Case Report, J Clin Exp Oncol Vol: 8 Issue: 1
Influence of Abemaciclib, a New CDK 4/6 Inhibitor, Decrease in Potassium Electrollytes and Urea in a Patient Suffering from Metastatic Breast Cancer
1Klinikum Landshut, 84034 Landshut, Germany
2Sana Clinic Pegnitz, 91257 Pegnitz, Germany
*Corresponding Author : Oliver Zorn
Head of the Department, Emergency Medicine, “Klinikum Landshut, 84034 Landshut, Landshut, Germany
Tel: 491729876144
E-mail: o_zorn@gmx.de
Received: March 11, 2019 Accepted: April 05, 2019 Published: April 15, 2019
Citation: Zorn O, Heirler F (2019) Influence of Abemaciclib, a New CDK 4/6 Inhibitor, Decrease in Potassium Electrollytes and Urea in a Patient Suffering from Metastatic Breast Cancer. J Clin Exp Oncol 8:1. doi: 10.4172/2324-9110.1000235
Abstract
A 46-year old patient suffering from metastatic breast cancer (FIGO IV D) underwent various chemotherapies and radiation treatments in the past. From November 4, 2018 the patient was treated with 300 mg Abemaciclib (2 × 150 mg). During therapy, her pain subjectively decreased. After 14 days the patient suffered nausea, emesis and exsiccation. She was therefore hospitalized and intensively monitored. Leukopenia and anemia on a common scale were treated in the usual way. Despite normal retentions, we detected a significant decrease in potassium and calcium. Surprisingly, we also detected a decrease in urea. Because of the continuous decrease in potassium and calcium, we substituted the electrolytes. After two weeks, all labor
Keywords: CDK 4/6 inhibitor, Breast cancer, Potassium, Calcium, Urea, Side effect
Case Report
A 46-year-old female patient, 77 kg, 165 cm (BMI: 29), unmarried, menarche at the age of 12, nullipara, non-smoker, living in a normal social milieu, with no relevant medical history, no previous operations, no hypertension, no heart failure, no diabetes and physical disability according to age.
In January 2014 the patient was R0-mastectomied, left-hand side, in a pT2 (3.2 cm), pN 1c (3/13, L1,V0, estrogenreceptor and progesteronreceptor positive, Her-2 negative, invasive ductal (NST), G 2 breast cancer, initial Ki 67 was 8%–10%. The clinical diagnostics (thorical x-ray, sonography of the liver and scintigraphy of the skeleton) were negative. In March 2014, antihormonal therapy started with tamoxifen 20 mg. ECOG: 1, Karnofsky: 90%.
In March 2014, postoperative radiotherapy of the thoracic wall was performed.
In August 2015, initial osteolysis in vertebral body, therefore radiotherapy was started. Because of instability, a minimally invasive fixateur intern was established.
In November 2015 increase in metastasis in other locations of the skeleton. Therapy with bisphosphonate and fluvestrant was started after radiotherapy.
In January 2017, after one year, stable state X-ray in December 2016 detected pulmonal metastasis. Chemotherapy was started (epirubicin, cyclophosphamid, paclitaxel).
In September 2018, brain metastasis and vertebral body metastasis were detected. New hitology: metastasis of the further known mamma carcinoma (ER: +, PR: +, HER-2: -, Ki 67: 40%).
September – October 2018: intrathecal application of chemotherapy (methotrexate).
In November 2018, therapy started with Abemaciclib 150 mg 1 – 0 – 1.
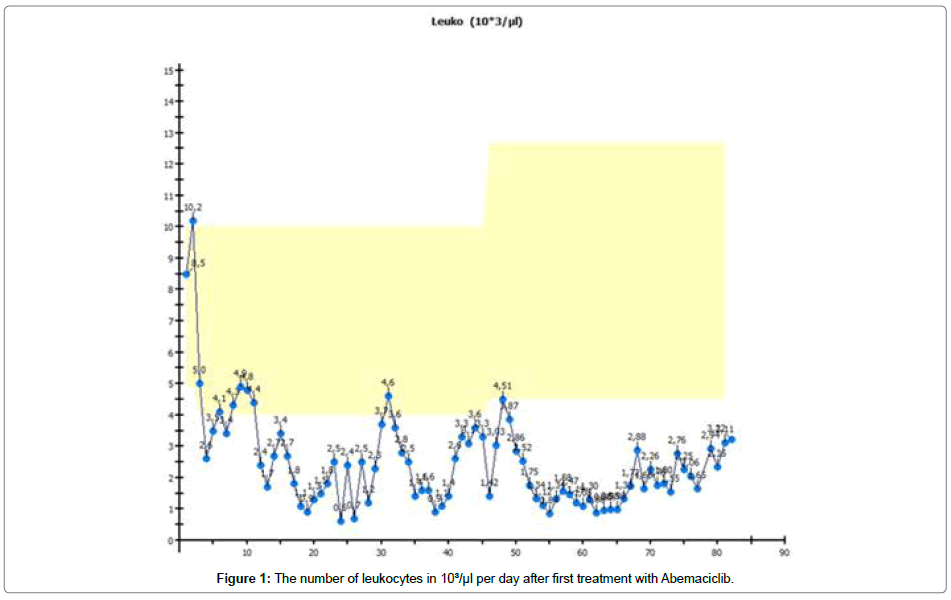
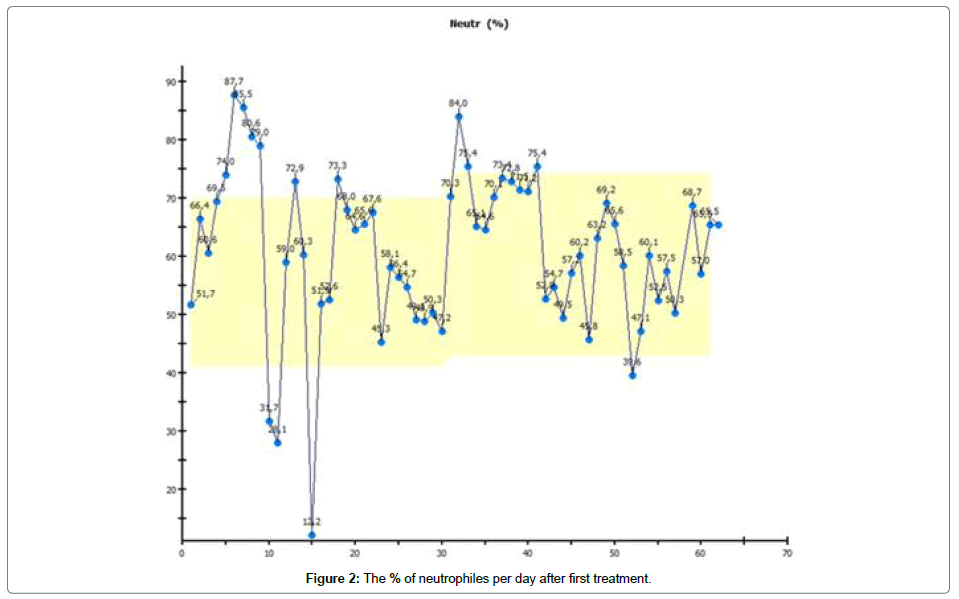
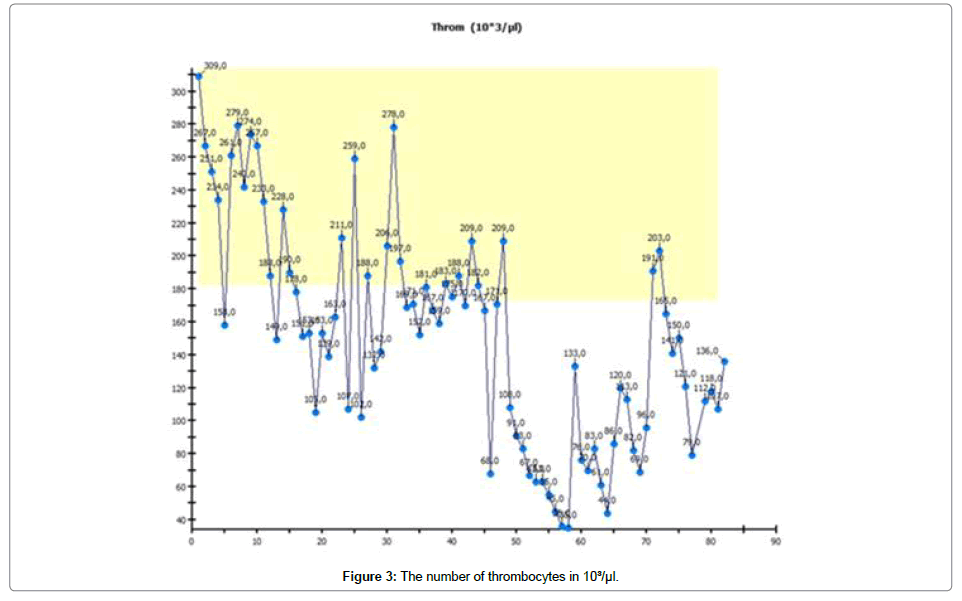
After 20 days of oral therapy, 2 × 150 mg daily, the patient suffered severe neutropenia, thrombopenia and anaemia, so she was isolated and treated with antibiotics, thrombocytes and erythrocyte-concentration infusion, shown in Figures 1-3 respectively.
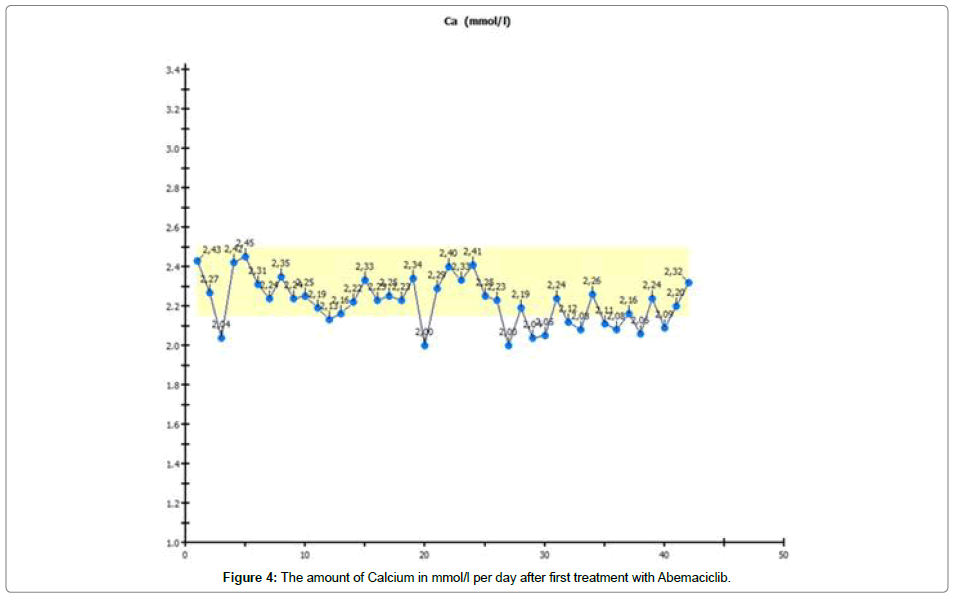
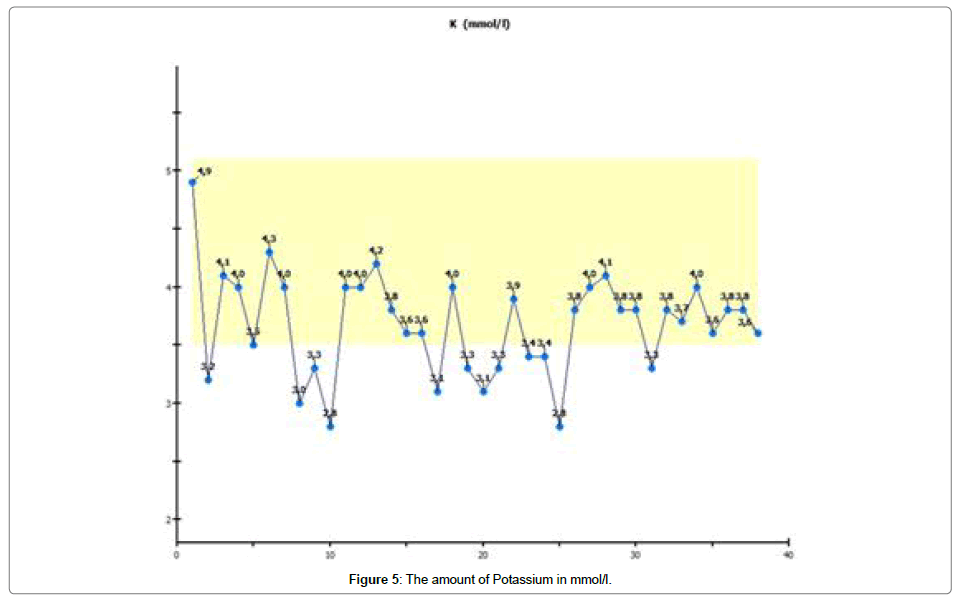
We conducted laboratory monitoring and detected a continuous decrease in potassium and calcium, so we substituted and controlled potassium and calcium every day. After 4 weeks, potassium and calcium normalized spontaneously, shown in Figures 4 and 5.
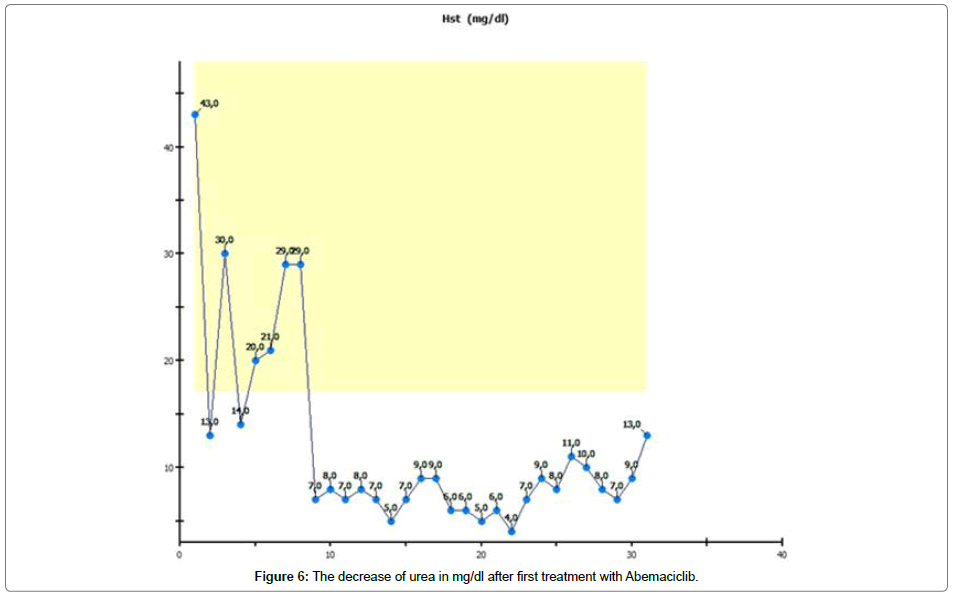
In order to control retention of the kidneys, we supervised creatinine and urea and found out that there was no change in the retention values. Surprisingly, we found that the urea value had undergone a very significant decrease, shown in Figure 6.
Discussion
A relatively new group of cancer therapeutics are cyclin-dependent kinases (CDK) inhibitors. In human beings, some 20 different CDKs regulate cell activity. CDK-inhibitors control cell proliferation. Abemaciclib is a cyclin-dependent kinase 4 and 6 inhibitor [1]. In combination with aromatase inhibitors and fulvestrant it is very effective in patients with metastatic breast cancer [2].
We use Abemaciclib because it can be administered orally in self-medication on a continuous basis. The dosage can be reduced. We expected the therapy to produce typical effects such as neutropenia anemia and thrombopenia [3,4]. These typical effects were seen in typical intensity and time range. In a patient with metastatic breast cancer under cytotoxic therapy, we also expected to see damage to large quantities of malignant cells [5]. Cells contain much more potassium than is to be found in the blood. Normally, massive cytolysis under cytotoxic therapy leads to an increase in potassium in the blood. Contrary to our expectations, we did not see an increase in potassium in our patient [6].
We observed a decrease in potassium, and this decrease was continuous. As a result, we had to substitute potassium. The same effect was seen with calcium, which we also had to substitute. Retention values were continuously within the normal range. In spite of a moderate increase in transaminases, the urea did not increase either.
The most surprising change in the patient’s laboratory values was a rapid and significant decrease in urea. Urea decreased to single figure values and returned untreated to a normal range four weeks after the last Abemaciclib administration [7,8].
If there is a spontaneous lag in some electrolytes and urea and if creatinine is continuously within the normal range, these materials must be located somewhere [9]. The only potential location of the electrolytes and urea is the highly replicant cells which accumulate this rough material for synthesis. During this phase, CDK 4/6 stopped cell replication.
Conclusion
We believe that the functioning of CDK 4/6 inhibitors is responsible for this effect. The reason for the intracellular accumulation of potassium, calcium and urea might be the discontinuation of cellular function at the end of the G1 phase, just before the synthesis phase starts. It might be that the cell attempts to accumulate material such as potassium, calcium and urea in preparation for the next step so as to have enough agents for synthesis.
Knowing this typical CDK 4/6 effect is important in terms of assessing the therapeutic effect. In the future, therefore, such laboratory values might be recorded in order to modulate Abemaciclib therapy. Further investigations are required.
References
- Barroso-Sousa R, Shapiro GI, Tolaney SM (2016) Clinical development of the CDK4/6 inhibitors ribociclib and abemaciclib in breast cancer. Breast Care (Basel) 11:167-173.
- DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, et al. ( 2015) CDK 4/6 inhibitor palbociclib (PD0332991) in Rbþ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 21:995-1001.
- Dickler MN, Tolaney SM, Rugo HS (2017) MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2-metastatic breast cancer. Clin Cancer Res 23:5218-5224.
- Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, et al. (2017) MONARCH 3, Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638-3646.
- Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, et al. (2016) Ribociclib as first-line therapy for HR-Positive, advanced breast cancer. N Engl J Med 375:1738-1748.
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14:130-146.
- Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: A changing paradigm. Nature Reviews Cancer 9: 153-166.
- O'Leary B, Finn RS, Turner NC (2016) Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13:417-430.
- Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, et al. (2016) Efficacy and safety of Abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung Cancer, and other solid tumors. Cancer Discov 6:740-753.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi