Research Article, J Vet Sci Med Diagn Vol: 11 Issue: 4
In Vitro Study of Curcuma, Honey, and Probiotics Combination as Candidates for Feed Additives to Replace Growth Promoter Antibiotics (AGP)
Marlin Cindy Claudya Malelak*, Agnesia Endang Tri Hastuti Wahyuni and Agustina Dwi Wijayanti
Department of Veterinary Science, Gadjah Mada University, Yogyakarta,Indonesia
*Corresponding Author:Marlin Cindy Claudya Malelak, Department of Veterinary Science, Gadjah Mada University, Yogyakarta, Indonesia; E-mail: sonyamalelak@gmail.com
Received date: 07 December, 2022, Manuscript No. JVSMD-21-49191; Editor assigned date: 10 December, 2022, PreQC No. JVSMD-21-49191 (PQ); Reviewed date: 27 December, 2022, QC No. JVSMD-21-49191; Revised date: 07 February 2022, Manuscript No. JVSMD-21-49191 (R); Published date: 01 April 2022, DOI: 10.4172/2325-9590.11.3.016
Citation: Malelak MCC, Wahyuni AETH, Wijayanti AD (2022) In Vitro Study of Curcuma, Honey, and Probiotics Combination as Candidates for Feed Additives to Replace Growth Promoter Antibiotics (AGP). J Vet Sci Med Diagn 11:4.
Abstract
Growth Promoter Antibiotics are used to prevent disease and promote growth and production in poultry. Repeated administration of feed can have a micro-organic resistance effect, accumulation of antibiotic residues in animal and environmental products and imbalance of normal micro-flora in the intestine. The antibacterial and carbohydrate content of some natural ingredients can be potential as a replacement candidate for AGP. This study aims to determine the role of a combination of curcuma, honey, and probiotics (Bacillus subtilis and Lactobacillus acidophilus) as AGP candiate in vitro. The antibacterial activity of the combination of curcuma and honey against pathogens (E. coli) and their use against probiotics was tested by disk diffusion method, while the calculation of optical density values to determine the minimum inhibitory concentration and minimum bactericidal concentration was carried out on E. coli. The inhibition ability of probiotics against pathogens is also done by the disk diffusion method. The disk diffusion test results showed the best combination of 25% curucuma aquades extract+100% Lombok honey with inhibition zone diameter (8.53 ± 0.03). Optical density values indicate this combination is able to inhibit and kill E. coli (DO 0.00 ± 0.002) and supports the growth of B. subtilis (DO 0.18 ± 0.002) and L. acidophilus (DO 0.25 ± 0.005) significantly better than positive control. MIC value of curcuma aquades extract and honey combination against E. coli is curcuma aquades extract 3.13%+Lombok honey 25%, and MBC value is curcuma aquades extract 6.25%+Lombok honey 25%. The combination of B. subtilis and L. acidophilus probiotics showed the largest inhibitory zone diameter against E. coli pathogen (7.30 ± 0.02 mm) compared to individual colonies. The combination of curcuma and honey, in addition to inhibiting also able to kill pathogens and support the growth of probiotics, so this formula can be used as one of the replacement candidates for AGP.
Keywords: Antibiotic growth promoter; E. coli; Honey;
Probiotics; Curcuma
Introduction
Antibiotics are used with the aim of increasing growth and production as well as preventing disease in poultry. These positive effects can change function when antibiotics are not in accordance with the recommendations and prescribed doses, so that they can have negative effects such as resistance to microorganisms, reduced use of good bacteria in the intestines, and antibiotic residues in animal products and the environment [1]. The Food and Agriculture Organization stated that the incidence of antibiotic resistance in both humans and animals is a major global problem and has now been recognized as a significant emerging threat to public health and food security (Food and Agriculture Organization, 2016). An alternative solution that is currently being carried out is the exploration of various natural materials to take advantage of the ability of antibacterial activity and increase livestock productivity [2-5]. Research on the combination of galangal, ginger, temulawak and honey with various concentrations has been shown to increase the productivity and carcass weight of broiler chickens. Feed supplementation with probiotics such as Lactobacillus, Bacillus, and Clostridium can increase growth, nutrient digestibility, and humoral immunity [6]. This research was designed to formulate a combination of ginger and honey and probiotics that has never been done before. The purpose of this study was to determine the role of the combination of ginger, honey, and probiotics (Bacillus subtilis and Lactobacillusacidophilus) as a candidate for in vitro AGP replacement.
Materials and Methods
The study was conducted in June 2019 on Veterinary Medicine Microbiology Laboratory, Departement of Veterinary Medicine, Gadjah Mada University. In this study, data from the disk diffusion method were analyzed by One-Way Analysis of Variance (ANOVA) and followed by the Post-Hoc Tukey test, optical density data from the dilution method were analyzed by Kruskal-Wallis and continued by the Mann-Whitney test. A significant difference from each treatment occurred when the P value <0.05. MBC determination was analyzed descriptively. Based on this framework, the following hypotheses can be formulated:
H1: The combination of curcuma and honey can inhibit the growth of E. coli and increase the growth of B. subtilis and L. acidophillus.
H2: The growth of B. subtilis and L. acidophilus can inhibit the growth of E. coli.
The research tools used were petri dishes, refrigerator, tweezers, ose, bunsen, microscope, syringe, microplate, microplate reader, digital caliper, centrifuge, and vortex. The materials used were Eschercia coli ATCC® 11775 (pathogen) and Bacillussubtilis ATCC® 6633 (probiotic) obtained from the Veterinary Center (BVET), and Lactobacillusacidophilus (probiotic) obtained from the collection of the UGM Inter-University Center (PAU), Yogyakarta, Indonesia, Broth Heart Infusion (BHI, MerckTM), phosphate buffered saline (pH 7.4, SigmaTM), Mueller Hilton agar (MHA, MerckTM), curcuma aquadest extract (concentration: 25%, 12.5%, 6.25%, 3.13%, 1.56%), Lombok honey (100% concentration), distilled water, blank disc (OxoidTM), chloramphenicol antibiotic disc (C 30 µg, OxoidTM).
The method used to test the antibacterial activity was Kirby-bauer disc diffusion in which the pathogenic and probiotic bacterial cultures were cultured on each medium resuspended with Phosphate Buffered Saline (pH 7,4, SigmaTM) to a concentration of 1.5 × 108 CFU/mL. A blank disc (OxoidTM) was dripped with 50 μl a combination of curcuma aquadest extract and honey then placed on the surface of MHA (MerckTM) media which had been cultured E. coli, B. subtilis, and L. acidophilus. Chloramphenicol disc (C 30 μg, OxoidTM) was used as a positive control, while sterile distilled water was used as a negative control and then incubated at 37°C for 24 hours. Observations were made by measuring the diameter of the inhibition zone and the growth zone around the disc. The zone of inhibition (clear zone) surrounding the disc describes the antibacterial activity of natural substances against pathogens, while the growth zone around the disc indicates the ability of natural ingredients to support the growth of probiotic candidates.
The next method is dilution to calculate optical density values on 96-well microplates on a round basis described in Clinical and Standard Institute M07-A9 and Clinical Institute Standard Institute. The combined extract with a volume of 25 μl (in 100 μl broth media, BHI for E. coli and B. subtilis culture media and MRS broth for L. acidophilus culture media) was placed in the first well, and then doubled dilution was carried out in each subsequent well until the lowest concentration of 1.56%. Ten microliters of each bacterial suspension with a concentration of 1.5 × 108 CFU/mL were added to each well. The broth media with various concentrations of herbal extracts was used as a negative control, while the broth media cultured with each bacterium with a concentration of 1.5 × 108 CFU/mL was used as a positive control [7]. The microplate was incubated at 37°C for 24 hours then the optical density value of the culture media was read on a microplate reader with a wavelength of 570 nm. Determination of MIC is the result of four times dilution from the initial concentration of the selected combination extract which is calculated for the optical density value. The optical density value which indicated the number 0.00 nm for E. coli was then cultured on MHA (MerckTM) media to determine MBC. MBC was determined using the lawning technique (spread method). The selected combination extract solution based on the results of the MIC test was taken from the microdilution plate well as much as 100 μl then spread on 15 ml of prepared MHA (MerckTM) media and then incubated at 37°C for 24 hours. Agar medium that is not covered with bacteria is designated as MBC.
Probiotics antagonist test against pathogens is also done by disc diffusion method. Probiotic candidate isolates that had previously been cultured in broth media (MerckTM) were then resuspended to a concentration of 1.5 × 108 CFU/ml, then cultured on MHA media (MerckTM) using the pour agar method [8]. 20 μl suspension of B. subtilis, L. acidophilus and their combination with a concentration of 1 × 106 CFU/ml was dropped onto a blank disc (OxoidTM). Positive control used is Chloramphenicol disc (C 30 μg, OxoidTM) while the disc is dripped PBS as a solvent used as a negative control suspension. The media was incubated at 37°C for 24 hours and the diameter of the inhibition zone (mm) was measured. All test methods were repeated three times to minimize biased results.
Results
Curcuma aqueduct extract with various concentrations combined with Lombok honey. This combination is expected to produce a combination of extract formulations that work synergistically in order to produce the best effect on bacterial growth. The combination of curcuma extracts is divided into 5 groups, combination of extracts 1: 25% aquadest extract +Lombok honey; extract combination 2: 12.5% aquadest extract+Lombok honey; combination of extracts 3: 6.25% aquadest extract+Lombok honey; extract combination 4: 3.13% aquadest extract+Lombok honey; extract combination 5: 1.56% distilled water extract+Lombok honey. The test results on the growth of the three bacteria are presented in (Tables 1-3).
| Type of bacteria | Curcuma aquadest extract concentration | Inhibition zone diameter (mm) | ||
|---|---|---|---|---|
| Lombok Honey (100%) | Control (+) Ch. Antibiotic | Control (-) Aquadest | ||
| E. coli | 0.25 | 8.53 ± 0.03 | 13 ± 0.03 | <6 |
| 12.5 % | 7.34 ± 0.02 | |||
| 6.25% | 6.65 ± 0.02 | |||
| 3.13% | 6.95 ± 0.04 | |||
| 1.56% | 6.96 ± 0.03 | |||
| B. subtilis | 0.25 | <6 | 14.58 ± 0.03 | <6 |
| 12.5 % | <6 | |||
| 6.25% | 6.94 ± 0.02 | |||
| 3.13% | 7.03 ± 0.02 | |||
| 1.56% | 7.06 ± 0.02 | |||
| L. acidophilus | 0.25 | <6 | 14.91 ± 0.04 | <6 |
| 12.5 % | <6 | |||
| 6.25% | <6 | |||
| 3.13% | <6 | |||
| 1.56% | <6 | |||
Table 1: The results of the antibacterial activity of the combination of aquadest curcuma extract and honey against E. coli.
| ANOVA | |||||
|---|---|---|---|---|---|
| Escherchia coli | |||||
| Sum of squares | df | Mean square | F | Sig. | |
| Between groups | 11.009 | 21 | 0.524 | 550.066 | 0 |
| Within groups | 0.042 | 44 | 0.001 | - | - |
| Total | 11.051 | 65 | - | - | - |
Table 2: Statistical analysis of Anova One-Way disc diffusion test results of a combination of curcuma aquadest extract and honey on the growth of E. coli.
| Multiple Comparisons | ||||||
|---|---|---|---|---|---|---|
| Escherchia coli Tukey HSD | ||||||
| (I) Herbs extract | (J) Herbs extract | Mean difference (I-J) | Std. Error | Sig. | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Combination extract M2+TW2 | Combination extract M2+TW3 | 1.19 | 0.02521 | 0 | 1.0936 | 1.2864 |
| Combination extract M2+TW4 | 1.88 | 0.02521 | 0 | 1.7836 | 1.9764 | |
| Combination extract M2+TW5 | 1.58333 | 0.02521 | 0 | 1.487 | 1.6797 | |
| Combination extract M2+TW6 | 1.57667 | 0.02521 | 0 | 1.4803 | 1.673 | |
Table 3: Statistical analysis of Tukey's Post-Hoc test results of the combination of curcuma aquadest extract and honey on the growth of E. coli.
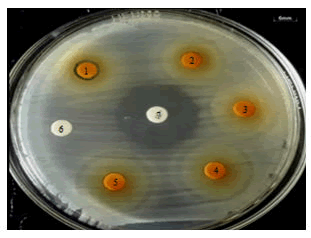
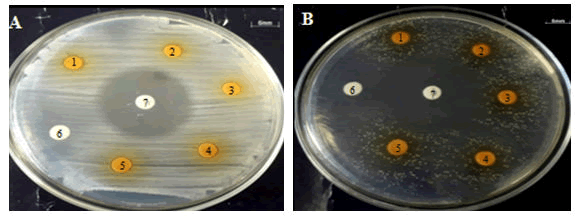
The results of the disk diffusion test showed that the diameter of the inhibition zone between the combination treatments of the concentration of aquadest extracts of curcuma and honey was significantly different (P<0.5). The combination of 25% curcuma aquadest extract+100% Lombok honey showed the largest inhibitory zone against E. coli and the growth zone of candidate probiotics B. subtilis and L. acidophilus, on the other hand, the positive control indicated the presence of an inhibitory zone against the probiotic candidate (Figures 1 and 2).
Figure 1: The results of the disc diffusion test of a combination of aquadest Curcuma extract+Lombok honey with a concentration of extract combination 1 (1), extract combination 2 (2), extract combination 3 (3), extract combination 4 (4), extract combination 5 (5), negative control (6), positive control (7) against E. coli.
Figure 2: The results of the disc diffusion test of a combination of Curcuma aquades extracts of+Lombok honey with a concentration of a combination of extracts 1 (1), a combination of extracts 2 (2), a combination of extracts 3 (3), a combination of extracts 4 (4), a combination of extracts 5 (5), negative control (6), positive control (7) against B. subtilis (A) and L. acidophilus (B).
The combination that showed the largest diameter for the growth of E. coli and supported the growth of B. subtilis and L. acidophilus was chosen as the extract combination for which the optical density value was calculated to determine the Minimum Inhibitory Concentration (MIC) and minimum killing concentration (MBC) for the growth of E. coli and to see the maximum ability of the combined extract to support the growth of B. subtilis and L. acidophilus (Tables 4-7).
| Combination of extracts (concentration before dilution) | Optical density value against bacteria (nm) | ||
|---|---|---|---|
| E. coli | B. subtilis | L. acidophilus | |
Lombok Honey+Curcuma aquadest extract 25% |
0.00 ± 0.002 |
0.18 ± 0.002 |
0.25 ± 0.005 |
Lombok Honey+Curcuma aquadest extract 12,5% |
0.01 ± 0.005 |
0.16 ± 0.004 |
0.25 ± 0.006 |
Positif control |
0.15 ± 0.005 |
0.11 ± 0.004 |
0.14 ± 0.004 |
Table 4: Optical density values on media enriched with curcuma aquadest extract and honey against bacteria.
| Kruskal-Wallis test | |
|---|---|
| Test statisticsa,b | |
| Escherchia coli | |
| Chi-Square | 46.122 |
| Df | 15 |
| Asymp. Sig. | 0 |
| Mann-Whitney test | |
| Test statisticsb | |
| Mann-Whitney U | 0 |
| Wilcoxon W | 6 |
| Z | -1.993 |
| Asymp. Sig. (2-tailed) | 0.046 |
| Exact Sig. (2*(1-tailed Sig.)) | 0.1001 |
a. Kruskal Wallis Test, b. Grouping Variable: Ekstrak herbal, 1. Not corrected for ties.
Table 5: Statistical analysis of Kruskal-Wallis and Mann-Whitney calculated the optical density values of herbs, honey, and probiotics on the growth of E. coli.
| Kruskal-Wallis test | |
|---|---|
| Test statisticsa,b | |
| Bacillus subtilis | |
| Chi-Square | 41.961 |
| Df | 15 |
| Asymp. Sig. | 0 |
| Mann-Whitney test | |
| Test statisticsb | |
| Mann-Whitney U | 0 |
| Wilcoxon W | 6 |
| Z | -2.087 |
| Asymp. Sig. (2-tailed) | 0.037 |
| Exact Sig. (2*(1-tailed Sig.)) | 0.1001 |
a. Kruskal Wallis Test, b. Grouping Variable: Ekstrak herbal, 1. Not corrected for ties.
Table 6: Statistical analysis of Kruskal-Wallis and Mann-Whitney calculated the optical density values of herbs, honey, and probiotics on the growth of B. subtilis.
| Kruskal-Wallis test | |
|---|---|
| Test statisticsa,b | |
| Lactobacillus acidophilus | |
| Chi-Square | 45.719 |
| Df | 15 |
| Asymp. Sig. | 0 |
| Mann-Whitney test | |
| Test statisticsb | |
| Mann-Whitney U | 0 |
| Wilcoxon W | 6 |
| Z | -1.964 |
| Asymp. Sig. (2-tailed) | 0.050 |
| Exact Sig. (2*(1-tailed Sig.)) | 0.1001 |
a. Kruskal Wallis Test, b. Grouping Variable: Ekstrak herbal, 1. Not corrected for ties.
Table 7: Statistical analysis of Kruskal-Wallis and Mann-Whitney calculated the optical density values of herbs, honey, and probiotics on the growth L. acidophilus.
In general, the optical density value of the combination of aquadest curcuma extract with honey which was analyzed by Kruskal-Wallis and Mann-Whitney statistics showed a significant difference (P<0.05) with the positive control. The calculation results explain that the combination of 12.5% curcuma aquadest extract (diluted four times to 3.13%) with 100% Lombok honey (diluted to 25%) indicates an increase in optical density value (0.01 ± 0.005) which can be interpreted that this combination of extracts is able to inhibit the growth of E. coli but has not been able to kill the bacteria. Different results were shown by the combination of 25% curcuma aquadest extract (diluted four times to 6.25%) with 100% Lombok honey (diluted to 25%) i.e. there was no additional optical density value (0.00 ± 0.002) which could be interpreted as the bactericidal effect works optimally on the growth of E. coli so that these bacteria cannot grow and develop properly. Based on the optical density values obtained, the MIC of the combination of curcuma aquadest extracts and Lombok honey is a combination of aquadest extract 3.13%+Lombok honey 25% and MBC a combination of curcuma aquadest extracts and Lombok honey is a combination of aquadest extracts of 6.25%+Lombok honey 25%. Follow-up tests were carried out to ensure and determine the Minimum Billing Concentration (MBC) using the dispersion method on a combined extract solution from the dilution method with an optical density value of 0.00. The test results proved that the combination of 25% curcuma aqueduct extract (diluted four times to 6,25%) with 100% Lombok honey (diluted to 25%) was able to kill E. coli as evidenced by the absence of bacterial colony growth on agar media (MHA). The results of the MIC and MBC test results for the combination of extracts are presented in (Table 8).
| Bacteria | Combination of curcuma aquadest extract+honey (%) | |
|---|---|---|
| MIC | MBC | |
| E. coli | Curcuma aquadest extract 3.13+Lombok honey 25 | Curcuma aquadest extract 6.25+Lombok honey 25 |
Table 8: MIC and MBC extract combination against E. coli.
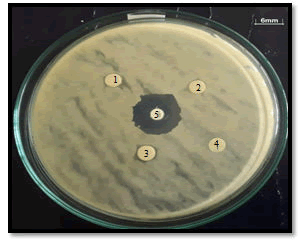
The addition of optical density values was also shown by the probiotic candidate’s B. subtilis and L. acidophilus. The combination of 12.5% curcuma aquadest extract (diluted four times to 3.13%) with 100% Lombok honey (four times dilution to 25%) on the growth of B. subtilis showed a lower optical density value (0.16 ± 0.004 ) compared the combination of 25% aquadest curcuma extract (four times dilution to 6.25%) with 100% Lombok honey (four times diluted to 25%) (0.18 ± 0.002), while the optical density value of both herbal combinations on the growth of L acidophilus showed almost the same value (0.25 ± 0.005; 0.25 ± 0.006). These results illustrate the ability of the combination of curcuma extract and Lombok honey to support the growth of prospective probiotics. The synergistic effect between the two natural ingredients causes the probiotic candidates B. subtilis and L. acidophilus to grow maximally as evidenced by the higher optical density value than the positive control (media broth). The inhibitory activity of probiotic candidates was also carried out using the Kirby-Bauer diffusion method to determine the ability of B. subtilis and L. acidophilus to inhibit the growth of E. coli. The resulting inhibition zone diameter data can be seen in (Table 9-11 and Figure 3).
| Probiotic candidate | Diameter of inhibition zone of probiotic candidate against E. coli(mm) |
|---|---|
| B. subtilis | 7.18 ± 0.02 |
| L. acidophilus | 6.95 ± 0.03 |
| Kombinasi | 7.30 ± 0.02 |
Table 9: The results of the disk diffusion test of B. subtilis, L. acidophilus and their combination on growth E. coli.
| ANOVA | |||||
|---|---|---|---|---|---|
| Escherchia coli | |||||
| Sum of squares | df | Mean square | F | Sig. | |
| Between groups | 11.009 | 21 | 0.524 | 550.066 | 0 |
| Within groups | 0.042 | 44 | 0.001 | - | - |
| Total | 11.051 | 65 | - | - | - |
Table 10: Statistical analysis of Anova One-Way disc diffusion test results of a combination of curcuma aquadest extract and honey on the growth of B. subtilis and L. acidophilus.
| Multiple Comparisons | ||||||
|---|---|---|---|---|---|---|
| Escherchia coli Tukey HSD | ||||||
| (I) Herbs extract | (J) Herbs extract | Mean difference (I-J) | Std. Error | Sig. | 95% confidence interval | |
| Lower bound | Upper bound | |||||
| Kombinasi probiotik | Probiotik B. subtilis | 0.12333 | 0.02521 | 0.002 | 0.0270 | 0.2197 |
| Probiotik L. acidophilus | 0.35333 | 0.02521 | 0 | 0.2570 | 0.4497 | |
Table 11: Statistical analysis of Tukey's Post-Hoc test results of the combination of curcuma aquadest extract and honey on the growth of B. subtilis and L. acidophilus.
Probiotic candidate B. subtilis, L. acidophilus and their combination showed the ability to inhibit the growth of E. coli. This was evidenced by the difference in the diameter of the inhibition zone of more than 6 mm around the disc containing B. subtilis, L. acidophilus, a combination of B. subtilis+L. acidophilus, and a positive control (Chloramphenicol), while the negative control showed no inhibition zone around the disc. The results of the calculation of the diameter of the inhibition zone which were analyzed with the One way Anova statistic showed a significant difference in each bacterium and also the combination of the two bacteria (P<0.05). B. subtilis showed a larger diameter of the inhibitory zone (7.18 ± 0.02) than L. acidophillus (6.95 ± 0.03), but the combination of the two probiotic candidates had the best inhibition zone diameter of (7.30 ± 0.02) and significantly different (P<0.05) compared to individual probiotic candidates.
Further testing to determine MIC and MBC against E. coli in this method was not carried out, because the calculation of the optical density value by the spectrophotometer was generated based on the level of turbidity of the test solution on the micro plate. The level of turbidity cannot be used to test the antagonistic ability of bacteria, because it can produce optical density values that are biased so that the determination of the antibacterial activity of probiotic candidates against E. coli is determined based on the diameter of the inhibition zone generated in the disk diffusion test.
Discussion
Differences in antibacterial activity of each test material can be influenced by 4 factors such as extract concentration, content of metabolites, extract diffusion power and the type of bacteria inhibited. Stated that one of the factors that influence the activity of antimicrobial substances is the concentration of antimicrobial substances [9-12]. The inhibitory power produced by antimicrobial materials will be higher if the concentration is also high. Another factor that can affect the results of the inhibition zone diameter using the Kirby-Bauer method is the ability of the extract to diffuse into the paper disc. The extract used in this test is included in the thick extract. The higher the concentration of the extract used, the higher the viscosity. The higher the viscosity of an extract, the lower the diffusion process of an antibacterial substance into the media so that it will affect the diameter of the inhibition zone.
Curcuma rhizome has a distinctive antimicrobial main compound, namely Xanthorrizol (XNT) from the terpenoid group which is larger (≥ 6%) than turmeric (≥ 3%). Curcuma generally contains antibacterial compounds which are included in the essential oil group. The antimicrobial activity of each type of essential oil can be influenced by the type and amount of active components it contains, the variety or cultivar, climatic factors and soil where it grows/area of origin, fresh or dried rhizome shape, as well as the extraction method and the type of solvent used [13-17]. Likewise, the production of other active ingredients such as terpenoid compounds in essential oils is also strongly influenced by the geographical conditions of the plant habitat as well as other specific influencing factors that are not yet known.
According to, extraction of distilled water, methanol, ethyl acetate, and n-hexane will produce a solution containing terpenoid compounds, phenols and alkaloids because the level of polarity of the solvent used is the same, from polar to semi-polar or non-polar solvents. The derivatives of phenolic compounds will interact with bacterial cells through an adsorption process that involves hydrogen bonds and can change the permeability of cell membranes [18]. Penetration of high levels of phenol into cells can cause protein coagulation and lysis of cell membranes, while low concentrations of phenolic compounds can form weak bonds and break down easily so that if phenol penetrates into cells it can cause protein coagulation and lysis of cell membranes occurs.
Another mechanism for the antibacterial role of essential oil elements is terpenoids which are thought to involve the breakdown of membranes by lipophilic components and curcumin which has a phototoxic effect on bacteria when exposed to light by producing hydrogen peroxide which can cause cytoplasmic membrane damage [19-22]. Terpenoids, phenols, and hydrogen peroxide are thought to act on bacteria by damaging the cytoplasmic membrane, this causes important inorganic ions, nucleotides, coenzymes, and amino acids to seep out of the cell, and prevent the entry of materials into the cell food or nutrients needed by bacteria to produce energy. The cytoplasmic membrane is in charge of carrying out energy metabolism in prokaryotic cells so that, if the cytoplasmic membrane is damaged, energy metabolism will not take place. This is what causes the inability of cells to grow and death cell causes.
Several studies have shown that in general, essential oils are more active against Gram-positive bacteria than Gram-negative bacteria, stated that the reaction caused by herbal phenolic compounds will affect the bacterial cell wall [23-25]. The simple arrangement of the cell wall in Gram-positive bacteria and the absence of an outer membrane cause antibacterial compounds to penetrate the cell wall and disrupt the cell wall biosynthesis process. Previous research related to the use of temulawak as a phytobiotic material has been widely carried out, but its ability to control the growth of E. coli (APEC) is still rarely in vitro studied.
The polyphenol content in herbal extracts is a compound that has high antioxidant activity to overcome free radicals and plays a role in overcoming oxidative stress generated by metabolic activity by providing a microaerophilic environment for probiotics. The content of essential oils in herbs is also able to stimulate and increase the growth of beneficial bacteria (eg, Lactobacilli and Bifidobacteria) in the intestines. Another opinion, states that the polysaccharide component is considered the most important active immune component.
Previous research on the ability of a combination extract of temulawak extract and red ginger extract to support the growth of L. acidophilus was proved that the combination of red ginger ethanol extract and temulawak aquadest extract was able to support the growth of this probiotic candidate and increase the ability of better adhesion of pathogenic bacteria to chicken intestinal epithelial cells [26]. Another explanation regarding the mechanism of compounds in herbs that supports the results of this study was conveyed, that differences in cell wall thickness of non-pathogenic and pathogenic bacteria affect the reactions caused by phenolic compounds. The cell walls of non-pathogenic bacteria will be dehydrated so that the pores will shrink, causing the cell wall permeability and membrane function to decrease so as to minimize damage to the probiotic cell wall.
The antibacterial activity of honey can be influenced by several factors such as high sugar content, low humidity, low pH, and hydrogen peroxide. Another opinion states that antibacterial activity in honey is influenced by osmolarity, pH, activity of peroxide and non-peroxide compounds. The mechanism of antibacterial activity related to the osmolarity of honey is due to the high osmotic power of honey, because 84% of the components of honey content are glucose and fructose while water is only around 15-21%. Osmolarity causes strong interactions between sugar molecules and water molecules and leaves fewer water molecules for bacteria, making bacterial growth difficult. Another factor in the form of hydrogen peroxide contained in honey produced by the glucose oxidase process is an important component that is able to inhibit bacterial growth. Honey also contains flavonoid compounds that can damage bacterial cell walls that react with alcohol groups on flavonoid compounds so that flavonoids can enter the cell nucleus and react with DNA and cause bacterial lysis and then die. These results are also in accordance with the research of which states that bitter honey has antibacterial activity against both Gram-negative and Gram-positive bacteria.
Honey contains about 80% carbohydrates consisting of monosaccharides, polysaccharides, and oligosaccharides. The content of oligosaccharides has been widely used in various food products with the aim of being a source of prebiotics which are undigested food components and provide benefits through microbial modulation that is useful for colon health (probiotics). The prebiotic activity of local honey oligosaccharide isolates from Sumbawa had higher prebiotic activity than inulin, which is a commercial prebiotic. The synergistic effect of Manuka honey (UMF 20+) which can increase the growth of probiotics and inhibit pathogens. Normal microflora such as Lactobacillus and bifidobacteria will ferment oligosaccharides in indigestible honey, for the benefit of bacterial metabolism so that it can provide benefits for the host body. The prebiotic content in honey can also maintain the growth and stability of the species.
The results of this study are in line with the results of who proved that the administration of a combination herbal formulation in broiler chickens has the potential to maintain a normal microflora balance in the digestive tract. The administration of a combination of galangal, ginger, temulawak, and honey in various concentrations was proven to increase productivity and carcass weight of broiler chickens. The best increase in broiler productivity was shown after administration of a combination of herbs for 17 days with a concentration of 2.5%. Another opinion stated that probiotics can also reduce the activity of acetyl coenzyme A carboxylase, the enzyme responsible for the rate of fatty acid synthesis, by producing statins as inhibitors of fat formation in the liver. The use of probiotics supplemented with prebiotics can increase energy and protein efficiency and can reduce blood cholesterol content than the partial use of probiotics and prebiotics. The addition of probiotics and prebiotics has no negative effect on broiler chickens so that they have the same growth as chickens given antibiotics, and can even increase antioxidant activity so that fat oxidation can be inhibited.
The test of antagonistic properties between probiotics and pathogens using the Kirby-bauer diffusion method was carried out to determine the ability of B. subtilis and L. acidophilus to inhibit the growth of E. coli. The antibacterial activity of probiotics is influenced by several important factors [27]. The main metabolites of acidic bacteria are short-chain fatty acids and lactic acid which can inhibit the growth of pathogenic bacteria in the intestines such as E. coli. Lactobacillus aciodophilus produces two bacteriocin components, namely bacteriocin lactacin B, and acidolin which is an extracellular component in the form of peptides or compounds in the form of antimicrobial proteins that can provide an antagonistic response by inhibiting the development of pathogenic organisms. The same thing was also shown by the inhibitory mechanism of B. subtilis which produces antibiotics that are toxic to other microbes such as iturin A which is a lipoprotein, subtilin which is a peptide compound, and bacitracin. Bacitracin is a polypeptide that works to inhibit the formation of cell walls.
Probiotics can be a potential alternative to antibiotics to inhibit growth and reduce colonization of enteric pathogens in the intestines of poultry. Research conducted stated that in vitroB. subtilis has better antagonistic activity against pathogens E. coli O157:H7 and S. thyphymurium than Lactobacilli. The use of probiotics such as B. subtilis, B. thuringiensis, and L. acidophilus through drinking water was reported to be able to replace the role of antibiotics, maintain the health of the digestive tract of livestock and reduce the number of E. coli. Another opinion, explains that a concentration of 107-108 CFU/g of Lactobacillus is effective in suppressing the growth of pathogenic bacteria significantly due to a decrease in acidity or pH from lactic acid production.
Conclusion
The best extract combination formulation was determined based on the diffusion method, calculation of optical density value, determination of MIC and determination of MBC. Curcuma aquadest extract 6.25%+Lombok honey 25% (concentration after four dilutions in the dilution method) was determined as the best combination capable of killing E. coli and supporting the growth of B. subtilis and L. acidophilus maximally in vitro. Curcuma aquadest extract with a concentration of 0.39% (3.9 mg/ml) was sufficient to inhibit the growth of E. coli while Lombok honey required 25% (250 mg/ml) to inhibit the growth of E. coli. The formulation with this concentration was also able to support the growth of B. subtilis and L. acidophilus. The growth of the combination of B. subtilis and L. acidophilus probiotics was also antagonistic to the growth of E. coli. Based on the above, it can be concluded that the combination formulation of curcuma aquades extracts and honey with these concentrations can be used as a substitute for AGP.
References
- Ajizah A, Thihana T, Mirhanuddin M (2018) Potensi ekstrak kayu ulin (Eusideroxylon zwageri T et B) dalam menghambat pertumbuhan bakteri Staphylococcus aureus secara in vitro. Bioscientiae 4. [Crossref][Google Scholar][Indexing at]
- Al Amrie AG, Ivan I, Anam S, Pitopang R (2014) Uji efektifitas ekstrak daun dan akar harrisonia perforata merr. Terhadap pertumbuhan bakteri Vibrio cholerae. Nat Sci J Sci Technol 3. [Crossref][Google Scholar][Indexing at]
- Ashayerizadeh, A, Dabiri N, Mirzadeh K, Ghorbani M (2011) Effect of dietary supplementation of probiotic and prebiotic on growth indices and serum biochemical parameters of broiler chickens. J Cell Anim Biol 5: 152-156. [Crossref][Google Scholar][Indexing at]
- Astrini D, Wibowo MS, Nugrahani I (2014) Aktivitas antibakteri madu pahit terhadap bakteri gram negatif dan gram positif serta potensinya dibandingkan terhadap antibiotik kloramfenikol, oksitetrasiklin, dan gentamisin. Acta Pharmaceutica Indonesia 39: 75-83.
- Burt S (2004) Essential oils: Their antibacterial properties and potential applications in foods-A review. Int J Food Microbiol 94: 223-253. [Crossref][Google Scholar][Pubmed]
- Cavallini DC, Bedani R, Bomdespacho LQ, Vendramini RC, Rossi EA (2009) Effects of probiotic bacteria, isoflavones and simvastatin on lipid profile and atherosclerosis in cholesterol-fed rabbits: A randomized double-blind study. Lipids Health Dis 8: 1-8. [Crossref][Google Scholar][Pubmed]
- Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564-582. [Crossref][Google Scholar][Indexing at]
- Dahl TA, Midden W, Hartman PE (1989) Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol 171: 2188-2194. [Crossref][Google Scholar] [Indexing at]
- Deniz G, Orman A, Cetinkaya F, Gencoglu H, Meral Y, et al. (2011) Effects of probiotic (Bacillus subtilis DSM 17299) supplementation on the caecal microflora and performance in broiler chickens. Revue Med Vet 162: 538-545. [Crossref][Google Scholar][Indexing at]
- El-Naggar, M. Y. (2004). Comparative study of probiotic cultures to control the growth of Escherichia coli O157: H7 and Salmonella typhimurium. Biotechnol 3: 173-180.
- Fuller R (1989) A review: Probiotics in man and animals. J Appl Bacteriol 66: 365-378.
- Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, et al. (2010) Dietary prebiotics: Current status and new definition. Food Sci Technol Bull Funct Foods 7: 1-19.
- Hernani Hayani E (2001) Identification of chemical components on red ginger (Zingiber officinale var. Rubrum) by GC-MS. 501–505.
- Hidayathulla S, Chandra KK, Chandrashekar K (2011) Phytochemical evaluation and antibacterial activity of Pterospermum diversifolium Blume. Int J Pharm Pharmaceut Sci 3: 201-207.
- Jagadesswari S, Vidya P (2010) Isolation and characterization of bacteriocin producing Lactobacillus Sp. from traditional fermented food. Electronic J Environ Agr Food Chemi 9: 575-581.
- Kwakman PH, Zaat SA (2012) Antibacterial components of honey. IUBMB Life 64: 48-55. [Crossref][Google Scholar][Pubmed]
- Luiz G, Aristides A, Oba A, Shimokomaki M (2012) The effects of biotic additives on growth performance and meat qualities of broiler. Int J Poult Sci 11: 599-604.
- Madan J, Singh R (2010) Formulation and evaluation of Aloevera topical Gels. Int J Ph Sci 2: 551-555.
- Manin F (2012) Potensi Lactobacillus acidophilus dan Lactobacillus fermentum dari saluran pencernaan ayam buras asal lahan gambut sebagai sumber probiotik. Jurnal Ilmiah Ilmu-Ilmu Peternakan 221-228. [Crossref][Google Scholar][Indexing at]
- Molan PC (2006) Using honey in wound care. Int J Clin Aromather France 3: 21-24. [Crossref][Google Scholar][Indexing at]
- Mountzouris K, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, et al. (2010) Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci 89: 58-67. [Crossref][Google Scholar][Pubmed]
- Parwata I, Dewi P (2008) Isolasi dan uji aktivitas antibakteri minyak atsiri dari rimpang lengkuas (Alpinia galanga L.). J Kimia 2: 100-104.
- Prakasita V, Asmara W, Widyarini S, Wahyuni A (2019) Combinations of herbs and probiotics as an alternative growth promoter: An in vitro study. Vet World 12: 614-620. [Crossref][Google Scholar][Pubmed]
- Qian Z, Wang S, Guang Y, Wen Z, LI H (2016) Development and evaluation of a herbal formulation with anti-pathogenic activities and probiotics stimulatory effects. J Integrate Agri 15: 1103-1111. [Crossref][Google Scholar][Pubmed]
- Rosendale DI, Maddox IS, Miles MC, Rodier M, Skinner M, et al. (2008) High‐throughput microbial bioassays to screen potential New Zealand functional food ingredients intended to manage the growth of probiotic and pathogenic gut bacteria. Int J Food Sci Technol 43: 2257-2267. [Crossref][Google Scholar][Indexing at]
- Rustama MM, Rahayuningsih SR, Kusmoro J, Safitri R (2005) Uji aktivitas antibakteri dari ekstrak air dan etanol bawang putih (Allium sativum L.) terhadap bakteri Gram negatif dan Gram positif. BIOTIKA Jurnal Ilmiah Biologi 4. [Crossref][Google Scholar][Indexing at]
- Zhang L, Cao G, Zeng X, Zhou L, Ferket P, et al. (2014) Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci 93: 46-53. [Crossref][Google Scholar][Pubmed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi