Research Article, Jva Vol: 9 Issue: 1
In vivo activity of pyrimidine-dispirotripiperaziniumin in the male guinea pig model of genital herpes
Novoselova EA1*, Alimbarova LM3, Monakhova NS1, Lepioshkin AY1, Ekins S2 and Makarov VA1
1A.N. Bach Institute of Biochemistry, Federal Research Centre, Fundamentals of Biotechnology, Russian Academy of Sciences, Leninskii Prosp. 33-2b, Moscow, 119071, Russia
2Collaborations Pharmaceuticals Inc, 840 Main Campus Drive, Lab 3510, Raleigh, NC 27606, USA
3Federal State Budgetary Establishment, Scientific Research Center of Epidemiology and Microbiology named after N.F. Gamalei, Ministry of Health of Russia, Gamaliel St, 18, Moscow, 123098, Russia
*Corresponding Author: Elena A. Novoselova
A.N. Bach Institute of Biochemistry, Federal Research Centre, Russian Academy of Sciences, Leninskii Prosp. 33-2b, Moscow, 119071, Russia
Tel: +7-916-6319870
E-mail: helen.novoselova@gmail.com
Received: December 30, 2019 Accepted: March 9, 2020 Published: March 16, 2020
Citation: Novoselova EA, Alimbarova LM, Monakhova NS, Lepioshkin AY, Ekins S, et al. (2020) In vivo Activity of Pyrimidine-Dispirotripiperazinium in the Male Guinea Pig Model of Genital Herpes. J Virol Antivir Res 9:1. doi:10.37532/jva.2020.9(1).193
Abstract
Herpes Simplex Virus (HSV-2) is a risk factor in the transmission of human immunodeficiency virus. While treatments exist for HSV they are either not completely effective or have a single target. We have described a novel pyrimidyl-di(diazaspiroalkane) derivative called 3,3’-(2-methyl-5-nitropyrimidine-4,6-diyl)-3,12-bis-6,9-diazadiazoniadispiro [5.2.5.2]hexadecane tetrachloride dihydrochloride (PDSTP) that targets heparan sulfate on host cells and has broad spectrum antiviral activity and lacks cytotoxicity. In previous studies we have confirmed the antiviral activity in vitro and now present the results of in vivo testing. We demonstrated that 10% ointment or 10% gel containing PDSTP administered topically or injected subcutaneously (twice daily for 5 days) has a therapeutic effect in the guinea pig model of genital herpes induced by HSV-2. PDSTP reduces symptom intensity, time of lesion resolution, mean disease duration and infectivity of HSV-2. The strongest effect was observed for the PDSTP solution administered parenterally, while the minimum effect was shown in the experiments when 10% gel was applied onto the lesion foci. Further experiments were performed which showed all the dosage forms had efficacy which was dependent on treatment initiation time. PDSTP performed comparably well against clinical symptoms versus acyclovir. Both PDSTP and acyclovir have different mechanisms so future work to study the effect of combination treatment is warranted.
Keywords: Antiviral activity; Dispiro compounds; Genital herpes; Heparan sulfate; Herpes virus infection; HSV
Abbreviations
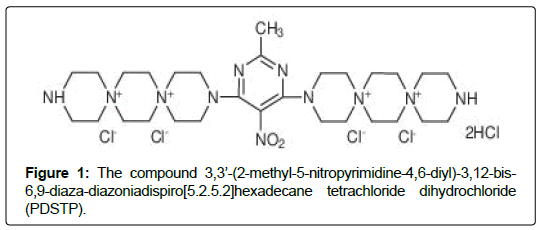
API: Active Pharmaceutical Ingredient; PDSTP: 3,3’-(2-methyl-5- nitropyrimidine-4,6-diyl)-3,12-bis-6,9-diaza-diazoniadispiro[5.2.5.2] hexadecane tetrachloride dihydrochloride
Introduction
During the 1980’s, genital herpes was an infection of epidemic proportions in many countries. Although there is evidence of a decline in the age-specific prevalence of HSV type 1 (HSV-1) infection, the seroprevalence of HSV type 2 has continued to increase [1]. HSV- 2 infections represent a risk factor for the transmission of Human Immunodeficiency Virus (HIV) infection [2,3] and an important clinical target.
Nucleoside analog therapy with drugs such as Acyclovir (ACV), its prodrug valacyclovir, famciclovir (a prodrug of penciclovir), and the second line drugs for resistant virus, e.g. foscarnet and cidofovir are used to treat acute infections, reduce viral shedding and disease associated with HSV reactivation. Despite the fact that HSV carries more than 80 genes that are required for its functionality, all the antiviral drugs against it are inhibitors of the viral DNA polymerase. Consequently, cross-resistance can occur [4]. Moreover, this therapy is completely ineffective [5,6]. Improving the efficacy of current HSV treatment therefore relies on the discovery of new antiviral compounds against different targets in the virus, preferably the earlier stages of the HSV viral life cycle such as viral attachment and entry.
A novel pyrimidyl-di(diazaspiroalkane) derivative has recently been identified to be a potent and broad-spectrum antiviral agent capable of inhibiting replication of various viral families in vitro [7-11] through specifically blocking heparan sulfate (HS) receptors on the host cell surface [12,13]. We have investigated the relationship between structure and antiviral activity for derivatives of dispirotripiperazine as well as examined cytotoxicity in vitro during a previous study [11]. As a result, the compound 3,3’-(2-methyl-5-nitropyrimidine-4,6-diyl)- 3,12-bis-6,9-diaza-diazoniadispiro[5.2.5.2]hexadecane tetrachloride dihydrochloride (PDSTP), (Figure 1) was found as the most promising due to its ratio of cytotoxicity and antiviral efficacy. The cytotoxicity (CC50) in GMK cells was >571.8 μM, the 50% inhibitory concentration (CI50) in L-929 cells with HSV-1 was >228.7 μM [11]. PDSTP is chemically stable and effectively blocked cellular heparan sulfate proteoglycans (HSPG), and thereby inhibited cell adhesion of the virus, leading to disturbance of the life cycle and reduced titer of the virus [11,13]. It is known that this mechanism is used by various viral families, such as type 1 and 2 herpesviruses (HSV-1, HSV-2) [14], papillomaviruses (HPV) [15], human cytomegalovirus (HCMV) [7], some strains of human immunodeficiency virus (HIV) [16], human respiratory syncytial virus (HRSV) [17], hepatitis B and C viruses (HBV and HCV) [18] and others.
In our previous experiments, dispiropiperazine compounds were found to be highly effective in vitro [11,19]. In the current study, we confirmed the promising antiviral activity of PDSTP in vitro. In addition, we have performed proof-of-concept experiments in guinea pigs infected with genital herpes (HSV-2) using different dosage forms (gel and ointment) of PDSTP solution compared to the standard drug acyclovir
Materials and Methods
Synthesis of PDSTP
The synthesis of PDSTP was performed as previously described by M. Schmidtke et al. [11].
Drug formulations applied in the in vivo studies
The active ingredient PDSTP in the form of 10% ointment was prepared using a 30% water–glycerol mixture with Tween 80 added and a 10% gel was prepared using 3% aqueous solution of Walocelhydroxypropyl methylcellulose for this study. Acyclovir (9-(2-hydroyethoxymethyl) guanine) available as Zovirax cream for external use (5%, GlaxoSmithKline, UK) was used as a reference drug. PDSTP for subcutaneous injection was diluted with sterile distilled water to reach the desired concentration.
Animals
Inbred male guinea pigs (weighing 250–300 g) were purchased from the Andreevka nursery of the Russian Academy of Medical Sciences (Moscow region, Russia). The animals were healthy, had a veterinary certificate of quality and health. The animals were housed in compliance with the sanitary rules for arrangement, equipment and maintenance of experimental biological clinics (vivariums). The experiments were performed in compliance with the Russiawide ethical regulatory documents, after they had been approved by the Ethics Committee of the I.I. Mechnikov Research Institute of Vaccines and Sera.
Viruses and cells
The Vero cell line (ATCC, American Type Culture Collection) was used in this study. Cells were cultured in the growth medium consisting of DMEM (Dulbecco’s modified Eagle’s medium, PanEco, Russia) supplemented with 10% inactivated fetal bovine serum (FBS, PanEco, Russia), 2 mM L-glutamine (Sigma, USA), antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin) and 2% FBS. The cells were incubated at 37°C in 5% CO2. Herpes simplex virus 2 (the State collection of viruses of N.F. Gamalei Scientific Research Center of Epidemiology and Microbiology Ministry of Health of Russia), strain VN, was used in the experiments. Viral titers in animal biomaterial (the contents of swab samples from the urogenital tract mucosa in guinea pigs) were assessed by cell culture using the conventional micro technique procedure [20] in 96-well culture plates (Costar) and expressed as logTCID50/ml. TCID50of the virus was defined as the minimal viral dilution causing 50% degeneration of the cell monolayer, while no degeneration of the cell monolayer was observed in the non-infected control (50% tissue culture infective dose, TCID50). The virus-containing suspension with infectious titer of 6.0 logTCID50/ml was used. All the viruses were kept in aliquots frozen at –70°C.
Study design
The efficacy of PDSTP using the guinea pig model of genital herpes was evaluated in two experiments.
In the first experiment, all animals were randomized random sampling into seven groups, with three guinea pigs per group (Table 1). In the second experiment, all animals were randomized into six groups, with three guinea pigs per group (Table 2).
| Group no. | Treatment | Route of administration | Time of first application with respect to infection* |
|---|---|---|---|
| 1 | 10% ointment | externally applied | 3 h post-infection |
| 2 | 10% ointment | externally applied | 48 h post-infection |
| 3 | 10% gel | externally applied | 3 h post-infection |
| 4 | 10% gel | externally applied | 48 h post-infection |
| 5 | API solution at a dose of 50 mg/kg/day | subcutaneous injection | 1 h pre-infection 3 h post-infection |
| 6 | Acyclovir | externally applied | 48 h post-infection |
| 7 | Negative control | infected, received no treatment | |
Table 1: Study design for the first experimental series [Studying the effect of API on frequency and level of HSV-2 isolation in guinea pigs depending on the dosage form of API being used].
| Group no. | Treatment | Route of administration | Time of first application with respect to infection* |
|---|---|---|---|
| 1 | 10% ointment | externally applied | 3 h post-infection |
| 2 | 10% ointment | externally applied | 48 h post-infection |
| 3 | API solution at a dose of 50 mg/kg/day | subcutaneous injection | 1 h pre-infection 3 h post-infection |
| 4 | Acyclovir | externally applied | 3 h post-infection |
| 5 | Acyclovir | externally applied | 48 h post-infection |
| 7 | Negative control | infected, received no treatment | |
Table 2: Study design for the second experimental series [Evaluation of the efficacy of different dosage forms by characterizing the infection process depending on time of therapy initiation after the infection].
In order to evaluate the efficacy of PDSTP and acyclovir using the genital herpes model, male guinea pigs were infected with viruscontaining culture fluid (HSV-2, VN) at a dose of 100 TCID50 by pipetting and subsequently rubbing it onto the pre-scarified penile skin. Scarification (area of 4–7 mm2) was performed using a lancet after the animals had been anesthetized with 1% lidocaine. The agents were administered subcutaneously and topically according to the treatment regimen: either 3 or 48 h post-infection. The agents were applied as a thin coat onto the lesion foci twice daily for 5 days (the application area was 1.3 cm2). PDSTP in the form of a solution for parenteral administration was studied using the standard treatment and prevention regimen: 1 h pre-infection and 3 h post-infection; 1 h pre-infection and 48 h post-infection. PDSTP was subcutaneously injected twice daily for 5 days.
Infection severity was evaluated daily prior to treatment and followed-up during the entire course of the disease using the following parameters: presence and degree of specific lesions (vesicles, pustules, ulcers, erosions, and crusts), edema, hyperemia and orchitis. The condition of the animals was assessed by a 4-point system as follows: 1. edema (1 - weak, 2 - moderate, 3 - severe, opening the penis is difficult, 4 - strong edema, it is impossible to open the penis); 2. hyperemia (1 - weak, 2 - average, 3 - severe, 4 - very strong); 3. rash (1 - small, vesicles, traces of erosion, 2 - erosion, crust, 3 - ulcer, crust, 4 - bloody or purulent ulcer); 4. neurological pathology (1 - weak paresis, 2 - hind limb paralysis, 3 - complete paralysis, 4 points - death). The condition of the animals reflected the amount of points scored in all four categories of evaluation. The maximum intensity of each parameter was 4 points. The total follow-up period lasted for 21 days. Efficacy of the agents was evaluated at the peak intensity of the pathological process using the conventional procedure. Reduced intensity of clinical symptoms and the therapeutic index (TI) were the criteria for evaluating the therapeutic effect of the agents. The therapeutic index was calculated using the formula:
TI=((A–B):A) x 100,
where TI is the therapeutic index (%); A is the total score in the control group; and B is the total score in the group of animals treated with an agent.
In order to confirm infection or determine the HSV-2 titers in guinea pigs, swab specimens were collected from the urogenital mucosa into sterile vials containing 1.0 ml DMEM culture medium. These procedures were performed at days 2, 4, 7, 9 and 11 postinfection in the first experiment and on days 5 and 7 postinfection in the second experiment.
Statistical analysis
The results were subjected to statistical processing by calculating the arithmetic mean (M = Σxi / n) and standard error of the mean (SE = σ / √ n, with σ = √ (Σ (x-X ̅)2/n). Evaluation of the statistical significance of differences for intergroup comparisons, a twosided Student’s t-test for independent groups, a Mann-Whitney U test was performed, the differences were considered statistically significant at p<0.05. Microsoft Excel 2011 was used for statistical calculations
Results and Discussion
Evaluation of efficacy of different dosage forms of PDSTP according to the characteristics of the infection process depending on the dosage form
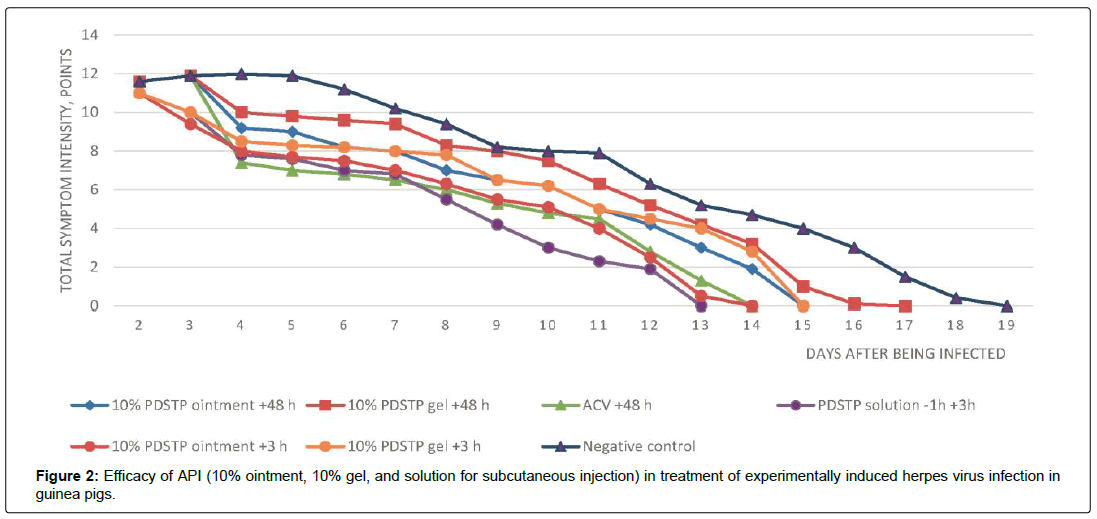
In the control group of untreated infected animals (group 7), typical local signs of genital herpes (vesicular elements and edema) developed 24–48 h after the animals had been infected. First, solitary vesicular eruption on an erythematous background was detected on the penile mucosa and the adjacent skin areas. As the disease was progressing, the number of vesicles increased, resulting in emergence of confluent lesions with the hemorrhagic contents, bleeding erosions, ulcers and moderate orchitis. The process was characterized by pronounced edema inflammatory events and slow healing or erosions and ulcers. The peak intensity of the symptoms was observed on day 4 post-infection. In the second experiment, the peak symptom intensity was observed on day 5 post-infection (Table 3). Figures 2–4 demonstrate the efficacy of PDSTP when used at early stages (3 h post-infection) and for the well-developed infection process (48 h post-infection), respectively. The effects of administration of PDSTP on the course of genital herpes in male guinea pigs differed in terms of intensity depending on dosage form.
| Group no. | Animal group (agent/time prior to (-) and after (+) the animal was infected) | Mean disease duration (MDD), days (M ± SE) | Total symptom intensity score, points | Mean symptom intensity score, points (M ± SE) | Therapeutic index, |
|---|---|---|---|---|---|
| 1 | (10% ointment /+3 h) | 13.5 ± 1.15** | 24 | 8.0 ± 1.0* | 33.3* |
| 2 | (10% ointment/+48 h) | 14.7 ± 0.57** | 27.3 | 9.1 ± 1.0* | 24.2* |
| 3 | (10% gel/+3 h) | 14.7 ± 1.15** | 25.8 | 8.6 ± 1.64* | 28.3* |
| 4 | (10% gel/+48 h) | 16.3 ± 1.15 | 30 | 10.0 ± 1.2 | 16.6 |
| 5 | (API solution/ -1 and +3 h) | 12.5 ± 2.5** | 23.1 | 7.7 ± 0.89* | 35.8* |
| 6 | (Acyclovir/+48 h) | 13.3 ± 1.52** | 22.2 | 7.4 ± 1.5* | 38.3* |
| 7 | (Negative control) | 18.3 ± 1.52 | 36 | 12.0 ± 0.97 | - |
Table 3: Efficacy of API for topical and parenteral administration for the guinea pig model of experimental genital herpes. The first experimental series.
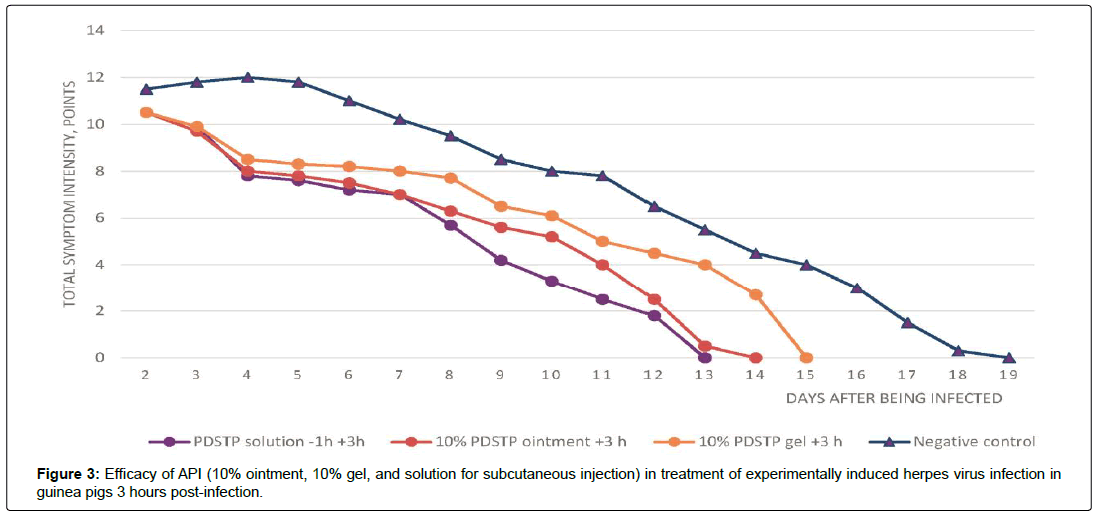
The most statistically significant treatment outcomes were obtained in the group of infected animals (group 5) subcutaneously injected with PDSTP solution according to the treatment and prevention regimen (1 h pre-infection and 3 h post-infection) and in the group treated with 10% ointment according to the treatment regimen (3 h post-infection) (group1). In accordance with these data, treatment with PDSTP as a solution for parenteral administration at a dose of 50 mg/kg/day resulted in a statistically significant therapeutic effect: symptom intensity decreased 1.6-fold and disease duration decreased considerably (by 5 days) compared to similar parameters in the group of untreated infected animals (the negative control). The infection process was characterized by moderate exudative inflammation and shorter time to resolution of lesions.
Administration of PDSTP as 10% ointment early after infection (group 1) reduced the symptom intensity 1.4-fold and decreased the mean disease duration as compared to the same parameters in the group of untreated infected animals (group 7). The animals treated with this dosage form of PDSTP within the early period recovered on average 3.6 days earlier than the untreated animals (the TI of PDSTP 10% ointment was 33.3%). Animals treated with the reference drug acyclovir, (group 6) had a mild course of infection, with no pronounced exudative inflammation, few eruptions, and no orchitis (the TI of acyclovir in was 38.3%).
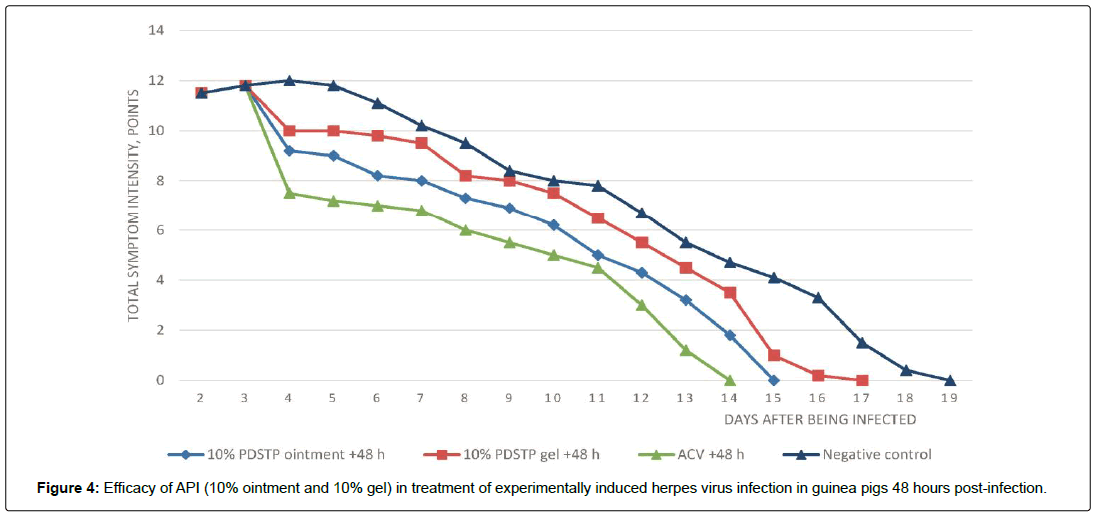
When analyzing the intensity of clinical symptoms for late therapy initiation (48 h post-infection), (Figure 4), it was found that administration of 10% ointment in infected animals with pronounced pathological symptoms (group 2) caused a statistically significant therapeutic effect of 24.2% (Figures 2 and 3). It should be mentioned that in terms of its TI, the PDSTP showed lower efficacy to that observed in the group treated with ointment within the early period after infection and in the group treated with acyclovir comparator drug (group 6) but was comparable to the reference drug group in terms of MDD.
10% gel is efficacious only if used early after infection. Thus, administration of gel 3 h after infection (group 3) resulted in a statistically significant TI (28.3%) and reduced MDD by 3.6 days compared to those in the untreated group. The efficacy of gel 3 h post infection was comparable to that of 10% ointment used 48 h post infection (group 2). It was also comparable to the efficacy of 10% ointment used 3 h post infection and the acyclovir reference drug in terms of its effect on MDD.
The experiments demonstrated that after treatment with 10% gel 48 h post-infection (group 4), intensity of local exudative inflammation did not differ significantly (p >0.05) from that in untreated animals. Administration of the gel caused no significant changes in the dynamics of development of the pathological process compared to the control group of untreated infected animals. In terms of its efficacy, gel was inferior both to the acyclovir reference drug (group 6) and 10% ointment used at early stage of infection (group 1) for all the evaluated parameters.
Studying the effect of PDSTP on frequency and level of HSV- 2 isolation in guinea pig depending on the dosage form
In order to determine the effect of PDSTP on frequency of HSV-2 virus isolation and its replication level in infected animals, we studied the swab samples collected from the lesions in animals at different periods after infection. A preliminary analysis of the swab samples collected from the urogenital area in non-infected animals prior to the experiment revealed herpes simplex virus in none of the animals. According to the virological data obtained by virus titration two days after the infection, the virus was isolated from swab specimens collected at the lesion foci in all experimentally infected animals, which proved the specificity of the observed clinical presentation of genital herpes. Virus isolation from the lesion foci was observed during 7 days after infection in the infected animals treated with PDSTP, either locally or subcutaneously (groups 1, 2, 3, and 5) and those treated with acyclovir reference drug (group 6). Meanwhile, virus isolation was observed up to day 9 (inclusive) in animals treated with 10% gel according to the treatment regimen 48 h post-infection (group 4) and in untreated animals (group 7). Examination of swab specimens collected from the lesion foci in infected animals after this period showed that virus was present in none of the animals across all groups.
It is noteworthy that the frequency of virus isolation 4 days postinfection in infected animals treated with 10% gel (groups 3 and 4) was similar to that in untreated animals (group 7) and was observed in all animals in these groups.
The frequency of virus isolation decreased by one-third in animals treated with acyclovir (group 6), 10% ointment 3 h post-infection (group 1), and PDSTP injected subcutaneously (group 5). Nine days after the infection, the virus was isolated only in group 4 (animals treated with 10% gel 48 h post-infection) and group 7 (untreated infected animals). It was found that administration of PDSTP in the form of ointment, gel, and solution (groups 1, 2, 3, and 5) 4 days postinfection reduced the replication level and infectivity of the virus isolated from swab specimens collected from the lesioned area by a factor of 30–50 compared to infectivity of the virus isolated from either untreated infected animals or those treated with 10% gel 48 h post-infection (group 4) (Table 4).
| Group no. | Animal group (agent / time prior to (-) and after (+) the animal was infected | Viral titers in animals | |||
|---|---|---|---|---|---|
| 1 | (10% ointment /+3 h) | 4.0 ± 0.5* | 2.5 ± 0.25* | 0 | 0 |
| 2 | (10% ointment/+48 h) | 4.0 ± 0.25* | 2.75 ± 0.25* | 0 | 0 |
| 3 | (10% gel/+3 h) | 4.25 ± 0.25* | 2.5 ± 0.15* | 0 | 0 |
| 4 | (10% gel/+48 h) | 5.25 ± 0.2 | 3.5 ± 0.3 | 2.0 ± 0.1 | 0 |
| 5 | (API solution/ -1 and +3 h) | 4.0 ± 0.1* | 2.25 ± 0.25* | 0 | 0 |
| 6 | (Acyclovir/+48 h) | 3.75 ± 0.1* | 2.0 ± 0.2* | 0 | 0 |
| 7 | (Negative control) | 5.75 ± 0.25 | 3.75 ± 0.3 | 2.25 ± 0.25 | 0 |
Table 4: Effect of API administered topically and subcutaneously on the titer of HSV-2 isolated from lesion foci in male guinea pigs depending on the dosage form
Experiments involving isolation and titration of the virus from swab specimens collected from animals infected with genital herpes in Vero cell culture demonstrated that the PDSTP administered topically and subcutaneously exhibited virus-specific activity. The dosage forms of PDSTP under study can be arranged in the following order as their efficacy decreases: group 5 (PDSTP solution -1 and +3 h) > group 1 (10% ointment +3 h post-infection) > group 3 (10% gel +3 h post-infection) > group 2 (10% ointment +48 h post-infection) > group 4 (10% gel +48 h post-infection).
Evaluation of the efficacy of different dosage forms by characterizing the infection process depending on time of therapy initiation after the infection
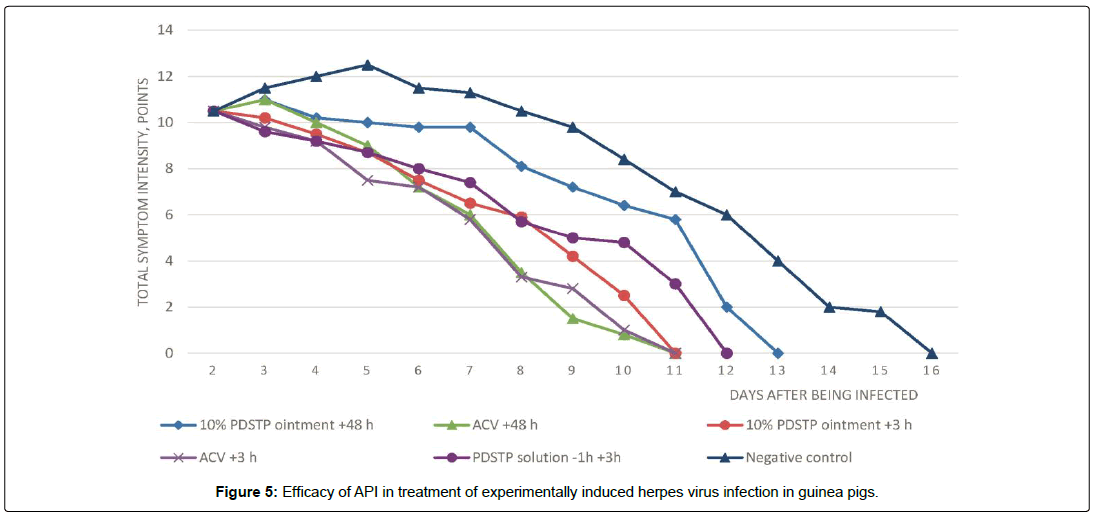
Table 5 summarizes the results of treating experimentally induced genital herpes in guinea pigs using PDSTP topically and subcutaneously. The efficacy of PDSTP administered at early stage and 48 h post-infection is shown in Figure 5.
| no. | Animal group (agent/time prior to (-) and after (+) the animal was infected) | Mean disease duration (MDD), days (M ± SE) | Total symptom intensity score, points | Mean symptom intensity score, points (M ± SE) | Therapeutic index, TI, % |
|---|---|---|---|---|---|
| 1 | (10% ointment /+3 h) | 11.0 ± 1.75** | 26.1 | 8.7 ± 1.0* | 30.4* |
| 2 | (10% ointment/+48 h) | 13.0 ± 0.5** | 30 | 10.0 ± 0.5 | 20.0* |
| 3 | (API solution/ -1 and +3 h) | 12.0 ± 0.9** | 25.5 | 8.5 ± 1.0* | 32.0* |
| 4 | (Acyclovir/+3 h) | 10.7 ± 2.5** | 22.5 | 7.5 ± 1.5* | 40.0* |
| 5 | (Acyclovir/+48 h) | 10.7 ± 1.15** | 25.5 | 8.5 ± 1.5* | 32.0* |
| 6 | (Negative control) | 15.7 ± 1.5 | 37.5 | 12.5 ± 0.5 | - |
Table 5: Efficacy of topically and parenterally administered API for the guinea pig model of experimentally induced genital herpes depending on treatment onset time after the infection
The most statistically significant treatment outcomes were obtained in the group of infected animals (group 3) that were subcutaneously injected with PDSTP solution at a dose of 50 mg/kg/ day according to the treatment and prevention regimen (1 h prior to and 48 h post-infection) and in the group of animals treated with 10% ointment according to the treatment regimen (3 h post-infection) (group 1). It was found that PDSTP administered subcutaneously resulted in a pronounced therapeutic effect, reduced the intensity of symptoms and the mean disease duration compared to these parameters in the group of untreated infected animals. The TI of PDSTP administered subcutaneously is 32.0%. The animals treated with this dosage form of PDSTP recovered on average 3.7 days earlier than the untreated animals (the TI of PDSTP is 32.0%).
The therapeutic index of 10% ointment for external use was 30.4%; TDD, 11.0 ± 1.75 days. The animals treated with the 10% ointment this dosage form of PDSTP on average recovered 4.7 days earlier than those receiving no treatment (the TI 30.4%). However, the efficacy of the ointment used at the early stage of the infection process is lower than that of the acyclovir reference drug (3 h, group 4) but is comparable in terms of its effect on disease duration. The TDD value in the infected animals treated with 10% ointment or the reference drug was 11.0 ± 1.75 days and 10.7 ± 2.5 days, respectively. A similar pattern was observed comparing the efficacy of the reference drug and 10% ointment at late treatment initiation in animals with pronounced manifestations of the pathological process: the TI of acyclovir (group 5) was 32.0%, TDD was 10.7 ± 2.5 days; while treatment with 10% ointment resulted in a statistically significant therapeutic effect (20.0%) in infected animals (group 2).
Evaluation of the effect of PDSTP on frequency and level of HSV-2 isolation in guinea pigs depending on dosage form
As one can see from the data listed in Table 6, the virus was isolated from swab specimens from the lesion foci at 5 days postinfection not only in all the untreated animals (group 6) but also in animals receiving treatment in groups numbers 1, 2, 3, and 4. In the group of animals that started treatment with the acyclovir reference drug early after infection, the frequency of virus isolation was somewhat lower, similar to the earlier studies. Seven days postinfection, the frequency of virus isolation remained unchanged in the group receiving no treatment (group 7) and in the groups treated with PDSTP injected subcutaneously (group 3) and with PDSTP in the form of 10% ointment 48 h post-infection (group 2).
| Group no. | Animal group (agent / time prior to (-) and after (+) infection) | Viral titers in animals | |
|---|---|---|---|
| logTCID50/ml (M ± SE) | |||
| day 5 | day 7 | ||
| 1 | (10% ointment /+3 h) | 3.4 ± 0.5* | 2.0 ± 0.25* |
| 2 | (10% ointment/+48 h) | 3.8 ± 0.5 | 2.75 ± 0.25 |
| 3 | (API solution/ -1 and +3 h) | 3.5 ± 0.7* | 2.5 ± 0.15* |
| 4 | (Acyclovir/+3 h) | 2.0 ± 0.4* | 1.25 ± 0.1* |
| 5 | (Acyclovir/+48 h) | 2.75 ± 0.7* | 2.0 ± 0.2* |
| 6 | (Negative control) | 4.75 ± 0.1 | 3.5 ± 0.15 |
Table 6: Effect of API administered topically and subcutaneously on titer of HSV-2 isolated from the lesion foci in male guinea pigs depending on treatment initiation time after the animals had been infected
Five days post-infection, administration of PDSTP in the infected animals (groups 1, 2, and 3) reduced the replication level and infectivity of the virus isolated from the swab specimens collected from the lesioned area on average at least ten-fold compared to infectivity of the virus isolated from untreated infected animals (group 6). Titers of the virus isolated from the swabs collected from animals treated with PDSTP (groups nos. 1, 2, and 3) were 3.4–3.8 logTCID50/ml, respectively, and 4.75 logTCID50/ml in untreated animals (group 6)(Table 6). Viral titer in infected animals treated with acyclovir reference drug (groups 4 and 5) reduced 100–500-fold compared to those in the control group (group 6) and were 2.0–2.75 log TCID50/ml.
Seven days post-infection, infectivity of HSV-2 isolated from the swab specimens collected from infected animals decreased regardless of the agent being used. Viral infectivity in infected animals treated with PDSTP on average decreased by 1.0–1.4 log compared to the previous study period and was comparable to that in treated animals.
The different dosage forms of PDSTP can be arranged in the following order as their efficacy decreases: group 3 (PDSTP solution / -1 and +3 h) > group 1 (10% ointment + 3 h post-infection) > group 2 (10% ointment + 48 h post-infection). However, the reference drug showed the best symptom presentation, which correlates with the data obtained by studying the frequency and the level of HSV-2 isolation
Conclusions
We studied the efficacy of PDSTP and compared it to acyclovir in the guinea pig model of genital herpes. We also demonstrated that 10% ointment and 10% gel containing PDSTP administered topically according to the treatment regimen (twice daily for 5 days) or injected subcutaneously according to the treatment and prevention regimen (twice daily for 5 days) have a therapeutic effect in the guinea pig model of genital herpes induced by HSV-2. PDSTP reduces symptom intensity, time of lesion resolution, mean disease duration, and infectivity of HSV-2. The strongest effect was observed for the PDSTP solution administered parenterally, while the minimum effect was shown in the experiments when 10% gel was applied onto the lesion foci. Therefore, the PDSTP solution and 10% ointment dosage forms were used for further studies. All the dosage forms showed that efficacy depends on treatment initiation time. These data correlate with the results of analysing frequency and intensity of virus isolation, which was determined by titration.
Although the data obtained showed that acyclovir was somewhat superior (the efficacy of the ointment is lower, but is comparable to that of acyclovir in terms of its effect on disease duration, p<0.05), the general efficacy of treatment of PDSTP was comparable to that of acyclovir when a combination of all parameters (intensity and duration of clinical symptoms) was evaluated.
It is known that the mechanism of action of pyrimidinedispirotripiperazinium compounds involves targeting the host cells instead of a direct effect on the pathogen and this can be an advantage of this novel class of agents. Further experiments will demonstrate whether we can slow the development of resistance to therapy with these derivatives. Taking into account the mechanism of action of PDSTP, it would be interesting to use a combination of this molecule with acyclovir and its derivatives. The findings in this study suggest that PDSTP will be highly efficacious in vivo. As HSV-2 infections have been shown to be a risk factor for transmission of HIV infection the use of PDSTP as a prophylactic for those at high risk of HIV infection may also be worthy of future evaluation
Funding
We kindly acknowledge funding from NIH/NINDS: 1R01NS102164-01, and RFBR 17-54-30007 invo New generation of non-nucleoside RT HIV inhibitors avoiding HIV-associated neurocognitive disorders.
References
- Russell DB, Sepehr NT, Jayne MR, Suzanne MG (2001) Seroprevalence of herpes simplex virus types 1 and 2 in HIV-Infected and uninfected homosexual men in a primary care setting. J Clin Virol 22: 305-313.
- Kenyon CR (2018) HIV prevalence by ethnic group covaries with prevalence of herpes simplex virus-2 and high-risk sex in Uganda: An ecological study PLoS ONE 13: e0195431.
- Looker KJ, Elmes JA, Gottlieb SL, Schiffer JT, Vickerman P, et al. (2017) Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 17: 1303–1316.
- Biswas S, Sukla S, Field HJ (2014) Helicase-primase inhibitors for herpes simplex virus: looking to the future of non-nucleoside inhibitors for treating herpes virus infections. Future Med Chem 6: 45–55.
- Wald A, Selke S, Warren T, Aoki FY, Sacks S, et al. (2006) Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis 33: 529–533.
- Vere Hodge RA, Field HJ (2013) Antiviral agents for herpes simplex virus. Adv in pharmacol 67: 1–38.
- Peaschke R, Woskobojnik I, Makarov V, Schmidtke M, Bogner E (2014) Prevents entry of human cytomegalovirus. Antimicrob Agents Chemother 58: 1963-1971.
- Selinka HC (2007) Use of a heparan sulfate-binding drug that efficiently inhibits papillomavirus infection for the analysis of cell surface events preceding internalization. J Virol 81: 10970-10980.
- Eymann-Häni, Leifer I, McCullough KC, Summerfield A, Ruggli N (2011) Propagation of classical swine fever virus in vitro circumventing heparan sulfate-adaptation. J Virol Methods 176: 85-95.
- Schmidtke M, Karger A, Meerbach A, Egerer R, Stelzner A, et al. (2003) Binding of a N,N-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically inhibits replication of viruses from different families. Virol 311: 134-143.
- Schmidtke M, Riabova O, Dahse HM, Stelzner A, Makarov V (2002) Synthesis, cytotoxicity and antiviral activity of N,N´-bis-5-nitropyrimidyl derivatives of dispirotripiperazine. Antiviral Res 55: 117-127.
- Artemenko AG, Muratov EN, Kuz'min V E, Kovdienko NA, Hromov AI, et al. (2007) Identification of individual structural fragments of N,N′-(bis-5-nitropyrimidyl)dispirotripiperazine derivatives for cytotoxicity and antiherpetic activity allows the prediction of new highly active compounds. J Antimicrob Chemother 60: 68–77.
- Schmidtke M, Wutzler P, Makarov V (2004) Novel opportunities to study and block interactions between viruses and cell surface heparan sulfates. Lett Drug Des Discov 1: 293-300.
- Shukla D, Spear PG (2001) Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108: 503–510.
- Selinka HC, Giroglou T, Sapp M (2002) Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virol 299: 279–287.
- Patel V, Ferguson M, Minor PD (1993) Antigenic sites on type 2 poliovirus. Virol 192: 361–364.
- Hallak LK, Spillmann D, Collins PL, Peeples ME (2000) Glycos-aminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol 74: 10508–10513.
- Jiang YF, He B, Ma J, Li NP, Gong GZ, et al. (2012) Comparison of the antiviral effects of entecavir and adefovir dipivoxil in chronic HBV infection: a randomized control trial. Acta Gastroenterol Belg 75: 316–321.
- Novoselova EA, Riabova OB, Leneva IA, Nesterenko VG, Bolgarin RN, et al. (2017) Antiretroviral activity of a novel pyrimidyl-di(diazaspiro-alkane) derivative. Acta Naturae 9: 112-114.
- Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Epidemio 27: 493-497.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi