Research Article, Jrgm Vol: 10 Issue: 3
Injectable Platelet Rich Fibrin with Mesenchymal Stem Cells
Chenaitia Hichem1, Messadi Yacine1, Kassir Radwan2, Silvestre Alain3, Guillaume Collet4, Gal Jocelyn5, Skhiri Taycir6, Gugenheim Jean6, Ben Amor Imed6*, Lecorgne Enora7, Gaggioli Cedric7, Ben Amor Virginie8
1Department of Nice Institut for Sport and Osteoarthritis, INSA, Nice, France
2Department of Bariatric Surgery, CHU Felix Guyon, Saint Denis, la Réunion, France
3Department of Center for Imaging of the Musculoskeletal System, INSERM U 1034, Bordeaux, France
4Department of Private Medical Laboratory, I-LAB, Nice, France
5Department of Centre Antoine Lacassagne, Statistic Department, Nice, France
6Department of Digestive Surgery, Teaching Hospital, Archet 2, Nice, France
7Department of Unite, INSERM : U 1081 (IRCAN), Nice, France
8Department of Radiology, Teaching Hospital, Pasteur 2, Nice, France
- *Corresponding Author:
- Ben Amor Imed
Department of Digestive Surgery
Teaching Hospital, Archet 2, Nice, France
E-mail: imedamorb@hotmail.com
Received: March 12, 2021 Accepted: April 12, 2021 Published: April 19, 2021
Citation: Hichem C, Yacine M, Radwan K, Alain S, Collet G, et al. (2021) Injectable Platelet Rich Fibrin with Mesenchymal Stem Cells. J Regen Med 10:3.
Abstract
The acceleration and the quality of post surgery healing remain a major issue in medical practice. The platelet rich plasma (PRP) has been widely investigated and used in many chirurgical feeld like orthopedics, dermatology, odontology and gynecology due to its properties and simplicity. Despite the increasing number of studies, there is no consensus regarding the classification used for different types of PRP. Other studies have shown that mesenchymal stromal cells (MSC) have been found in human pererefiric blood. The quantity and quality of MSCs found in the PRP, seem to directly influence the healing results. We hypothesize that current PRP protocols are deleterious for MSCs so that it would be more effective to decrease the speed and duration of centrifugation. Thus the objective of our study was to determine the best centrifugation speed and time to optimize the composition of PRP, particularly MSC, platelet and leukocyte concentration. We have shown that peripheral blood could be considered as a source of MSCs for regenerative medicine. We develop the concept of “soft centrifugation” to obtain a higher concentration of MSCs in the PRP from a peripheral blood sample. Our study shows that injectable platelet rich fibrine with mesenchymal stromal cells (IPRF with MSC) is easy and cheap to produce with minimal effort and can be prepared at the point of care.
Keywords: Platelet Rich Fibrin; Mesenchymal Stem Cells; Orthopedics; Dermatology
Keywords
Platelet Rich Fibrin; Mesenchymal Stem Cells; Orthopedics; Dermatology
Introduction
Multipotent progenitor cells and more precisely mesenchymal stromal cells (MSC) have been isolated from adipose tissue, liver, tendons, synovial membrane, amniotic fluid, placenta, umbilical cord, teeth [1-4] and have also been found in human peripheral blood [5]. MSCs display the morphology and surface phenotype of bone marrow resident mesenchymal cells and are capable of differentiating into bone, cartilage, fat, myoblasts neurons and astrocytes in vitro and in vivo [2,3,6,7] . These cells derived from bone marrow may constitute a source of autologous cells with critical clinical import. Following transplantation in vivo, MSCs migrate to distant organ and integrate within the new parenchyma, acquiring the identity of resident cells structurally and functionally [5].
The preparation techniques for platelet rich plasma (PRP) have in common collecting peripheral blood, followed by centrifugation [8]. PRP has been widely investigated and used in orthopedics [9,10], dermatology [11], odontology [12] and gynecology [13] due to its properties and simplicity to obtain this product. Despite the increasing number of studies, there is no consensus regarding the classification used for different types of PRP. There are a wide variety of commercially approved kits available with many different centrifugation protocols resulting variable cellular concentrations. These systems change greatly with regard to the blood volume, the use of an anticoagulant, the number of spins, the centrifugation speed and time, and the plasma volume, resulting in PRP with different concentrations of cells [14]. Interestingly, some studies found a functional interest to inject MSCs issue from bone marrow aspiration in knee osteoarthritis [15-17] and confirmed an articular cartilage regeneration using arthroscopy and histology [18]. Clinical studies in patients with knee osteoarthritis demonstrate clinical improvement several months after injection of PRP in the joint, but this treatment shows no superiority compared to the intra-articular injection of hyaluronic acid [19-22]. This lack of result could be explained by the small amount of MSCs in PRP production.
We hypothesize that current PRP protocols are deleterious for MSCs so that it would be more effective to decrease the speed and duration of centrifugation. Thus the objective of our study was to determine the best centrifugation speed and time to optimize the composition of PRP, particularly MSC, platelet and leukocyte concentration.
Methods
MSC concentration
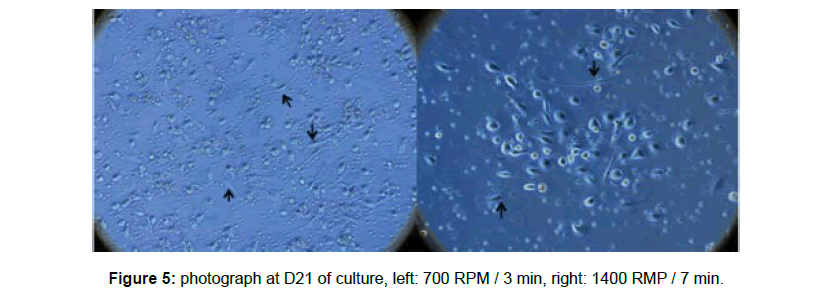
Blood samples were collected with the informed consent of 15 healthy volunteers donors (60 total samples). The Institutional Ethic commity / Review Board (IRB) of Nice hospital determined that this study did not require IRB review or approval. All donors were free of any infectious disease and did not have any abnormal consumption of nicotine or alcohol. The subjects were not presently under any medications. Peripheral blood samples were collected from the antecubital vein in sterile tubes (10 ml) with anticoagulant (ACD-A). The samples were centrifuged using the laboratory centrifuge (Centrilab 80-2C®) at a constant temperature of 22°C with 3 different centrifugation rate (revolution per minutes (rpm); centrifuge rotational radius (r), relative centrifugal force RCF (g) = 11,18 x 10-6 x r x N rpm2) and 2 different times : 700 rpm (37g), at 1400 rpm (148g), and at 2100 rpm (333g) for 3 and 7 min respectively by the same centrifuge machine.
Then, three layers are formed because of its density: The bottom layer consisting of Red Blood Cells (RBCs), the middle layer consisting of platelets and white blood cells (WBC) named buffy coat and the top supernatant plasma named Platelet Poor Plasma (PPP) layer. Remove buffy coat and PPP in new sterile tube, then a fluorescence-activated cell sorting analysis was done. All the procedures were realized in sterile conditions.
Immunofluorescence or Fluorescence-Activated Cell Sorting (FACS) Analysis
For all 15 patients, supernatant plasma were diluted in fetal bovine serum, then we separated cells on Ficoll density gradient with a centrifugation (RFC 200g; 2000 rpm, 10 min). The mononuclear cell (MNC) fraction was collected and washed in saline solution, then counting by flow cytometry.
MSCs were characterized according to the criteria defined by the International Society for Cellular Therapy by flow cytometry to confirm the expression of CD73, CD90, CD105, and CD146 as well as documenting lack or very low expression of CD11b or CD14, CD34, and CD45 (Dominici et al. 2006). In our study the Cell surface markers were evaluated through direct or indirect immunofluorescence : CD 44, CD 73, CD 90, CD 105 positive, CD 34 and CD 45 negative (Markers BD Bioscience).
Colony-Forming Unit-Fibroblast Assay
For six patients at random we try to obtain Colony Forming Unit Fibroblast (CFU-F). We separated cells in the supernatant plasma on Ficoll density gradient with a centrifugation (RFC 200g; 2000 rpm, 10 min), and the MNC fraction was collected and washed in saline solution. This MNC fraction is put in growth medium, containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 100 units/ mlPenicillin/Streptomycin (Pen/Strep, Gibco, Grand Island, NY, USA) in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, NY, USA) and MEM NEAA (100X) Minimum Essential Medium Non-Essential Amino Acids (Glycine, L-alanine, L-Asparagine, L-Aspartic Acid, L-Glutamic, L-Proline, L-Sérine) (Gibco, Grand Island, NY, USA) and cultured in a humidified atmosphere at 37°C with 5% CO2.
The medium was replaced twice a week and non-adherent cells were discarded. After two weeks one colony of adherent fibroblastlike cells was noticed in one of the seeded specimens. When the colony reached the approximate size of 5 cm2, cells were detached and seeded in a new flask in growth medium. Cells reached confluence after 10 days. Following first confluence, cells were passaged regularly. After reaching confluence, cells were subcultured routinely in growth medium, using Trypsin/EDTA solution (PAA Laboratories). The populations of MSCs were expanded for up to 40 passages. Colony- Forming Units-Fibroblastic (CFU-F) assays were performed by plating MSCs (P3 to P6) in 6 well plates at 10, 50 and 100 cells/well in growth medium, in two replicas. After 14 days under standard culture conditions, the cells were fixed with ice-cold methanol and stained with 0.3% crystal violet. The number of visible colonies (more than 50 cells) was counted.
We seeded 1 × 105 MNCs and 5 × 103 cells, obtained after each passage, in 8 cm diameter Petri dishes containing complete α-MEM. After 10 days of culture, we counted fibroblastic colonies with more than 50 cells, using a May Grünwald-Giemsa coloration (Merck, Darmstadt, Germany, http://www.merck.com).
Platelet and leukocyte concentrations
Blood samples were collected with the informed consent of 20 healthy volunteers donors (60 total samples). The subjects who were systemically healthy, not presently under any medications and any medical problem were included in the study. An informed consent was obtained from all the participants included in the study. Pregnant women, people aged fewer 18 years, patient with a disorder of comprehension whatever the reason (example language barrier), patient under trusteeshipor guardianship were systemically excluded.
Peripheral blood samples were collected from the antecubital vein in sterile tubes (10 ml) with anticoagulant (ACD-A). The samples were centrifuged using the laboratory centrifuge (Centrilab 80-2C®) using the same protocol, at a constant temperature of 22°C with different centrifugation rate but onetime: 700 rpm (37 g), at 1000 rpm (75 g), and at 1400 rpm (148 g) but only during 3 min. Baseline platelet and leukocyte count was assessed for all the subjects following which PRP was prepared. The platelet and leukocyte count in the PRP was determined using coulter counter (Unicel DXH 800 coulter cellular analyse system, Beckman Coulter®). All the procedures were realized in sterile conditions.
Statistical analysis
The mean values and frequency were calculated for the demographic variables. Data was analysed with statistical software SPSS version 11. Results are expressed as means ± SD. The significance of the differences between the means was analyzed using one-way and two way analyses of variance (ANOVA) with Tukey multiple comparisons test (α = 0.05). Thereby, significant differences were marked as significantif P values were less than 0.05 (*P < 0.05) and highly significantif P values were less than 0.01 (**P < 0.01), 0.001(***P < 0.001) or 0.0001 (****P < 0.0001).
Results
Part A
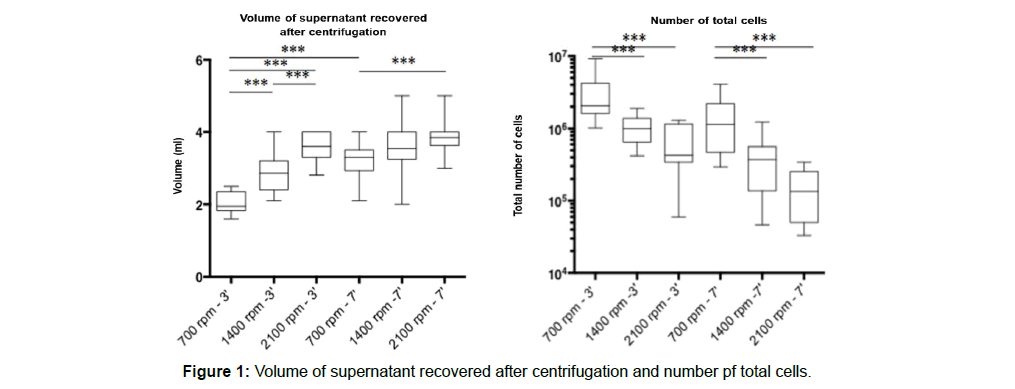
The age range of the 15 subjects included in the study was 25–53 years (37 years average age) that comprised of 8 males and 7 females. The plasma supernatant volume raises and cellular concentration decreases with the speed of centrifugation. The mononuclear cells detected in the FACS analysis are significantly more important when the speeds are low (Figure 1).
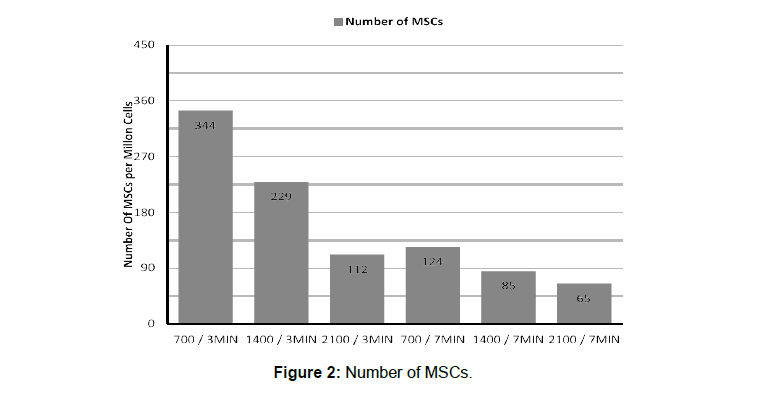
The higher rate of MSC was detected with the lowest centrifugation force and the shortest time (75g, 700 rpm and 3 min). The time and the speed of centrifugation decrease MSC concentration. MSC were 2 to 3 times lower when centrifugation lasted 7 min compared to 3 min (P < 0.0001). The MSC level was respectively 2 and 3 time higher with a centrifugation speed at 2100 rpm compared to 1400 and 700 rpm (P < 0.001) (Figure 2).
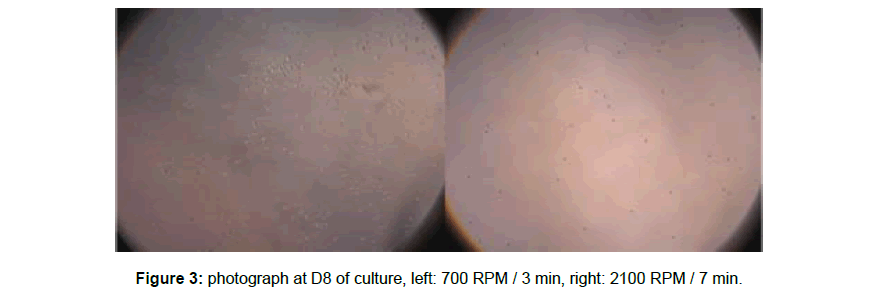
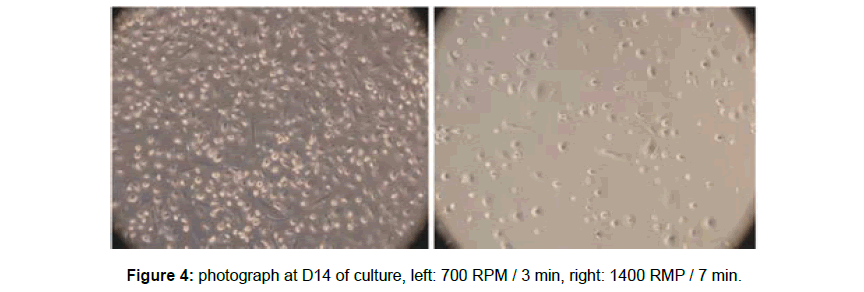
The cell cultures (CFU ?) of the samples centrifuged at low speed and for a short time observed with optical microscopy were more prolific compared with those resulting from high speeds and long time (2100 rpm and 7 min) (Figures 3-5). We observed the same tendency for cells that adhere to the plastic and possess pseudopods (Figure 3-5).
Part B
The age range of the 20 subjects included in the study was 19– 65 years (34 years average age) that comprised of 10 males and 10 females.
Total platelet number
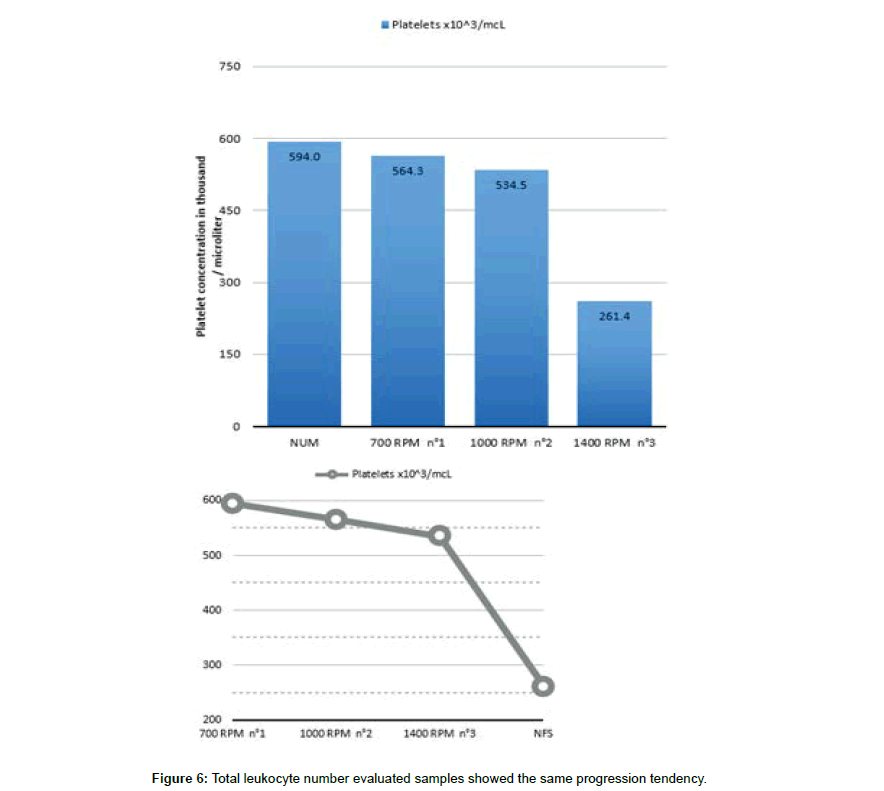
The platelets concentration in the PRP obtained by simple centrifugation of whole blood was always above baseline. The total platelet number within the buffy coat and PPP showed a significant increase with RCF reduction (Figure 6). The lowest platelet concentration was found in the protocol with the slowest centrifugation. The number of platelet was 11% higher when the rpm was about 700 compared to the protocol with 1400 rpm. All the evaluated samples showed the same progression tendency (Figure 6).
Total leukocyte number
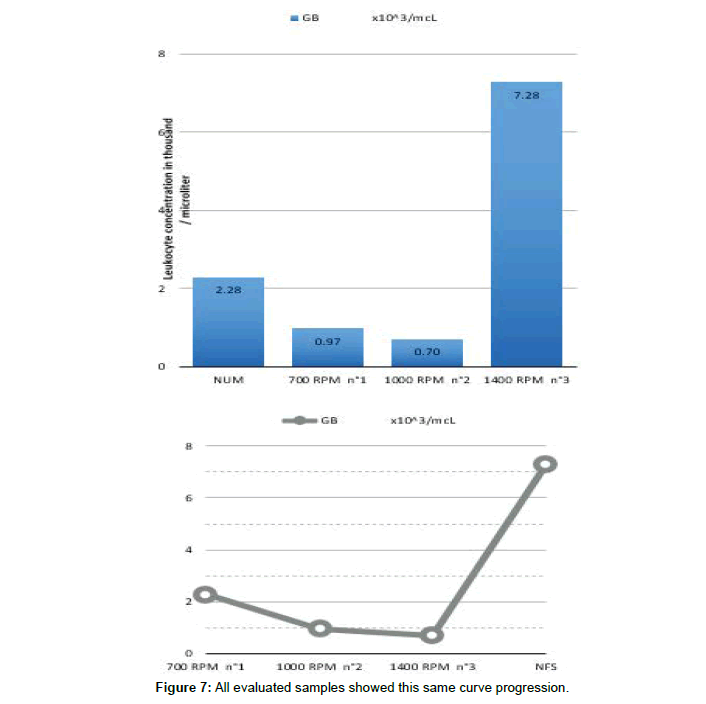
The leukocyte concentration from buffy coat and PPP is always below the whole blood leukocyte concentration. Reducing the RCF led to an increase of the total leukocyte number within the buffy coat and PPP. The protocol which was centrifuged with the highest RCF (148 g, 1400 rpm and 3min), showed only 700 leukocytes/ μl, whereas the third protocol (37 g, 700 rpm and 3min), revealed the highest number of leukocytes (2300 leukocytes/μl (ecart type ?) (Figure 7). All evaluated samples showed this same curve progression (Figure 7).
Discussion
We have demonstrated for the first time that the amount of MSC in the PRP depends on the speed and duration of the centrifugation. The maximum number of MSC is obtained via a single spin technique and a centrifugation of 3 min with 700 rpm.
Arguments in favor of the technique used
Since for over 20 years PRP has been used safely in a variety of conditions with promising results. The PRP is an interesting therapeutic tool because its components have a great similarity with the resources necessary for the natural healing process. Scientific researches that seek to explain the beneficial mechanisms of this treatment have focused on the action of platelets, white blood cells and growth factors [23,24]. So far, nobody has assumed that the MSCs contained in the PRP play a primordial role in the healing mechanism. We have demonstrated the presence of MSCs in the PRP prepared with usual speeds and durations, a procedure easy to obtain, cheap and without anticoagulant. We were also able to get a higher concentration of MSCs by decreasing the speed and the duration of the centrifugation. We used a single spin technique, instead of a double-spin technique, because the first spin (a hard spin) separates the red blood cells from the plasma, the latter layer contains platelets, white blood cells, and clotting factors [24]. The usual technique carry out a second round (a soft ride) to separates platelets and white blood cells, but excluding white blood cells does not seem useful as they are important to the healing process. Some studies on MSCs show that they attenuate the inflammatory response of the immune system [25-27]. Although all clinical studies on PRP use these two steps, the second spin could have a detrimental impact on its composition by removing white blood cells and destroying MSCs. MSCs have probably not been observed previously in the PRP because the double centrifugation as well as high speeds and durations are deleterious for these cells. Our results could also be of interest in MSCs preparation techniques for cellular therapy. Today, the isolation and the culture of MSCs may require up to 3 centrifugations with a total duration of 50 minutes [28,29]. The slow centrifugation method could be a way to improve their performances.
Our results suggest that the “low speed centrifugation concept” provides a higher platelet and leukocyte concentration, as seen in a recent work [30]. These authors developed the “low speed centrifugation concept”. These findings are also in agreement with the observations of other studies [31,32], who reported that a lower centrifugation speed and time favoured platelet separation and thereby PRP enrichment. These parameters also allow better conservation of growth factors and cytokines [30,33]. Using low speeds and low time provides a platelet concentrate that does not need anticoagulant. Then the product obtained doesn’t interfere with hemostasis, one of the parts of the healing and is still liquid so that it can be injectable. As this PRP is rich in MSCs, we propose to call it plasma rich mesenchymal stem cells (PRMSC). Future research should investigate the best compromise between time and centrifugation rate to obtain the best cell activity and thus improve the regeneration potential of PRMSC.
Concerning safety procedure, any concerns of immunogenic reactions or disease transfer are eliminated because PRP enriched with MSC is prepared from autologous blood. No studies have documented that PRP or mesenchymal stem cell infusion promotes adverse events including hyperplasia, carcinogenesis, or tumor growth [28- 35]. Nevertheless, the use of PRP enriched with MSCs must respect usual contraindications including the presence of a tumor, metastatic disease, active infections, pregnancy or breastfeeding.
Are plasma rich mesenchymal stem cells (PRMSC) a cellular therapy product?
The presence of non-hematopoietic stem cells in the bone marrow (BM) was first suggested by Friedenstein [34]. Pittenger isolated MSC from BM by density gradient centrifugation to eliminate unwanted cell types and only 0.001% to 0.01% of the cells isolated from the density interface were mesenchymal stem cells [6]. These cells are able to differentiate into a number of mesenchymal phenotypes, including bone, cartilage, fat, myoblasts, neurons and astrocytes in vitro and in vivo. They are isolated in vitro by using their adhesion capacities on plastic of the culture flasks and by their strong proliferation potentials. So far, the majority of clinical studies have used high doses of MSCs by culturing bone marrow biopsies. MSCs have been studied for clinical research protocols in hematology to avoid transplant rejection after myeloablative therapy. Injections are given intravenously with doses between 1x106/kg and 10x106/ kg and combined with hematopoietic stem cells [35,28,36]. A MSCs dose between 25x106 to 125x106 was administered by intra-articular injection into the knee joint to test pain reduction in osteoarthritis and rheumatoid arthritis [37,38]. They have been studied intravenously at 100×106 cells/infusion for their anti-inflammatory effects in COPD [39], in patients with locally advanced or metastatic breast cancer [29] and in neuromyelitis optica spectrum disorder [40]. CMSs were also used to form the bone structure. In bone defect CMSs were placed on macroporous hydroxyapatite scaffolds, implanted at the lesion sites and in osteogenesis imperfect they were intravenously infused [41,42]. The highest doses of CSMs have been used to treat patients with stroke and myocardial infarction. One to ten billion cells were injected into the middle cerebral artery or intra-carotidally and by catheterization of the coronary artery respectively [43-45]. Nevertheless, the procedure of BM extraction is traumatic and the amount of material extracted is limited. Therefore, exploring new sources for obtaining such cells is of great interest.
Other organs were used to obtain MSCs. Human umbilical cord blood-derived MSCs have often been used in clinical trials for aplastic anemia [46], Rheumatoid Arthritis [47] and atopic dermatitis [48]. Some studies report intra-articular injection of MSCs obtained from adipocytes in patients with knee osteoarthritis [49-51]. In the clinical trial of Saw and coworkers, autologous peripheral blood precursor cells were collected with an automated cell separator (apheresis) by central venous access in patients beforehand stimulated by hematopoietic growth factor and the product was injected into the operated knee joint [51,53]. With our method, the slow centrifugation can collect at mean 120 MSCs per 10 ml of peripheral blood. To our knowledge, only two clinical trials have tested the efficacy of stem cells taken from a simple peripheral blood sample without culture before intra-articular injection but they were isolated in a CS3000 plus Fenwal (USA) separator which selected only CD34+ cells [54,55]. Although injected cells were not just MSCs, functionnal scores and imaging exams improved in across all scales.
In bone marrow mesenchymal stem cells occur at very low frequencies of 2 to 5 MSCs/1x106 mononuclear cells. The number of cells isloated per blood sample is limited and ex-vivo expansion is imperative in order to reach clinically meaningful cell numbers. Faced with the growing interest of MSCs for regenerative medicine, the isolation and purification techniques have been improved [56- 58]. Rapid proliferation of these cells in vitro allows its expansion by a factor of 103 within 14 to 21 days of culture [58]. If these methods improve, a simple blood sample could replace the others invasive collection methods to use MSCs as treatment.
The cell multiplication technique is not necessary to make PRMSC. The number of MSCs remains low, but in a cell environment suitable to the repair of lesions. PRMSC could therefore be used routinely in the current indications of the PRP because it comes out of the definition of tools for cell therapy. Indeed, 1) there is no substantial manipulation of SMCs or cell multiplication, 2) the autologus product is injected directly after obtaining. Nevertheless, as MSCs are used to replace failing cells, bioethics laws should rule.
Limitations
Fibrinogen, the major component of the blood coagulation process, play a crucial role in tissue repair by providing an interim matrix that supports cell adhesion, proliferation and migration involved in wound repair. It has also been shown that fibrin binds MSCs with hight affinity [59]. In our study, we have focus on cellular components present in PRP, whereas it would have been easy to obtain fibrinogen concentration.
The Growth Factors, major components of tissue healing, have not been measured, but Choukroun and Ghanaati proves that their concentrations follow the concentration of platelets [30]. Our method of soft centrifugation probably enriches PRP-based fluid matrices with growth factors.
An important bias of our study was the need for rapid and prolonged centrifugation before switching to the flow cytometry, which could potentially have significantly decreased the number of MSCs. The number of MSCs obtained in our results is probably underestimated.
The characteristics of MSCs has been defined by the International Society for Cellular Therapy. They include a set panel of cluster of differentiation, plastic adherence and differentiation capacity along the mesenchymal lineages. Surface markers CD29, CD44, CD73, CD90, CD105, CD166 are positifs and CD14, CD31, CD34, CD45 are negatifs [60]. Due to the limiting techniques of the flow cytometer, we have been constrained for the number of clusters to analyze. We characterized the expression of CD44, CD73, CD90, CD105, CD146 as well as the lack or very low expression of CD11b or CD14, CD34, CD45. We did not focus about CD29 because CD90 is also a cell adhesion molecule [61]. We left CD31 because it was not in the criteria of Cesselli et al. who found the first CSMs in the peripheral blood [5]. CD34 and CD45 are almost ubiquitously related to hematopoietic cells [62,63]. Clinical studies used different differentiation clusters to characterize MSCs, but CD44+, CD73+, CD90+ and CD105 are frequently choosen [28,64,37]. Many phenotypically distinct progenitor cells have been described in the peripheral blood MSCs in both laboratory animals and humans [65,66]. New subpopulation is progressively identified. For exemple, CD271+ MSCs have a greater chondrogenic potential [67] and CD106+ MSCs have a greater immunoregulatory activity [68]. Knowledge about MSCs is constantly evolving and the determination of differentiation clusters is still in progress. Most importantly MSCs possess an ability to form colonies comprising non refringent spindle-shaped cells deriving from a single cell. We evaluated the cloning efficiency (number of CFU-F/5,000 seeded cells) of MSCs obtained from peripheral blood and we obtained few fibroblastic colonies (3.5 ± 1.5 CFU-F/5,000 seeded cells), which is the obvious proof that these cells are indeed stem cells.
Conclusions
Our results suggest that peripheral blood could be considered as a source of MSCs for regenerative medicine. We develop the concept of “soft centrifugation” to obtain a higher concentration of MSCs in the PRP from a peripheral blood sample. Our study shows that injectable platelet rich fibrine with mesenchymal stromal cells (IPRF with MSC) is easy and cheap to produce with minimal effort and can be prepared at the point of care. Future studies are necessary to evaluate possible clinical benefits of PRMSC compared to classic PRP.
References
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71-74
- Bianco P, Gehron Robey P, (2000) Marrow stromal stem cells. J Clin Invest 105: 1663-1668
- Beyer N, Silva ML (2006) Mesenchymal stem cells : Isolation, in vitro expansion and characterization. Handb Exp Pharmacol 174: 249-282.
- Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res Rev 5: 91-116
- Cesselli D, Beltrami AP, Rigo S, Bergamin N, D'Aurizio F, et al. (2016) Multipotent progenitor cells are present in human peripheral blood. Circ Res. 104(10): 1225-1234
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multineage potential of adult human mesenchimal stem cell. Science 284:143-147.
- Jori FP, Napolitano MA, Melone MA, Cipollaro M, Cascino A, et al. (2005) Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem 94: 645-655
- Whitman DH (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 55(11): 1294-1299
- Sadabad HN, Behzadifar M, Arasteh F, Behzadifar M, Dehghanl HR (2016) Efficacy of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: a systematic review and meta-analysis. Electron Physician. 8(3): 2115-2122
- Mi B, Liu G, Zhou W, (2017) Platelet-rich plasma versus steroid on lateral epicondylitis: meta-analysis of randomized clinical trials. Phys. Sportsmed. 45(2): 97-104
- Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, et.al (2015) The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Trans. Med. 4(11): 1317-1323
- Del FM, Bortolin M, Taschieri S, Weinstein R (2011) Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J. Periodontol. 82(8): 1100-1111
- Dawood AS, Salem HA (2018). Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice. Clin Exp Reprod Med. 45(2): 67-74
- Lana JF, Purita J, Paulus C, Huber SC, Rodrigues BL, et al. (2017) Contributions for classification of platelet rich plasma - proposal of a new classification: MARSPILL. Regen Med. 12(5): 565-574
- Saw KY, Anz A, Siew-Yoke JC, Merican S, Ching-Soong Ng, et al. (2013) Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 29(4): 684-694.
- Skowroński J, Skowroński R, Rutka M (2012) Cartilage lesions of the knee treated with blood mesenchymal stem cells - results. Ortop Traumatol Rehabil. 14(6): 569-577
- Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, et al. (2011) Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 27(4): 493-506
- Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, et al. (2015) Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial. Am J Sports Med. 43(7):1575-1582
- Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, et al. (2012) Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 23(13): 229
- Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, et al. (2011) Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 27(11): 1490-1501
- Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA, et al. (2017) Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis. Am J Sports Med. 45(2): 339-346
- Campbell KA, Erickson BJ, Saltzman BM, Mascarenhas R, Bach BR (2015) Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatment of Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses. Arthroscopy. 31(10): 2036-2045.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy. 8(4) 315-317
- Sampson S, Gerhardt M, Mandelbaum B (2008) Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 1: 165-174
- Teng X, Chen L, Chen W, Yang J, Yang Z (2015) Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 37: 2415-2424
- Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M (2015) Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell Immunol. 293:113-121
- Curtis BJ, Shults JA, Boe DM, Ramirez L, Kovacs EJ (2018) Mesenchymal Stem Cell Treatment Attenuates Liver and Lung Inflammation after Ethanol Intoxication and Burn Injury. Alcohol.
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, et al. (2005) Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 11: 389-398.
- Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, et al. (2002) Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 30: 215-222
- Choukroun J, Ghanaati S (2018) Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low-speed centrifugation concept. Eur J Trauma Emerg Surg. 44:87-95.
- Sabarish R, Lavu V, Rao SR (2015) A Comparison of Platelet Count and Enrichment Percentages in the Platelet Rich Plasma (PRP) Obtained Following Preparation by Three Different Methods. J Clin Diagn Res. 9: 10-2.
- Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, (2014) Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 176060
- Amable PR, Carias RB, Teixeira MV, Cruz PI, Granjeiro JM, et al. (2012) Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 4:67.
- Friedenstein AJ, Gorskaja JF, Kulagina NN (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol; 4: 267-274.
- Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI (1995) Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant.16: 557-564.
- Zhang X, Li JY, Cao K, Lu H, Hong M, et al. (2010) Cotransplantation of HLA-identical mesenchymal stem cells and hematopoietic stem cells in Chinese patients with hematologic diseases. Int J Lab Hematol. 32: 256-264.
- Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, et al. (2016) Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther.18: 301
- Shadmanfar S, Labibzadeh N, Emadedin M, Jaroughi N, Azimian V, et al. (2018) Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 20: 499-506.
- Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, et al. (2013) A placebo-controlled, randomizedtrial of mesenchymal stem cells in COPD. Chest. 143: 1590-1598.
- Fu Y, Yan Y, Qi Y, Yang L, Li T, et al. (2016) Impact of Autologous Mesenchymal Stem Cell Infusion on Neuromyelitis Optica Spectrum Disorder: A Pilot, 2-Year Observational Study. CNS Neurosci Ther. 22: 677-685
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, et al. (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 344: 385-386
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, et al. (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 5: 309-313.
- Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, et al. (2012) Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 43: 2242-2244.
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, et al. (2002) Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 106: 1913-1918
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, (2004) Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 94: 92-95.
- Wang H, Wang Z, Zheng X, Ding L, Zhu L, et al. (2013) Hematopoietic stem cell transplantation with umbilical cord multipotent stromal cell infusion for the treatment of aplastic anemia. A single-center experience. Cytotherapy. 15: 1118-1125.
- Park EH, Lim HS, Lee S, Roh K, Seo KW, et al. (2018) Intravenous Infusion of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Rheumatoid Arthritis: A Phase Ia Clinical Trial. Stem Cells Transl Med. 7: 636-642.
- Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, et al. (2017) Clinical Trial of Human Umbilical Cord Blood-Derived Stem Cells for the Treatment of Moderate-to-Severe Atopic Dermatitis: Phase I/IIa Studies. Stem Cells. 35: 248-255.
- Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, et al. (2017) Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-YearFollow-up Study. Am J Sports Med. 45: 2774-2783.
- Jo CH, Lee YG, Shin WH, Kim H, Chai JW, et al. (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 32: 1254-1266
- Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, et al. (2016) ADIPOA Consortium. Adipose MesenchymalStromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl Med. 5:847-856.
- Saw KY, Anz A, Siew Y, Jee C, Merican S, et al. (2013) Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy. 29:684-694.
- Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, et al. (2011) Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 27: 493-506.
- Skowroński J, Skowroński R, Rutka M (2012) Cartilage lesions of the knee treated with blood mesenchymal stem cells - results. Ortop Traumatol Rehabil. 14: 569-577
- Jancewicz P1, Dzienis W, Pietruczuk M, Skowroński J, Bielecki M, et al. (2004) Osteochondral defects of the talus treated by mesenchymal stem cell implantation--early results. Rocz Akad Med Bialymst. 49 (1): 25-27.
- Zhang M, Huang B (2012) The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res Ther. 3:48.
- Martin I, De Boer J, Sensebe L (2016) MSC Committee of the International Society for Cellular Therapy. A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy. 18: 613-620.
- Rojewski MT, Fekete N, Baila S, Nguyen K, Fürst D, et al. (2013) GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 22: 1981-2000.
- Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, et al. (2006) Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 37: 967-976.
- Dominici M, Le Blanc K, Mueller I, (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315-317.
- Nakamura Y, Muguruma Y, Yahata T, Miyatake H, Sakai D, et al. (2006) Expression of CD90 on keratinocyte stem/progenitor cells. Br. J. Dermatol. 154:1062-1070.
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A, et al. (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 32:1380-1389.
- Shivtiel S, Kollet O, Lapid K, Schajnovitz A, Goichberg P, et al. (2008) CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 205: 2381-2395.
- Götherström C, Westgren M, Shaw SW, Aström E, Biswas H, et al. (2014) Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med. 3: 255-264.
- Moniche F, Montaner J, Gonzalez-Marcos JR, Carmona M, Piñero P, et al. (2014) Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGF-BB, and MMP-2 serum levels in stroke patients: results from a clinical trial. Cell Transplant. 23(1): 57-64.
- Zhang Y, Huang B (2012) Peripheral blood stem cells: phenotypic diversity and potential clinical applications. Stem Cell Rev. 8: 917-925.
- Mifune Y, Matsumoto T, Murasawa S, Kawamoto A, Kuroda R, et al. (2013) Therapeutic superiority for cartilage repair by CD271 positive marrow stromal cell transplantation. Cell Transplant. 22:1201-1211.
- Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, (2013) CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 8: 59354.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi