts around and within the central (inner 1/2) and peripheral (outer 1/2) zones and individual core fibers of the synthetic ligaments.
Results: Sheath and core segments had amounts of cellular infiltration which ranged from minimal to moderate which consisted primarily of fibroblasts with rare multinucleate giant cells present and no evidence of lymphoid or inflammatory cellular infiltrates. Peripheral zone fibroblast ingrowth ranged from minimal to moderate and infiltration consistently decreased into the central (inner ½) zones. There was a minimal amount of fibroblast infiltration and encircling of individual core fibers. Infiltrating fibroblasts deposited collagen matrix and mild to moderate amounts of reticulin fibers within the hypercellular regions of the sheath with lesser amounts in the core segments.
Conclusions and Clinical Relevance: The structure and composition of this synthetic ligament supported variable amounts of fibroblast ingrowth and activity while inducing a minimal amount of inflammatory cell infiltration.
Keywords: Cranial cruciate ligament; Synthetic ligament; Intra-articular; Ligament; Canine; Dog
Introduction
Cranial cruciate ligament (CrCL) disease is the most common cause of lameness in dogs and has an estimated economic impact on pet owners in excess of a billion dollars annually [1,2]. The most commonly used surgical treatments include extracapsular and tibial osteotomy techniques which are in contrast to human orthopedics where graft-based intracapsular anterior ligament reconstructions are most often employed. However, the harvesting of such autografts causes significant patient morbidity and they typically require four to six months to undergo enough ligamentatization in order to achieve adequate strength. Synthetic ligaments (SL) have been investigated over the past four decades in human orthopedics as an alternative to grafts because they require no harvesting, little preparation and provide superior initial strength. Such major advantages have continued to drive SL research and development despite the consistently poor long-term results reported with their past use [3].
Intracapsular graft reconstruction techniques are rarely used to treat canine CrCL disease but continue to be investigated because of their potential advantages over more commonly used surgical treatments [4,5]. However, the degenerative and inflammatory joint environment often present with CrCL disease may adversely affect the long-term strength and viability of autogenous intracapsular grafts in dogs. By contrast, an appropriate SL could be a better intracapsular option than a tissue graft by not only providing superior initial strength but also by being less sensitive to an unfavorable joint environment.
In order to maintain long-term stifle stability an SL must have mechanical properties similar to those of the native CrCL, be resistant to wear and be amenable to good tibial and femoral fixation. Additionally, it needs to be highly biocompatible in order to minimize tissue reactions and support patient tissue integration. Tissue ingrowth is necessary to maximize patient tolerance, strengthen the SL fixation and to decrease fiber shearing and rupture by improving the viscoelastic properties of the implant [6-10]. Many of the longterm SL failures reported in humans were attributed to the inability of those implants to support cellular ingrowth [3]. For tissue integration to occur, a SL must be composed of materials with intrinsic chemical properties that possess a surface charge and tension and porosity compatible with cell entry and adhesion and a structure that provides a scaffold to support tissue growth [6-10].
The goal of this study was to histologically analyze retrieved SLs implanted in canine stifles for the treatment of naturally occurring CrCL disease and to report the observed cellular ingrowth and response.
Methods
Fifty SLs were implanted in 50 client-owned dogs as part a prospective clinical study between January 2011 and April 2012 [11]. The SLs retrieved from five dogs that experienced postoperative complications which necessitated their removal and one dog that died of unrelated causes (Table 1) were evaluated in this present study.
| Breed | Age (years) | Days SL in situ before removal | Reason for Retrieval |
|---|---|---|---|
| Mix | 11 | 224 | Ruptured SL |
| Lab Ret | 7 | 298 | Died of ruptured splenic hemangiosarcoma |
| Mix | 2 | 306 | Partial loss of SL fixation |
| Lab Ret | 5 | 306 | Partial loss of SL fixation |
| Boxer | 5 | 238 | Partial loss of SL fixation |
| Lab Ret | 1.5 | 121 | Ruptured SL |
Table 1: Patient and Synthetic Ligament (SL) Retrieval Information.
For the inclusion in the prospective clinical study owners were counseled on established surgical treatments versus the study procedure. Care was taken to emphasize that the study treatment was novel and that while the implant appeared to have favorable mechanical and biological properties based on company-sponsored Avalon Medical, Stillwater, MN, USA test results, it had not been tested in a clinical setting. A written consent form was signed by each owner prior to enrollment of their dog. Owners who participated in this study received all surgery-related care at no cost.
The SL was a six mm wide, two mm thick and 330mm long composite polymeric device composed of a multifilamentous ultrahigh molecular weight polyethylene terephthalate core contained within a braided porous polytetrafluoroethylene sheath with a pore size of greater than 30 microns. The two components were joined together using 0 polyethylene terephthalate suture and 4cm of crossstitch pattern made in both ends of the SL. Avalon Medical, Stillwater, MN, USA All SLs were implanted using the same previously described surgical technique [11] were retrieved by removing the interference screws and screw - spiked washer posts and pulling them out of the tibial and femoral bone tunnels by hand. All samples were placed in 10% neutral buffered formalin and submitted for histologic evaluation.
The formalin fixed SLs were embedded in paraffin, sectioned at six microns of thickness and stained with hematoxylin and eosin using standard histologic staining protocols. Additionally, Masson’s trichrome, Alcian blue, and Reticulin stains were used to assess degree and pattern of fibroblast ingrowth, and production of glycosaminoglycans and collagen. Vimentin staining was performed to determine if the cells present were of mesenchymal tissue origin. Multiple transversely and longitudinally oriented sections of the SL and any interface membrane present were examined histologically via normal light microscopy by two board-certified pathologists (DWG and DG). The intraarticular and bone tunnel locations of the device were sectioned and assessed together. The histologic evaluation involved both a qualitative description and semiquantitative assessment of each dog’s cellular response to the implanted SL.
The qualitative assessment described the various cell types present within the SL and surrounding interface membrane and a distribution of any associated fibroblastic response. A semiquantitative assessment of the degree of inflammation present was performed by developing a scoring system for the amounts of various types of inflammatory cells present, overall inflammatory grade, amount of granulation tissue present, and the amount of mature fibrous connective tissue present (fibroplasia). Inflammatory and giant cell scores were assigned by determining the percentage of these cells relative to the total population of all cellular infiltrates in a given specimen (Table 2).
| Inflammation | ||
|---|---|---|
| Tissue/ Cells | Score | |
| Neutrophil, lymphocyte, and plasma cell | Total number of each cell type in a given specimen divided by the total number of inflammatory cells | 0 = 0 1 = 1-24% 2 = 25-49% 3 = 50-74% 4 = 75-100% |
| Overall inflammation |
Semi-quantitative assessment based upon percentage of nucleated cells per total tissue area in most extreme zone of inflammation present on a given slide | 0=None present 1=Minimal 2=Mild 3=Moderate 4=Marked |
| Giant cells | Assessment of number and types of giant cells within inflammatory lesions | 0 = 0 1 = 1-24% 2 = 25-49% 3 = 50-74% 4 = 75-100% |
| Granulation tissue | Semi-quantitative assessment of overall amount of granulation tissue present | 0=None present 1=Minimal 2=Mild 3=Moderate 4=Marked |
| Fibroblast Ingrowth | ||
| Zone | Score | |
| Central fibroblast ingrowth | Semi-quantitative assessment of fibroblast ingrowth based upon evaluation of inner ½ of SL in longitudinal section | 0=None Present 1=Minimal 2=Mild 3=Moderate 4=Marked |
| Peripheral fibroblast ingrowth | Semi-quantitative assessment of fibroblast ingrowth based upon evaluation of outer ½ of SL in longitudinal section | As above |
| Individual core fiber fibroblast ingrowth | Semi-quantitative assessment of fibroblast encircling of individual core fibers in transversely sectioned SL | As above |
| Total fibroblast ingrowth | Semi-quantitative assessment of percentage of overall fibroblast ingrowth in transversely sectioned SL | As above |
Table 2: Histologic Scoring System.
A similar semiquantitative scoring system was used for the distribution and amount of fibroblasts around and within the SL. Longitudinal sections of the SL were divided into central (inner 1/2), and peripheral (outer 1/2) zones for assessment of fibroblast scores. Additionally, transverse sections of the SL were assessed for the complete encircling of individual core fibers by fibroblasts. Lastly, a total fibroblast ingrowth score was determined by the percentage of overall fibroblast ingrowth in the transversely sectioned SL (Table 2).
Results
The median time that elapsed between SL placement and explantation was 249 days (range, 121-306 days).
Staining with hematoxylin and eosin revealed that sheath and core segments had moderate amounts of cellular infiltration that consisted primarily of fibroblasts with rare multinucleate giant cells present and no evidence of lymphoid or inflammatory cellular infiltrates. Additionally, capillary ingrowth was noted into the sheath but it did not extend into the SL core fibers.
All fibroblasts, multinucleate giant cells, and epithelioid macrophages present exhibited positive cytoplasmic vimentin staining consistent with mesenchymal tissue origins.
Summaries of the semiquantitative assessments of the cellular responses to the implanted SLs are provided in Table 3.
| Histologic Parameters | Mean Score (Range) |
|---|---|
| Inflammatory grade | 1.6 (1-3) |
| Neutrophils | 0.29 (0-2) |
| Lymphocytes | 1.0 (0-2) |
| Plasma Cells | 0.48 (0-1) |
| Histiocytes | 1.5 (1-3) |
| Giant cells | 0.88 (0-2) |
| Granulation tissue | 0.78 (0-2) |
| Mature fibrous tissue | 1.8 (1-3) |
Table 3: Inflammation Scores.
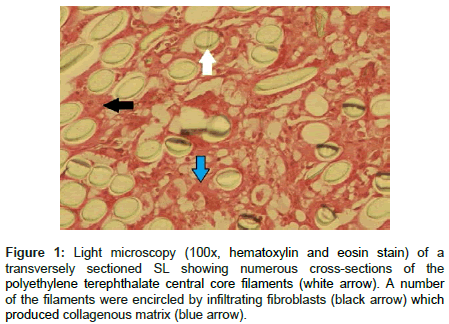
There was overall mild SL peripheral zone (outer ½) fibroblast ingrowth that ranged from minimal to moderate and infiltration consistently decreased into the central (inner ½) zones of the SLs. Fibroblasts did infiltrate to and encircle individual core fibers but did so in generally minimal amounts (Figure 1). Summaries of the SL fibroblast ingrowth scores are provided in Table 4. Positive Masson’s trichrome staining present within the sheath and core of the SLs confirmed that infiltrating fibroblasts had deposited collagen matrix. Reticulin stains revealed mild to moderate amounts of reticulin fiber deposition occurred around individual cells and at the cell-SL interface within the hypercellular regions of the sheath while minimal amounts were present in the core segments. Additionally, there was a mild to moderate amount of Alcian blue staining of glycosaminoglycans and/ or mucopolysaccharides present in the sheath and core sections.
Figure 1: Light microscopy (100x, hematoxylin and eosin stain) of a transversely sectioned SL showing numerous cross-sections of the polyethylene terephthalate central core filaments (white arrow). A number of the filaments were encircled by infiltrating fibroblasts (black arrow) which produced collagenous matrix (blue arrow).
| Sectioned SL Zones | Mean Score (Range) |
|---|---|
| Central | 1.1 (0-2) |
| Peripheral | 2.2 (1-3) |
| Individual fibers | 1.0 (0-2) |
| Total ingrowth | 1.0 (0-2) |
Table 4: Synthetic ligament (SL) fibroblast ingrowth scores.
Further analysis of the collected data did not reveal any correlation between the duration of the SL implantation and the cellular response or infiltration grades and scores present between dogs (i.e. a longer duration of implantation did not necessarily increase scores).
Discussion
In the present study, SLs that were retrieved following implantation to treat naturally occurring canine CrCL disease were histologically evaluated for degree of cellular response and infiltration utilizing qualitative descriptions and semiquantitative assessment scores. Fibroblasts adhered to and infiltrated the external sheath fibers, surrounded the inner core filaments and produced and deposited extracellar matrix in the form of polysaccharides and collagen III fibers. These observations, combined with the low numbers of multinucleate giant cells and lymphoid and inflammatory cellular infiltrates observed, support that this device is biocompatible and can facilitate active tissue ingrowth. A number of unfavorable biologic responses associated with but not limited to poor biocompatibility include severe synovitis, bone tunnel osteolysis, and ganglionic-type foreign body reactions have been reported following implantation of SLs in humans. Cellular ingrowth into an SL is in part necessary to avoid such issues and provide long-term patient tolerance. It also improves SL viscoelastic properties and bone tunnel fixation strength [6,12]. While the tissue integration reported in this study supports some degree of biocompatibility of this SL, whether the amounts recorded were sufficient to provide the purported benefits cannot be concluded. To the authors knowledge, there are no reports that describe what the minimum desired amount of cellular infiltration is. Additionally, there was no histologic evidence of osseointegration. Formation of layers of interposed scar tissue between such a device and surrounding bone instead of osseointegration has been implicated as a major cause for SL fixation failures [13]. This may explain why the partial loss of SL internal fixation was a common complication that necessitated revision surgery in our patients [11]. However, without a control group, it is not possible to say whether the degree of replacement of glycoasaminoglycans by collagen, indicating delayed osseointegration, was more than expected. It‘s also possible that early superficial and delicate osseointegration did occur but was removed when the SLs were pulled out of their bone tunnels. Ultimately, the presence or absence of osseointegration would be best determined by examining the SL in situ.
Several limitations of this study are noteworthy. The histologic scoring methods used were inherently subjective, however, we attempted to mitigate this issue by using 2 pathologists who were unaware of any of the clinical data related the specimens. A separate analysis of the extraosseous and intraosseous portions of the SL was not performed but could have yielded additional relevant information since these two distinct zones likely experience different cellular responses. Additionally, evaluation of synovial tissue would likely have yielded some additional relevant information relative to patient tolerance. It‘s possible that while mild to moderate inflammatory cell infiltration was noted in the SL, a more generalized synovial inflammatory response could have occured as has been described associated with other intraarticular SLs.
Conclusion
This report describes the cellular ingrowth and response to a SL used to replace the CrCL in dogs with naturally occuring disease. The structure and composition of this SL supported variable amounts of fibroblast ingrowth and activity while inducing a mild to moderate amount of inflammatory cell infiltration. These findings may support a reasonable biologic compatability for this SL, an important characteristic for such an implant to have.
Declarations
Ethics and consent
For the inclusion in the prospective clinical study owners were counseled on established surgical treatments versus the study procedure. Care was taken to emphasize that the study treatment was novel and that while the implant appeared to have favorable mechanical and biological properties based on company-sponsored test results, it had not been tested in a clinical setting. A written consent form was signed by each owner prior to enrollment of their dog. Owners who participated in this study received all surgeryrelated care at no cost. Adherence to a high standard of veterinary care was maintained throughout this study.
Competing interests
Matthew Barnhart is a paid lecturer for Securos and receives royalties from the sales of some of their products. The remaining authors declare no conflicts of interests.
Authors contributions
MB was responsible for the clinical study from which the samples were acquired and for preparation of this manuscript. D.Getzy and D.Gardiner were responsible for all histologic analysis and grading
Availability of data and materials
All collected data is presented in this manuscirpt and raw data is avaible upon request.
References
- Wilke V, Robinson D, Evans R, Rothschild M, Conzemius M (2005) Estimate of the annual economic impact of treatment of cranial cruciate ligament injury in dogs in the United States. J Am Vet Med Assoc 227: 1604-1607.
- Hayashi K, Manley PA, Muir P (2004) Cranial cruciate ligament pathophysiology in dogs with cruciate disease: a review. J Am Anim Hosp Assoc 40: 385-390.
- Johnson D (2008) Why synthetic ligaments failed. In: Prodromos. The anterior cruciate ligament: reconstruction and basic science.
- Leighton RL (1999) Preferred method of repair of cranial cruciate ligament rupture in dogs: survey of ACVS Diplomates specializing in canine orthopedics. Vet Surg 28: 194.
- Biskup J, Freeman A, Camisa W, Innes J, Conzemius M (2014) Mechanical properties of canine patella-ligament-tibia segment J Am Vet Med Assoc 43: 136-141.
- Trieb K, Blahovec H, Brand G, Sabeti M, Dominkus M, et al. (2004) In vivo and in-vitro cellular ingrowth into a new generation of artificial ligaments. Eur Surg Res 36: 148-151.
- Nau T, Lavoie P, Duval N (2002) A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. J Bone & Joint Surg (Br) 84-B: 356-360.
- Wang CL, Hsiao CK, Hsu AT, Dung CZ, Chang CH (2012) Biocompatibility and mechanical property of LARS artificial ligament with tissue ingrowth. J Mech Med Biol 12: 1-13.
- Kock HJ, Sturmer R, Letsch R, Schmit-Neuerburg KP (1994) Interface and biocompatibility of polyethylene terephthalate knee ligament prostheses. Arch Orthop Trauma Surg 114: 1-7.
- Seitz H, Marlovits S, Scwendenwein I, Müller E, Vécsei V (1998) Biocompatibility of polyethylene terephthalate (Trevira hochfest) augmentation device in repair of the anterior cruciate ligament. Biomaterials 19: 189-196.
- Barnhart MD, Maritato K, Schankereli K, Wotton H, Naber S (2016) Evaluation of an intra-articular synthetic ligament for treatment of cranial cruciate ligament disease in dogs: a 6-month prospective clinical trial. Vet Comp Orthoped Traumatol 29: 491-498.
- Hong L, Yunsheng G, Yang W, Jia J, Kai G, et al. (2011) Hydroxyapatite coating enhances polyethylene terephthalate artificial ligament graft osseointegration in the bone tunnel. Int Orthop 25: 1561-1567.
- Guidoin MF, Marois Y, Bejui J, Poddevin N, King MW, et al (2000) Analysis of retrieved polymer fiber based replacements for the ACL. Biomaterials 21: 2461-2474.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi