Research Article, J Virol Antivir Res Vol: 9 Issue: 2
Immunological and Virological Effects of Novel Prakasine Nanomedicine in HIV-Infected Patients in South India: A Preliminary Study
Prakash S.K*
Department of Naval AIDS Research Centre,Namakkal, Tamilnadu, India
*Corresponding Author: Prakash S.K
Department of Naval AIDS Research Centre,Namakkal, Tamilnadu India
E-mail: drskprakash@gmail.com
Received: April 20, 2020 Accepted: July 02, 2020 Published: July 08, 2020
Citation: Prakash SK (2020) Immunological and Virological Effects of Novel Prakasine Nanomedicine in HIV-Infected Patients in South India: A Preliminary Study. J Virol Antivir Res 9:2. doi: 10.37532/jva.2020.9(2).195
Abstract
Objectives: While HIV remains incurable, one method of eliminating latent virus is, sensing dormant HIV then target the infected cells. The Cytotoxic T-Lymphocytes (CTL) play a major role in it. Several nanoparticles were reported to induce CTL, renewal of stem cells, immune cells and cytokines as well. It is hypothesised, if CTL is stimulated with nanoparticles HIV reservoir would be eradicated. To test this hypothesis this study was performed. This is the modification of 500 years old traditional medicine. Methods: Prakasine (PRK-NP) is the 10 - 50 nm size, spherical shape, nanoparticle. Consenting ART-naive (n=14), male and female, aged between 25 - 50 years, with weight loss about average 5 kgs, lethargic, HIV RNA ≤ 100000 copies/ml, CD4~500 cells /µl HIV patients and healthy individuals (n=4) were enrolled in this study and divided as positive control (n=4), treatment group (n=10) and negative control (n=4). The treatment group only administered 1gram of PRK-NP thrice daily for three years in Naval AIDS Research centre, Namakkal. Body weight (BW), viral load (VL), HIV1 proviral DNA, CD4, CD8, complete blood cell (CBC), liver function tests (LFT), kidney function tests (KFT) and physical neurological examination were analysed once in three months. Wald chi-square test was performed for statistical analysis. Results: In all patients in treatment group (n=10) clinical symptoms disappeared. The mean BW gain about 10 kgs, mean rise of CD4 and CD8 are 296 and 225 respectively (p=0.0001, Wald chi-square test) show that the PRK-NP has induced immune cells growth in treatment group. The VL came to Less than Detectable (LDL) level, but HIV-1 proviral DNA detected in five patients and in another five patients, the Proviral DNA not detected. Whereas in positive control VL raised from 27250 to 181500, CD4 decreased from 827 to 402 and CD8 reduced from 750 to 467, the Proviral DNA is detected in all the times. In negative control, all the time, the VL is LDL, proviral DNA is not detected and there are no significant changes in CD4 and CD8. In all patients in treatment group and in both positive and negative control, CBC, LFT, KFT and physical neurological examination arenormal. Discussion: PRK-NP reveals no side-effects and possesses therapeutic efficacy.
Keywords: Nanomedicine, Prakasine, HIV Cure, HIV treatment, alternative to ART, Immunotherapy and Immunostimulant drug
Introduction
Mercury has been shown as miraculous metal in Tamil Traditional Medicine (TTM) [1-5]. It has various kinds of physical and chemical properties than any other metals [6-13]. Mercury and its compounds have medicinal properties and it is being used for various diseases in various preparations in TTM [14-19]. Several countries banned mercury in medicine due to its toxicity and side effects [20-33]. Mercury is still in use in dentistry in many countries [34]. Mercury was used for syphilis treatment from 16th - 20th century [35]. Mercury is still being used in alternative medicine systems especially in Chinese, Ayurvedic and Siddha (Traditional Tamil Medicine) effectively. Even though, modern medicine says that mercurial preparations are toxic, the traditional medicines play an effective role in the treatment of ailments in humans, animals and birds. Hence, the question is being raised! Which produces the real mercury toxicityonly single compounds or only certain type of mercurial compounds? Can we produce a non-toxic or less toxic mercury compounds like anti-snake venom from venom, vaccines from same viruses by using nanotechnology and biotechnology? This is the milliondollar question. If we can produce less toxic or non-toxic mercury compound, certainly, it will be useful for therapeutics because from 16th century to 20th century, the whole world was depended only on mercurial for incurable diseases then stopped because of its toxicity. If we nullify the toxicity by nano-biotechnological strategies again it will come into existence for the treatment of diseases. As an author, I have been observing last two decades in my TTM practice to various diseases and admired about mercurial preparations comprising plant extracts, minerals in TTM did not produce any side effects rather they have given only beneficial effects. Unfortunately, no publications available for that, but there is separate department available in Tamilnadu government and this kind of preparations are desperately prescribed and supplied to the patients suffering with several ailments in each and every government hospital of Tamilnadu, India. With this background, unpublished reports, results from my patients, my experience and observations of various combinations and its apparent chemical molecules present in it to produce therapeutic effects have given me an enthusiasm to explore this and produce non or less toxic mercurial nano medicine based on hypothesis and my proposed modern scientific theory.
When we look into insight of toxicity and side effects, these will fall under only two categories. One is, the mercury atom attaches with the sulphur atom of the biomolecules and causes toxicity. The another one is stagnation of metal compounds in to the cells or cellular compartments without getting eliminated after ingestion by any route. Here based on reaction of molecules In-vivo and observations made among many reactions and various compounds, a wide scientific theory is proposed on hypothesis, that is,
1.If metal atoms are attached with sulphur atoms with coordinated covalent bond gives raise to metallic sulphur complex, if this metallic sulphur complex is attached further with organic compound (ligand), the organometallic complex will be formed, which will not produce any toxicity or less toxicity.
2.This metallic sulphur complex will form di-sulphide bond in In vivo and evince therapeutic action with less adverse effect.
3.Some metals particularly mercury attaches with sulphur and form covalent bond even when more other elements present in a biochemical reaction. The bond between Hg and S cannot be broken by biochemical reactions both In-vivo and In-vitro.
This proposed theory is here after called as “Prakash Theory of Metal Drugs”. Thus, the synthesised metal nano medicine would have potential for exploitation in the biomedical field. To test this hypothesised theory, the highly toxic metal mercury was selected and the present study was performed. Mercury and sulphur atom were covalently attached, again this molecule was attached with one sulphur containing biomolecule in Ex-vivo made into nanoscale level and has been developed as drug. As per the theory the mercury gets into system and come out as HgS or its complexes after evincing the biological role. So, there will be no toxicity by this drug. My previous works proved this theory and Prakasine play as immunotherapy as well [36-39].
Materials and Methods
Ethical Statement
The particular regimen of Prakasine nanomedicine is from the approved system being under medical practice more than 500 years in my state and the formulation is already in our country’s Traditional Knowledge Digital Library (TKDL). I obtained informed consent and this study was performed as per Helsinki declaration.
Nano medicine Synthesis
Prakasine (PRK-NP) nanoparticle was synthesised with processed mercury, processed sulphur and methionine (ligand) according to patent application number CHE/5776/2014 and as reported in my previous works [36-39] and employed for this present study.
Nano medicine Therapy
The HIV individuals those who voluntarily sought the treatment from Naval AIDS Research center, Namakkal were randomly selected. Some 14 individuals who were not ready to go for HAART due to its adverse effects were enrolled in this longitudinal study. Among the 14, four HIV individuals neither to take PRK-NP nor to take HAART due to their various situations were kept as positive control as per their request to monitor the HIV status in the absence of any treatment. Some four HIV negative individuals having sexual exposure with unknown persons psychologically demanded the PRK-NP were kept as negative control to monitor their status with the intention of initiating treatment if they become positive.
The inclusion criteria are both male and female, aged between 25 - 50 years, with weight loss about average 5 kgs, lethargic, HIV RNA ≤100000 copies/ml, CD4~500 cells /μl and four were non-HIV infected individuals. The exclusion criteria are paediatric age group, pregnant women, opportunistic infections, diabetics, hypertension, any other diseases and HAART. They were administered with PRKNP at the dose level of one capsule containing 1000 mg thrice in a day for 36 months. The viral load, HIV proviral DNA, CD4, CD8 profile, Complete Blood Count (CBC), Liver Functions Tests (LFT), Renal Function Tests (RFT) and physical neurological examination were performed every three months once for all. All assays were performed in triplicate. Wald chi-square test was performed for statistical analysis.
Results
The results of PRK-NP nano medicine therapy have shown that the clinical symptoms like lethargies disappeared completely in all patients of treatment group, in positive control the clinical symptoms increased. Whereas in negative control no significant changes noticed. The mean BW gain about 10 kgs in treatment group, whereas in the positive control the BW average 5 kgs reduced but in negative control no significant changes.
Viral load
In treatment group the VL has reduced from 59330 (mean value) (Table 1) to Less than detectable limit (LDL) when compare to the before treatment. In positive control the VL has increased from 27250 (mean value) to 181500 (mean value) when compare to the before treatment. Whereas in negative control all the time were LDL.
| PATIENT NUMBER | BEFORE TREATMENT | AFTER 6 MONTHS | TREATMENT 12 MONTHS | 18th month | 24th month | 36th month |
|---|---|---|---|---|---|---|
| Treatment group | 90000 | 53000 | 11000 | 5500 | 770 | LDL |
| 94300 | 69500 | 15400 | 9000 | 1500 | LDL | |
| 43000 | 21500 | 10300 | 5500 | 900 | LDL | |
| 52000 | 23000 | 14000 | 7000 | 1400 | LDL | |
| 63000 | 29000 | 17000 | 9000 | 1000 | LDL | |
| 15000 | 6700 | 2900 | 1100 | 700 | LDL | |
| 80000 | 18000 | 11000 | 6500 | 900 | LDL | |
| 90000 | 35000 | 17500 | 7000 | 600 | LDL | |
| 16000 | 7500 | 300 | LDL | LDL | LDL | |
| 50000 | 25000 | 12500 | 5000 | 500 | LDL | |
| Positive Control | 27000 | 110000 | 145000 | 130000 | 160000 | 230000 |
| 19000 | 49000 | 63000 | 78500 | 110000 | 150000 | |
| 29000 | 55000 | 85000 | 111000 | 148300 | 201000 | |
| 34000 | 57000 | 76000 | 111500 | 126600 | 145000 | |
| Negative Con- trol | LDL | LDL | LDL | LDL | LDL | LDL |
| LDL | LDL | LDL | LDL | LDL | LDL | |
| LDL | LDL | LDL | LDL | LDL | LDL | |
| LDL | LDL | LDL | LDL | LDL | LDL |
Table 1: Quantification of of viral load (Copies of Viral RNA per ml of Serum).
CD4 cells
The CD4 counts of the treatment group has increase from the 632 (mean value) to 928 (mean value) (Table 2) compare to before treatment but in positive control it has reduced from 827 (mean value) to 402 (mean value). In the negative control there is no significant changes
| PATIENT NUMBER | BEFORE TREATMENT | AFTER | TREATMENT | 18th month | 24th month | 36th month |
|---|---|---|---|---|---|---|
| 6th month | 12th month | |||||
| Treatment group | 690 | 710 | 990 | 980 | 1000 | 1010 |
| 680 | 880 | 1080 | 950 | 1000 | 1050 | |
| 510 | 940 | 950 | 930 | 960 | 980 | |
| 890 | 1100 | 1100 | 950 | 1000 | 1090 | |
| 580 | 990 | 990 | 980 | 999 | 1000 | |
| 670 | 1010 | 1000 | 1020 | 1000 | 1100 | |
| 690 | 980 | 950 | 800 | 800 | 800 | |
| 510 | 760 | 760 | 770 | 750 | 750 | |
| 520 | 790 | 750 | 750 | 760 | 750 | |
| 580 | 800 | 770 | 770 | 760 | 750 | |
| Positive Control | 1000 | 850 | 750 | 700 | 600 | 500 |
| 750 | 560 | 510 | 490 | 410 | 360 | |
| 760 | 750 | 680 | 569 | 448 | 360 | |
| 800 | 750 | 590 | 550 | 480 | 390 | |
| Negative Control | 1000 | 980 | 1000 | 1020 | 1000 | 990 |
| 900 | 990 | 910 | 900 | 990 | 970 | |
| 950 | 1000 | 950 | 1000 | 980 | 990 | |
| 1100 | 1080 | 1100 | 1200 | 1000 | 1010 |
Table 2: Cd4 cell counts of patients before and during the course of treatment (cells/ l of blood).
CD8 Cells
The CD8 counts of the treatment group has increase from the 769 (mean value) to 994 (mean value) (Table 3) compare to before treatment but in positive control it has reduced from 750 (mean value) to 467 (mean value). In the negative control there is no significant changes.
| PATIENT NUMBER | BEFORE TREATMENT | AFTER | TREATMENT | 18th month | 24thmonth | 36thmonth |
|---|---|---|---|---|---|---|
| 6thmonth | 12thmonth | |||||
| Treatment group | 760 | 770 | 1000 | 790 | 750 | 1000 |
| 750 | 770 | 780 | 770 | 1030 | 990 | |
| 800 | 810 | 750 | 750 | 760 | 890 | |
| 760 | 770 | 760 | 1200 | 760 | 1000 | |
| 759 | 767 | 768 | 768 | 787 | 980 | |
| 800 | 810 | 820 | 810 | 1810 | 1000 | |
| 769 | 750 | 1300 | 750 | 760 | 1050 | |
| 770 | 760 | 780 | 760 | 780 | 980 | |
| 760 | 760 | 800 | 1000 | 1320 | 1020 | |
| 770 | 760 | 1050 | 780 | 750 | 1030 | |
| Positive Control | 740 | 770 | 810 | 760 | 770 | 550 |
| 750 | 760 | 770 | 780 | 800 | 410 | |
| 760 | 800 | 860 | 850 | 630 | 400 | |
| 750 | 770 | 770 | 810 | 450 | 510 | |
| Negative Control | 1020 | 760 | 770 | 780 | 770 | 1000 |
| 800 | 790 | 780 | 890 | 798 | 790 | |
| 890 | 900 | 1000 | 1000 | 770 | 900 | |
| 910 | 1080 | 910 | 900 | 800 | 900 |
Table 3: Cd8 cell counts of patients before and course of treatment (cells/ l blood).
Complete Blood Cell Counts (CBC)
The hemogram (Table 4) shows the CBC values of all the patients of treatment group, positive control and negative control. There are no significant changes of CBC before and after the treatment of all groups.
| PIN | W.B.C | P | L | E | M | HB | RBC | PC | PCV | E.S.R | W.B.C | P | L | E | M | HB | RBC | PC | PCV | E.S. R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | X105 | % | % | % | % | % | X105 | % | |||||||||
| 1 | 7500 | 50 | 40 | 6 | 4 | 13 | 5 | 3 | 40 | 5 | 9000 | 41 | 45 | 4 | 10 | 14 | 6 | 4.2 | 40 | 5 |
| 2 | 4000 | 60 | 30 | 7 | 3 | 10 | 4 | 1.3 | 27 | 7 | 10000 | 45 | 40 | 6 | 9 | 13 | 5 | 2.3 | 40 | 5 |
| 3 | 5500 | 55 | 35 | 5 | 5 | 11 | 4 | 2 | 45 | 6 | 9000 | 46 | 44 | 4 | 6 | 14 | 4 | 3 | 45 | 4 |
| 4 | 4500 | 60 | 35 | 7 | 5 | 12 | 4 | 3 | 30 | 6 | 10000 | 45 | 45 | 2 | 8 | 15 | 5 | 4 | 45 | 5 |
| 5 | 5000 | 55 | 37 | 7 | 4 | 12 | 3 | 3 | 30 | 6 | 10000 | 50 | 40 | 3 | 7 | 14 | 5 | 3.6 | 40 | 4 |
| 6 | 7000 | 60 | 30 | 7 | 3 | 12 | 4 | 2 | 30 | 7 | 9000 | 40 | 45 | 5 | 10 | 15 | 6 | 4 | 50 | 4 |
| 7 | 4000 | 60 | 30 | 6 | 4 | 11 | 3 | 3 | 28 | 7 | 9000 | 40 | 45 | 5 | 10 | 14 | 5 | 4 | 40 | 4 |
| 8 | 5500 | 55 | 35 | 5 | 5 | 12 | 4 | 2 | 30 | 6 | 8500 | 47 | 40 | 3 | 10 | 15 | 5 | 3.5 | 50 | 5 |
| 9 | 6000 | 60 | 30 | 6 | 4 | 12 | 4 | 3 | 30 | 7 | 9000 | 45 | 42 | 3 | 10 | 14 | 6 | 4 | 45 | 4 |
| 10 | 7000 | 60 | 30 | 5 | 5 | 11 | 3 | 2 | 27 | 6 | 9500 | 45 | 42 | 3 | 10 | 14 | 5 | 4 | 50 | 5 |
| 11 | 4000 | 60 | 30 | 6 | 4 | 11 | 4 | 3 | 30 | 6 | 9000 | 43 | 45 | 3 | 9 | 15 | 5 | 3 | 45 | 4 |
| 12 | 6500 | 65 | 27 | 4 | 4 | 12 | 4 | 3 | 30 | 6 | 9000 | 43 | 45 | 4 | 8 | 14 | 5 | 4 | 50 | 5 |
| 13 | 7000 | 60 | 30 | 5 | 5 | 12 | 4 | 3 | 30 | 6 | 9500 | 43 | 45 | 4 | 8 | 15 | 6 | 4 | 45 | 5 |
| 14 | 6000 | 65 | 25 | 5 | 5 | 12 | 3 | 2 | 30 | 5 | 9000 | 42 | 45 | 3 | 10 | 14 | 4 | 4 | 45 | 4 |
| 15 | 6000 | 61 | 23 | 5 | 6 | 11 | 3 | 2 | 29 | 5 | 6000 | 41 | 44 | 2 | 11 | 13 | 3 | 4 | 45 | 4 |
| 16 | 6500 | 60 | 24 | 5 | 5 | 12 | 4 | 3 | 30 | 5 | 7000 | 43 | 45 | 4 | 10 | 15 | 6 | 4 | 45 | 5 |
| 17 | 7000 | 61 | 30 | 5 | 5 | 12 | 4 | 3 | 30 | 6 | 7000 | 43 | 45 | 4 | 11 | 15 | 6 | 4 | 45 | 5 |
| 18 | 6000 | 60 | 30 | 5 | 5 | 12 | 4 | 3 | 30 | 6 | 6000 | 43 | 45 | 5 | 12 | 14 | 6 | 4 | 30 | 3 |
Table 4: Haemogram of the hiv-1 infected and normal individuals before and after treatment (36 months). The patients 1-10 are treatment group, patients 11-14 are positive control and 15-18 are negative control.
Proviral DNA
The HIV-1 Proviral DNA is detected in all the 10 patients in treatment group before the initiation of the treatment (Table 5) but after the treatment 5 patients that is patient numbers 1, 4, 5, 9 and 10 has come to the level of “not detected”. This is due to the significant effect of the treatment with PRK-NP.
| Patients Numbers | Before Treatment | After Treatment |
||||
|---|---|---|---|---|---|---|
| 6th month | 12th month | 18th month | 24th month | 36th month | ||
| Treatment Group | Detected | Detected | Detected | Detected | Not Detected | Not Detected |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Not Detected | Not Detected | Not Detected | |
| Detected | Detected | Detected | Detected | Not Detected | Not Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Not Detected | Not Detected | Not Detected | |
| Detected | Detected | Detected | Detected | Detected | Not Detected | |
| Positive control | Detected | Detected | Detected | Detected | Detected | Detected |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Detected | Detected | Detected | Detected | Detected | Detected | |
| Negative control | Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | Not Detected |
| Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | |
| Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | |
| Not Detected | Not Detected | Not Detected | Not Detected | Not Detected | Not Detected |
Table 5: Status of the hiv-1 proviral dna of infected and normal individuals before and after treat- ment (36 months). The patients 1-10 are treatment group, patients 11-14 are positive control and 15-18 are negative control.
Neurological Study
The neurological studies were performed for all the patients in all the group (Table 6) to assess the neurotoxicity of PRK-NP. The study reveals that there is no neurotoxicity.
| Patients Numbers | Before Treatment | After Treatment |
|---|---|---|
| Treatment Group | Normal | Normal |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Positive control | Normal | Normal |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal | |
| Negative Control | Normal | Normal |
| Normal | Normal | |
| Normal | Normal | |
| Normal | Normal |
Table 6: Neurological examination (mental status, cranial nerves, motor, sensory, reflexes, coordination, gait and for neurological clinical symptoms).
As the measure of another safety study, in all the patients, in treatment group, positive control and negative control, LFT and KFT are normal in all the times. The results are statistically significant (p=0.0001, Wald chi-square test).
Discussion
Even though the existing state of art mercury compounds are toxic, the PRK-NP was developed by novel theory, strategy and also by TTM protocols as nontoxic by its elemental articulations, particle sizes and administered to humans since it did not produce any toxicity in animal studies conducted in my previous works [36- 39]. My previous studies in birds, zebrafish ,mice and in human had revealed that the PRK-NP is able to increase the humoral immunity against Newcastle disease, increases the immunostimulatory (IL1α, IL1β, IL2, IL6, IL8, IL12, TNFα, IFNγ) cytokine gene expressions, the maximum tolerance dose is 900 mg/kg body weight and potential immunotherapy for HIV respectively.
The PRK-NP is the combination of TTM molecules and biomolecules; therefore, these are novel chimeric molecules for therapeutic use. Since the TTM is the oldest system of medicine in Tamilnadu and approved by government of India, the references cited in this manuscript are in India’s Traditional Knowledge Digital Library (TKDL) website (http://www.tkdl.res.in/tkdl/LangDefault/ siddha/Sid_Advancesearch.asp?GL=Eng) both in translated version of English and prior art version of Tamil including its formulator preparations and therapeutic uses, so no need of present regulatory approvals to use in human beings. Many of the TTM ingredients possesses methionine, therefore, methionine was directly added in pure form to make this chemical complexes easily in this synthesis. The TEM, XRD and SEM studies revealed these particles are in nanoscale, the sizes are about 5 nm -50 nm [36-39].
There are other metal complexes already produced in nanoscale level and reported to have therapeutic effect [40-45]. Already several nanoparticles were employed in HIV research both for diagnostic and treatment purposes but none of them have given encouraging results to treat HIV patient’s alternative to ART successfully [46-50]. Different micronized HAART drugs are heavily toxic that is being administered to HIV patients since there is no alternative for them [51-53]. Since there are no available regulatory bodies for nano medicines only few drugs in Nano form are under Human clinical trials [54]. Already methionine metal complexes had been synthesised and reported but they are not nanoparticles [55,56].
Even though many nanoparticles used as medicine there is no specific theory to synthesis like Prakash theory of metal drugs. From zero side effects of PRK-NP and its appreciable results in human HIV patients, the theory is proven to be effective. This study shows that the nano mercury locked with sulphur atom or atoms will not produce any toxicity, side effects rather beneficial effects. Before this study there is one evidence with mercury preparations developed by TTM protocols were administered to HIV/AIDS patients in government Tambaram Sanatorium Hospital by Dr. Deivanayagam and the patients were recovered successfully without any adverse effects [57].
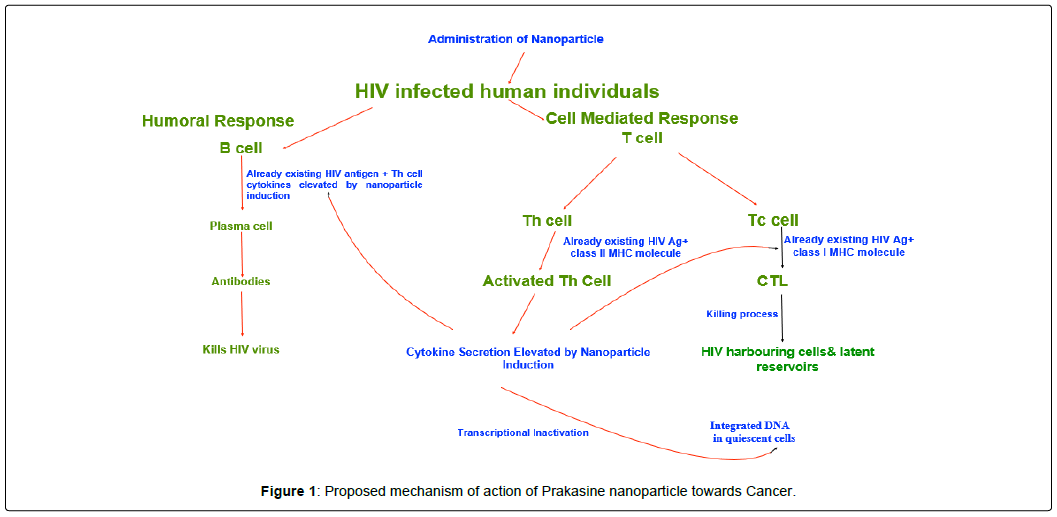
We could understand from results of this study that the PRKNP possess the effects to reduce the viral load and eliminate HIV proviral DNA in some patients with increase of CD4 and CD8 cells. Also, we could observe that the PRK-NP has increased the weight gain and reduced the symptoms. It is assumed since there are no significant changes in the parameters like liver profile, renal profile, CBC, neurological examination that the PRK-NP does not have any adverse effects as they are novel and in nanoscale level. PRK-NP was tested for anti-HIV activity but they did not possess the same In vitro (unpublished data) but they are producing good immunological anti-HIV results during the treatment to HIV patients due to immunostimulatory cytokines gene expression. They might evince the anti-HIV action In-vivo by sensing dormant HIV then target the infected cells. The Cytotoxic T-Lymphocytes (CTL) might play a major role in it (Figure 1). Several nanoparticles were reported to induce CTL, renewal of stem cells, immune cells and cytokines as well. Further studies to be warranted to confirm this.
It is very important in this study that the mercury atoms were detoxified with sulphur locks and again attached with sulphur containing amino acid DL-Methionine Ex-vivo to prevent the adverse and toxic effects by arresting the reactions of mercury with sulphur atoms of sulphur containing amino acids because all the mercury compounds reacts with sulphur containing amino acids In-vivo particularly the mercury atom attaches with sulphur atom and disturb the functions of that amino acid that is why the mercury toxicity is being developed by inactivating sulphur or sulfhydryl groups of enzymes and thus interfering with cellular metabolism and function as mercury readily forms covalent bond with sulphur [58]. The PRKNP is not interfering with cellular metabolism and function as other mercury compounds. Therefore, this is safe and has the potency to treat HIV patients with further studies.
Conclusion
Since no drugs have the potency so far towards the HIV cure, this PRK-NP may be useful for the HIV patients as an alternative to ART and cure as well with numerous further studies.
Acknowledgement
This is to acknowledge Dr.P.Revinselvan MBBS, Naval AIDS Research Centre general physician for conducting this study in human individuals and generating the data.
Conflict of Interest
This is to declare that this study does not have any conflict of interest.
References
- Thiyagarajan G (1992) Department of Indian Medicine and Homeopathy, Chennai;167-199
- Agasthiyar (1964) Agasthiyar paripooranam 400 Rathina Nayakar & sons, Thirumagal vilakku press, Chennai: 40
- Matchamuni (1985) Matchmuni perunool 700 (Matchmuni big therapeutic book 700 formulations) CCRAS publications, Chennai:113-114
- Kannusami P (1993) C.Vaithiya Chinthamani (Treatment Protocols).Rathina Nayakar & sons, Thirumagal vilakku press,Chennai:216- 217
- Kannusami P (1875) C.Paramparai Vaithiyam (Traditional Treatment).Rathina Nayakar & sons, Thirumagal vilakku press,Chennai:327- 329
- Lide (2005) Magnetic susceptibility of the elements and inorganic compounds CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press
- Stuart Berg F (1974) Hydrargyrum. Random House Webster's Unabridged Dictionary
- Hammond (2005) The Elements. in Lide,CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press
- Senese F (2007)
- Norrby LJ (1991) Why is mercury liquid? Or, why do relativistic effects not get into chemistry textbooks?" J.Chemi Edu.68: 110
- Lide (2005) CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRCPress.126.
- "Why is mercury a liquid at STP?". General Chemistry Online at Frostburg State University Retrieved 1 May
- Pulippani (1969) Pulippani vaithiyam 500 (Pulippani treatment 500 formulations).Rathina Nayakar & sons, Thirumagal vilakku press,Chennai: 72- 7
- Agasthiyar (1970) Agathiyar vaithia ratina chrukkam 300 (Agathiyar treatment abstracts 300 formulations) Ed: Dr.Radhakrisnan.Pub: Rathina Nayakar & sons, Thirumagal vilakku press,Chennai:125-127
- Therayar (1979) Therayar Sekarappa (Therayar treament collections). Pub:CCRAS publications,Chennai:163-164
- Agasthiyar (1881) Agathiyar amutha kalai gnanam 1200 (Agathiyar wisdom of medical practices 1200 formulations). Ed.Thirumalai Vaithiya Linga Desigar.Pub:Kuppusamy Naidu Sakalakalanilaya press:228
- Bogar (1994) Bogar 700 (Bogar treatment practices 700 formulations).Ed.Ramachandran:Thamarai Noolagam;,Chennai:54-55
- Bogar (1975) Bogar 7000 (Bogar treatment practices 7000 formulations). Ed. Ganghadhara Thevar: Palani Thandayuthabani Devasthanam Publications, Directorate of Indian Systems of Medicine; Chennai:182-183
- Gmelin (2012) Leopold.Hand book of chemistry.1852. Cavendish Society. pp. 103 (Na), 110 (W), 122 (Zn), 128 (Fe), 247 (Au), 338 (Pt). Retrieved 30 December
- Greenwood, Norman N, Earnshaw (1997) Alan Chemistry of the Elements (2nded).
- Butterworth Heinemann
- United States Environmental Protection Agency (2008) "Mercury: Laws and regulations".16 April. Retrieved
- United States Court of Appeals for the District of Columbia Circuit (2008) Argued 6 December 2007, Decide 8 February 2008. Retrieved 30 May
- Boston Globe (2012) "Oldest, dirtiest power plants told to clean up" 22 December 2011. Retrieved 2 January
- Howard Berkes 2012) NPR Retrieved 2 January
- International Joint Commission on the (1979)
- "Reductions in Mercury Emissons"
- United States Environmental Protection Agency (2007) "Clean Air Mercury Rule" Retrieved
- Electronic Equipment (2003) "Directive 2002/95/EC on the Restriction of the Use of Certain Hazardous Substances in Electrical
- EIA Track (2008) "Mercury compounds in European Union Retrieved
- Jones H. (2007) "EU bans mercury in barometers, thermometers” Reuters. Retrieved
- EU Business (2008) Archived from the original on
- "Norway to ban mercury" Retrieved
- Berg, T, Fjeld, E, Steinnes E (2006) "Atmospheric mercury in Norway: contributions from different sources". The Science of the total environment 368: 3-9
- Torun B (2006) Atmospheric mercury in Norway: Contributions from different sources.368:3-9
- S.K. Prakash (2017) Effect of Feed Supplementation of Mercury Nanoparticles on Immunostimulant of Live Lentogenic Newcastle Disease Vaccine in Layer Birds. Indian Vet. J, 94:11-13
- Nanomedicine and Biotechnology (press) (1973) Prakasine nanoparticle as a potential drug candidate for cancer treatment by gene expression stimulation. Journal of Artificial Cells,
- Eastern Europe and Central Asia AIDS Conference (2016) Immunological and Virological Effects of Prakasine Nanomedicine in HIV Eradication: A preliminary Study. Moscow, World Trade Centre, March:23-25
- Central Asia AIDS Conference (2018) Prakasine as the potential drug towards the HIV elimination.6th Eastern Europe, Moscow, World Trade Centre:18-20
- Edlich, Richard F (2006) Banning Mercury Amalgam. US FDA
- The local Sweden to ban mercury (2012) Retrieved on 30 December
- Jaypee Brothers Publishers (2002) Sorter Essentials of Dental Materials
- George CR (2011) Mercury and the kidney.J Nephrol.Suppl 17:26-32
- Wenjie s, Ding Q, Yihua M,Yan c (2014) Enhanced stability and antibacterial efficacy of a traditional chinese medicine-mediated silver nanoparticle delivery system.Int J Nanomedicine:9:5491–5502
- Mohan S, Sheraz R. Markar, George B (2015) Application of gold nanoparticles for gastrointestinal cancer theranostics: A systematic review.Nanomedicine: Nanotechnology, Biology and Medicine.11:2083–2098
- Jonathan S. Rink, Michael P. Plebanek Shad Thaxton (2013) Update on Current and Potential Nanoparticle Cancer Therapies.38. Curr Opin Oncol.25:646–651
- Catherine A, Heinz M, Iseult L (2013) Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine.8:449-467.
- S Jain, Hirst D, Sullivan J (2012) Gold nanoparticles as novel agents for cancer therapy. Br J Radiol.85:101–113
- Erik C, Lauren A, Megan A, Mostafa A (2012) Size matters: gold nanoparticles in targeted cancer drug delivery.Ther Deliv.3:457–478
- Anil K, Bhargavi M, Xing-Jie L (2011) Gold Nanoparticles: Promising Nanomaterials for the Diagnosis of Cancer and HIV/AIDS. Journal of Nanomaterials:17
- Khoza S (2011) Highly active antiretroviral therapy: Does it Sound toxic?J Pharm Bioallied Sci.3:142–153
- Carin E (2011) Common Adverse Effects of Antiretroviral Therapy for HIV Disease.Am Fam Physician.83:1443-1451
- Alexandra C, Bernard H, David A (2009) Cooper and Andrew Carr. A new era of antiretroviral drug toxicity.Antiviral Therapy.14:165–179
- Hood JL, Jallouck AP, Campbell N, Ratner L, Wickline SA (2013) Cytolytic nanoparticles attenuate HIV-1 infectivity. Antiviral Therapy.19: 95 -103
- Singh H, Singh J (2012) Synthesis and characterisation of new lead (II) and organotin (IV) complexes of Schiff bases derived from histidine and methionine. International Journal of Inorganic Chemistry.7
- Tewodros M, Ashley M (2010) Emerging nanotechnology approaches for HIV/AIDS treatment and prevention.Nanomedicine (Lond).5:269–285
- Deivanayagam C N (2000). "Traditional Medicine: Siddha therapy in HIV Disease – a South Indian Experience". World AIDS Conference, Durban. Tambaram Sanatorium, Chennai, India: Govt. Hospital of Thoracic Medicine. Retrieved.12-25
- Goodman, Louis S, Alfred Gilman, Laurence L. Brunton Goodman & Gilman's the Pharmacological Basis of Therapeutics. New York: McGraw-Hill.1753-1763
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi