Research Article, J Regen Med Vol: 10 Issue: 5
Intravenous Administration Of Stembells in The Acute Phase After Myocardial Infarction Reduces The Pro-Inflammatory Status Of The Intramyocardial Vasculature in A Rat Model
Maikel J Oedayrajsingh Varma1,2*, Hans WM Niessen1,3, Umit Baylan1, Linde Woudstra1, Elisa Meinster1, Annemieke Van Dijk1, Paul AJ Krijnen1, Marco N Helder4, S Marieke Van Ham5, Nico De Jong6, Lynda JM Juffermans1, Casper G Schalkwijk7, Suat Simsek8,9
1Department of Pathology, Amsterdam UMC, Location VUMC, Amsterdam, The Netherlands
2Pathan BV Pathology Laboratory, Rotterdam, The Netherlands
3Department of Cardiac Surgery, Amsterdam UMC, Location VUMC, Amsterdam, The Netherlands
4Department of Oral and Maxillofacial Surgery / Oral Oncology, Amsterdam UMC, location VUMC, Amsterdam, The Netherlands
5Department of Immunopathology, Sanquin Research and Landsteiner Laboratory, Academic Amsterdam University Medical Center and Swammerdam Institute for Life Sciences, University of Amsterdam, Amsterdam, The Netherlands
6Department of Biomedical Engineering, Erasmus Medical Center, Rotterdam, The Netherlands
7Internal Medicine, Maastricht University Medical Centre, Netherlands and Cardiovascular Research Institute Maastricht (CARIM), Netherlands
8Department of Internal Medicine, Symbiant, Northwest Clinics, Alkmaar, The Netherlands
9Department of Internal Medicine, Amsterdam UMC, Location VUMC, Amsterdam, The Netherlands
*Corresponding Author: Maikel J Oedayrajsingh-Varma
Department of Pathology, Amsterdam UMC, Location VUMC, Amsterdam, The Netherlands
Tel: 0681936410
E-mail: m.varma@pathan.nl
Received: July 24, 2021 Accepted: August 13, 2021 Published: August 20, 2021
Citation: Varma MJO, Niessen HWM, Baylan U, Woudstra L, Meinster E, et al. (2021) Intravenous Administration of Stembells in the Acute Phase after Myocardial Ä°nfarction Reduces the Pro-inflammatory Status of the Ä°ntramyocardial Vasculature in a Rat Model. J Regen Med 10:5.
Abstract
Background: Acute myocardial infarction (AMI) induces a pro-inflammatory status of the intramyocardial vasculature in which N?-(carboxymethyl)lysine (CML), an advanced glycation end product, and NOX2, a source of reactive oxygen species (ROS), play an important role. As such they form attractive therapeutic targets to prevent future heart failure development. Previously, we found that adipose tissue derived stem cells coupled to antibody-targeted microbubbles (the so-called StemBells (StB)) improved cardiac function when administered intravenously, also when applied early after AMI in a rat model. Its effect on the intramyocardial microvasculature however is unknown. In the present study we have analyzed its effect on both CML and NOX2 depositions in the intramyocardial vasculature in a rat AMI model.
Methods: AMI was induced by ligation of the left coronary artery followed by reperfusion in male Wistar rats. In a subset of these rats, intravenous StB were administered at day 1 [n=8] (group StB day 1), day 7 [n= 7] (group StB 7) or days 1 and 7 [n=7] (group StB day 1+7) post-AMI. Animals were sacrificed at day 42 post-AMI. The effect on CML (immunohistochemical score and/or the number of blood vessels with different intensity scores) and NOX2 positivity (the number of positive blood vessels) was then analyzed using immunohistochemistry.
Results: AMI induced a significant increase in both CML (from 0.08 ± 0.01 /mm2 in controls up to 0.3 ± 0.05/mm2 in the AMI group) and NOX2 positivity of the intramyocardial vasculature (from 0.1 ± 0.01 in non-infarcted control rats to 0.53 ± 0.08 in AMI rats). StB therapy significantly decreased NOX2 (0.11 ± 0.02 treated on day 1 post-AMI, 0.19 ± 0.02 when treated on day 7 post-AMI and 0.25 ± 0.04 with administration at day 1 and day 7 post-AMI), without significant differences between the three StB groups) and CML positivity (immunohistochemical score: 0.13 ± 0.03 when treated at day 1 post-AMI, 0.17 ± 0.02 when treated on day 7 post-AMI and 0.13 ± 0.01 with treatment on day 1 and day 7 post-AMI) of the intramyocardial vasculature in all three groups. A significant decreasing effect of StB therapy on the lowest CML intensity (score 1) was found in all three StB groups, on the intermediate (score 2) in the StB day 7 and StB day 1+7 group, and finally on the highest CML intensity (score 3) in the StB day 1+7 group only.
Conclusion: StB therapy reduced the pro-inflammatory status of the intramyocardial vasculature post- AMI when applied in the acute phase post-AMI.
Keywords: NOX2, AGE, Stem cells, Myocardial infarction, Inflammation.
Introduction
Heart failure (HF) development after acute myocardial infarction (AMI) is a leading cause of morbidity and mortality in the western world [1,2]. Post-AMI inflammation is playing an important role in the development of HF. One of the best characterized advanced glycation end products (AGE), Nε-(carboxymethyl)lysine (CML) [3] has been described in the intramyocardial vasculature before but also subsequent to AMI [4-6]. This suggest that AGEs not only play a role in the induction of AMI but also in the jeopardizing inflammatory response post-AMI and as such could even play a role in the process of re-infarction and/or HF development. CML accumulation has been associated with increased oxidative stress [7] and it has been shown that its accumulation coincides with the activation of NOX2 proteins, a source of reactive oxygen species (ROS) in the heart [8,9]. Inhibiting CML and/or NOX2 in the intra myocardial vasculature might thus form a putative therapy to prevent cardiac complications post-MI.
Adipose derived stem cells (ASC), have emerged as an important element of regenerative therapies [10] partly due to their capacity of paracrine secretion of a broad selection of anti-inflammatory cytokines, chemokines, and growth factors making them clinically attractive as an anti-inflammatory and immunomodulatory therapy [11-13]. To improve stem cell therapy, we have coupled ASCs to gasfilled microbubbles that were coated with antibodies to Intercellular Adhesion Molecule (ICAM- 1 ), to create the so-called StemBells (StB). ICAM-1 is increased on activated endothelial cells in the heart post-AMI and CD90 (an ASC surface marker). These injected StB, can be pushed against the vessel wall using ultra-sound to increase their adherence [14]. Intravenously injected StB significantly improved cardiac function on the long term (42 days post-AMI) in a rat model of AMI, even when applied at 1-7 days post-AMI when the post- AMI induced inflammatory response is especially high. However, intravenous injected StB did not show an effect on infarct size [15].
In this study we have analyzed whether StB could influence the pro-inflammatory status of the intramyocardial vasculature by analyzing its effect on both CML and NOX2 depositions in the intramyocardial vasculature in a rat AMI model.
Materials and Methods
The rat AMI model
We used tissue specimen of a recent study in which the effect of the StB application was studied in a rat AMI model [16]. In short, eight-week old male Wistar rats (300-400 g, Harlan, the Netherlands) [17] were anaesthetized using fentanyl/fluanison (0.5 ml/kg) and midazolam (5 mg/kg). Subsequently, AMI was induced via ligation of the left anterior descending coronary artery (LAD). After 40 minutes the ligation was removed to allow reperfusion. All animal experiments were performed according to national guidelines and were approved by the Institutional Animal Care and local Animal Ethical Committee of the Vrije Universiteit (VU) University Medical Centre (Amsterdam, the Netherlands).
Stem Bell treatment
StB were assembled using stem cells that were isolated from adipose tissue that was harvested from the inguinal fat pads of 18 male Wistar rats (Harlan, Horst, the Netherlands) [16]. Subsequently, biotinylated mouse-anti-rat-CD90 antibodies (1 μg; BD Biosciences, Franklin Lakes, NJ) and biotinylated mouse-anti-rat- ICAM-1 antibodies (1 μg; Acris antibodies, Herford, Germany) were coupled to biotinylated microbubbles using streptavidin (1 mg/ml; Sigma-Aldrich) as a linker. To create StB, the antibodyloaded microbubbles were then coupled to the ASCs, as described previously [13,16].
The rats were separated in 5 different groups: a control group (rats without AMI and without StB therapy, n=7), an AMI group (rats with AMI, without StB therapy, n=6), and three therapy groups (StB groups): rats with AMI and with StB therapy given either on day 1 post-AMI ( n=8), on day 7 post-AMI (n=9) or both on days 1 and day 7 (1+7) post-AMI (n=7). The StB (1 x 106 in 600 μl DMEM) were administered in the tail vein. Rats without therapy received a control injection of 600 μl DMEM. The rats were sacrificed 42 days post-AMI, and the hearts were excised and fixed in 4% formaldehyde and then paraffin embedded for immunohistochemical analysis.
Immunohistochemical staining for CML and NOX2
For immunohistochemical analysis, paraffin embedded heart slides, 4-μm thick, were deparaffinized for 10 minutes in xylene followed by dehydration with ethanol and incubation in methanol / H2O2 (0.3%) for 30 minutes to block endogenous peroxidases. Antigen retrieval for CML staining was performed by incubating the slides in 0.1% pepsin buffer for 30 minutes at 37ºC, for NOX2 staining by boiling the slides in a TRIS/EDTA buffer (pH 9.0) for 10 minutes. All sera and antibodies were diluted in Normal Antibody Diluent Solution (ImmunoLogic). The slides were incubated for 1 hour with a mouse anti-human CML antibody (1:500 dilution) [18] or a mouse anti-human NOX2-antibody [19]. After washing in PBS, the slides were incubated with a biotin-conjugated rabbit-anti-mouse antibody (1:500 dilution, Dako) for 30 minutes, followed by subsequent washing with PBS. The slides were then either incubated with streptavidin–HRP complex (1:500 dilution, Dako) for CML staining, or with the ABC-kit (1:100 dilution, Vector Lab, Burlingame, CA) for NOX2 for 1 hour. After visualization with 3,3’ diaminobenzidine (DAB, Dako), the slides were stained with hematoxylin, dehydrated and covered.
Tissue analysis
Two cross-sections were examined per heart, one section approximately 2 mm above the tip of the apex and the other approximately 2 mm below the site where the LAD was ligated. Quantification of the immunohistochemical staining of CML was performed as follows: for the intensity scoring each CML-positive blood vessel was given a score of 1= weak positive; 2=moderately positive; or 3=strongly positive. Additionally, the surface area of the tissue was measured using QPRODIT [18]. To obtain the immunohistochemical (IH) score per mm2, first each intensity score (1, 2 and 3) was multiplied by the number of vessels positive for this score. These multiplication scores were added and divided by the tissue area resulting in an immunohistochemical score per mm2 [19]. NOX2-positive blood vessels were counted and related to the surface area of the analyzed tissue (measured using QPRODIT) [18].
Statistical Analysis
Data analysis was performed with Prism v.4.0 (Graphpad Software, La Jolla, CA). One-way ANOVA combined with Tukey posthoc test was used to analyze the differences between groups. Data values in text are displayed as mean ± standard error. P<0.05 was considered statistically significant for all the performed analyses.
Results
CML depositions in the intramyocardial vasculature
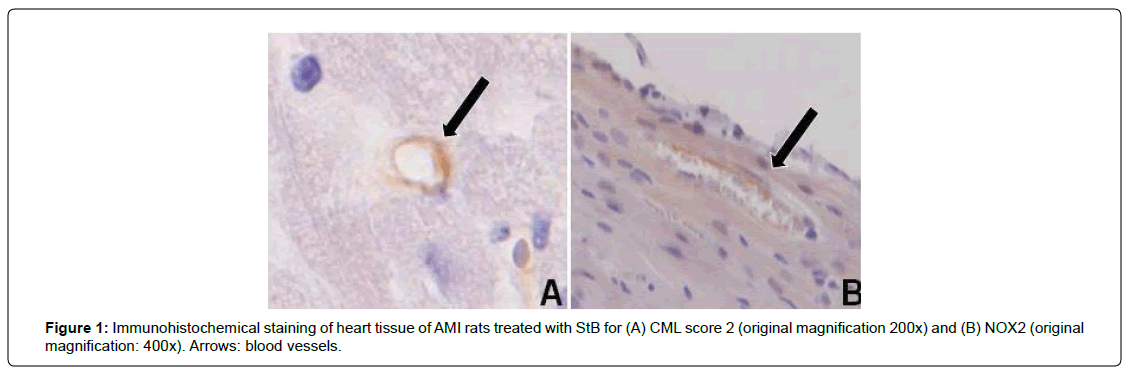
CML depositions in the intramyocardial vasculature (Figure 1) were quantified at day 42 post-AMI in the ventricles. AMI of the anterior wall induced a significant increase in CML IH score(from 0.08 ± 0.01 /mm2 in controls up to 0.3 ± 0.05/mm2 in the AMI group (p<0.05, Figure 2). In all three StB groups, the CML IH-score was significantly lower than in untreated AMI rats ( 0.13 ± 0.03 (StBtreatment on day 1 post-AMI), 0.17 ± 0.02 (StB-treatment on day 7 post-AMI) and 0.13 ± 0.01 (StB- treatment on days 1+7 post-AMI)). We found no significant differences between the different StB treated groups, neither between the three StB treated groups and the control group.
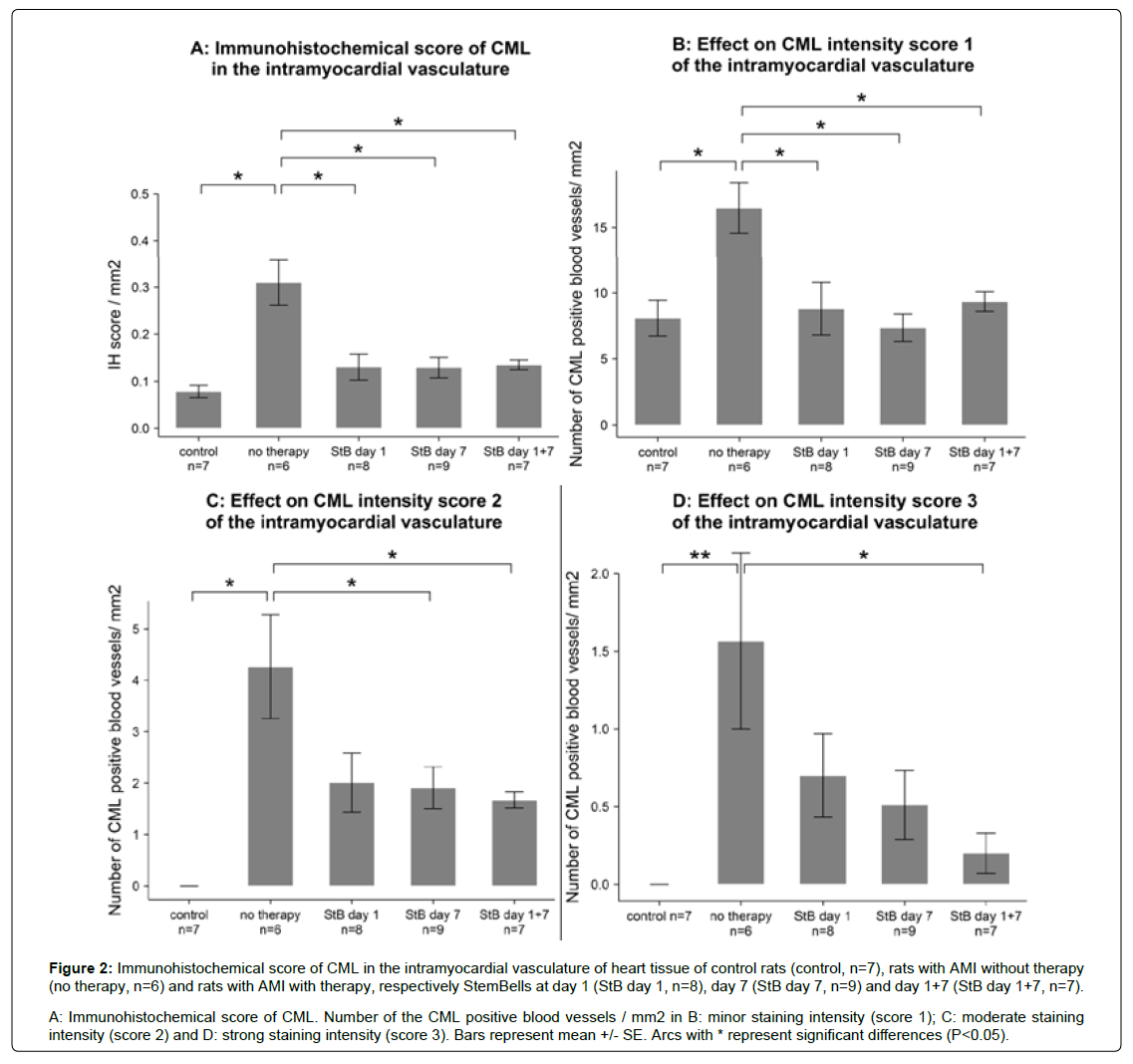
Figure 2: Immunohistochemical score of CML in the intramyocardial vasculature of heart tissue of control rats (control, n=7), rats with AMI without therapy (no therapy, n=6) and rats with AMI with therapy, respectively StemBells at day 1 (StB day 1, n=8), day 7 (StB day 7, n=9) and day 1+7 (StB day 1+7, n=7).
A: Immunohistochemical score of CML. Number of the CML positive blood vessels / mm2 in B: minor staining intensity (score 1); C: moderate staining
intensity (score 2) and D: strong staining intensity (score 3). Bars represent mean +/- SE. Arcs with * represent significant differences (P<0.05).
We subsequently analyzed the effects of StB therapy on the number of blood vessels positive for a particular CML intensity score (Figure 2). AMI induced a significant increase in the numbers of weak, moderate and strong CML-positive blood vessels. The number of weak CML-positive vessels with a score 1 was significantly reduced to control levels in all three StB therapy groups, without differences between the three StB groups (Figure 2). The number of moderate CML-positive vessels was decreased significantly only by StB treatment on day 7 and on days 1+7 post-AMI. Finally, the number of strong CML-positive vessels was only significantly reduced by StB treatment on days 1+7 post AMI.
NOX2 positive blood vessels
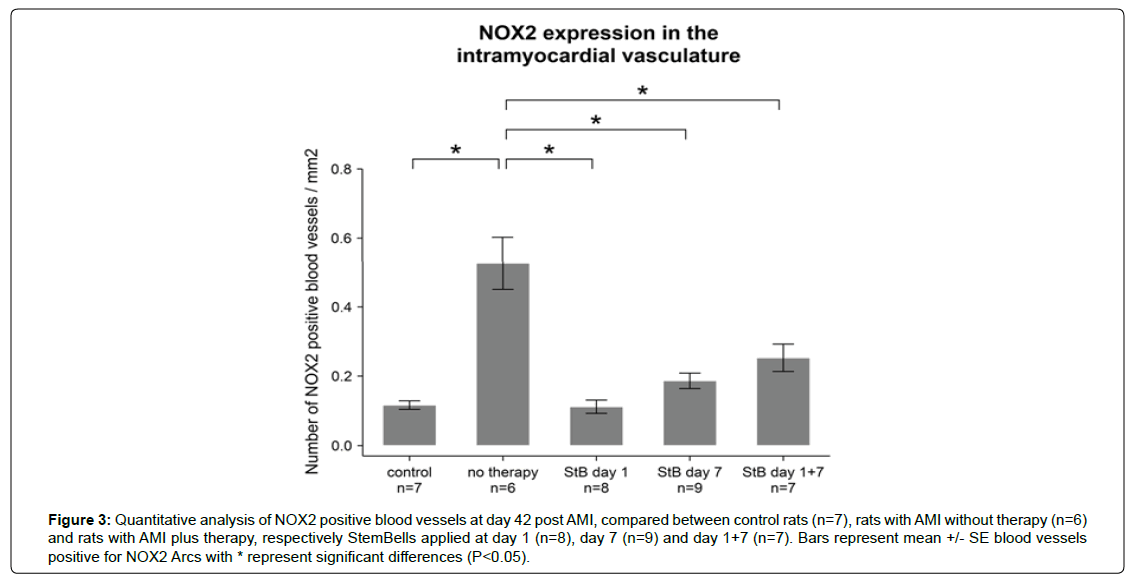
AMI induced a significant increase in the number of NOX2- positive blood vessels from 0.1 ± 0.01 in non-infarcted control rats to 0.53 ± 0.08 in AMI rats (Figures 1 and 3). In all three StB therapy groups the number of NOX2 positive blood vessels decreased significantly (to 0.11 ± 0.02 (StB-treatment on day 1 post-AMI), 0.19 ± 0.02 (StB-treatment on day 7 post-AMI) and 0.25 ± 0.04 (StB-treatment on days 1+7 post-AMI)). No significant differences between the three StB groups were measured. The number of NOX2 positive blood vessels in the three StB groups was not significantly different compared with control rats.
Figure 3: Quantitative analysis of NOX2 positive blood vessels at day 42 post AMI, compared between control rats (n=7), rats with AMI without therapy (n=6) and rats with AMI plus therapy, respectively StemBells applied at day 1 (n=8), day 7 (n=9) and day 1+7 (n=7). Bars represent mean +/- SE blood vessels positive for NOX2 Arcs with * represent significant differences (P<0.05).
Discussion
AMI can result in heart failure in which post-AMI inflammation is playing an important role [20,21]. In the present study we have found that AMI induced an increased accumulation of CML and of NOX2 in the intramyocardial vasculature. This increase was significantly decreased by StemBell (StB) therapy, irrespective of the time point of StB application.
Previously we have shown that microvascular CML levels were increased in the heart of rats on day 5 after AMI [18]. In the present study we observed increased levels of CML and NOX2 in the small intramyocardial blood vessels 42 days after the induction of AMI, indicating that this AMI-induced increase in CML is present for an extended time. Similarly, in patients who died from AMI, increased CML levels in the intramyocardial arteries were observed both in acute and chronic phase after AMI [18]. Moreover, as was found previously in the rat and patient study [18], the increased presence of CML, as well as NOX2, was not limited to the infarct area but was observed throughout the ventricles in the non-infarcted myocardium. In patients with diastolic heart failure CML levels were increased in the cardia microvasculature [22] and were associated with the clinical severity and outcome in heart failure patients [23,24]. Also, endothelial-specific overexpression of NOX2 was shown to induce hypertrophy, fibrosis in angiotensin II-treated mice leading to diastolic dysfunction [25]. Taken together, these observations may point to a role for microvascular CML and NOX2 in the chronic remodeling of the heart after AMI.
Mesenchymal Stem Cells secrete paracrine factors, which can reduce cell death, fibrosis and inflammation after AMI [26-28]. We now found that STBs decreased the AMI-induced pro-inflammatory status of the intramyocardial vasculature [18,29,30] as detected/ indicated by a decrease of CML and NOX2 positivity in the intramyocardial blood vessels. We analyzed heart tissue from a previous rat study in which we found that StB therapy improved cardiac function without having an effect on infarct size [15]. The finding that StB therapy did reduce both CML and NOX2 in the intramyocardial vasculature could indicate that the improvement of cardiac function by STB is due, at least partly, by a reduction of the pro-inflammatory status of the heart after AMI. Indeed, in a clinical study a correlation was found between elevated serum CML levels and the severity and mortality in heart failure, at least in diabetes [24]. Furthermore, it was shown that individuals with a high AGE serum levels have an 5-fold increased risk of post-infarction HF [31,32]. The observed reduction of NOX2 positive blood vessels by StBs therapy in theory could also explain its beneficial effect on heart function.
The long-term cardiac function improvement of StB administration in a rat AMI model was independent of the time point of StB administration [16]. Although it was suggested that application of stem cells at an early time point post AMI is less effective related to the detrimental cardiac inflammatory response [33,34]. We found however that even early application of StB therapy resulted in decreased NOX2 expression and CML immunohistochemical score. Indeed, Richardson [35] also demonstrated in a rat model of AMI that repeated bone marrow-derived MSC intervention in both the acute (immediately after AMI) and the sub-acute period (1 week after AMI) resulted in additional improvement of ventricular function beyond single dose, including an increase in arteriolar density in the infarct zone and remote segments.
Conclusion
In conclusion, we show that early StB application reduced CML and NOX2 positivity of the intra myocardial vasculature and as such might prevent heart failure development post MI.
References
- Mackay J, Mensah GA (2004) The atlas of heart disease and stroke. The atlas of heart disease and stroke. World Health Organization.
- Roger VL, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. (2011) Heart disease and stroke statistics-2011 update. Circulation. Am Heart Assoc 123: 18-209.
- Furth AJ (1997) Glycated proteins in diabetes. British J Biomed Sci 54: 192–200.
- Schleicher ED, Wagner E, Nerlich AG (1997) Increased accumulation of the glycoxidation product N (epsilon)-(carboxymethyl) lysine in human tissues in diabetes and aging. J Clin Invest Am Soc Clin Investig 99: 457–68.
- Nakamura Y, Horii Y, Nishino T, Shiiki H, Sakaguchi Y, et al. (1993) Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. The American journal of pathology. Am Society for Investi Pathol 143:1649.
- Nerlich AG, Schleicher ED (1999) Ne-(carboxymethyl) lysine in atherosclerotic vascular lesions as a marker for local oxidative stress. Atherosclerosis. Elsevier 144: 41-7.
- Kislinger T, Huber B, Qu W, Taguchi A, Du Yan S, et al. (1999) N e-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Bio Chem ASBMB 274: 31740-9.
- Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, et al. (2006) Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. Am Heart Assoc 113: 1235–43
- Guimarães EL, Empsen C, Geerts A, van Grunsven LA (2010) Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepato 52:389-97.
- Varghese J, Griffin M, Mosahebi A, Butler P (2017) Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem cell research & therapy. BioMed Central 8: 45.
- Müller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, et al. (2006) Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Molec Cellular Cardio 41: 876–84
- Van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, et al. (2009) Comparison of transplantation of adipose tissue-and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. NIH Public Access 87: 642.
- Bertolini F, Lohsiriwat V, Petit JY, Kolonin MG (2012) Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1826: 209-14.
- Kokhuis T, Skachkov I, Naaijkens B, Juffermans L, Kamp O, et al. (2015) Intravital microscopy of localized stem cell delivery using microbubbles and acoustic radiation force. Biotechnology and bioengineering. Wiley Online Library 112: 220–7.
- Woudstra L, Krijnen P, Bogaards S, Meinster E, Emmens R, et al. (2016) Development of a new therapeutic technique to direct stem cells to the infarcted heart using targeted microbubbles: StemBells. Stem Cell Res 17: 6-15.
- Emmens RW, Oedayrajsingh-Varma M, Woudstra L, Kamp O, Meinster E, et al. (2017) A comparison in therapeutic efficacy of several time points of intravenous StemBell administration in a rat model of acute myocardial infarction. Cytotherapy 19: 131-40.
- Van Dijk A, Naaijkens B, Jurgens W, Nalliah K, Sairras S, et al. (2011) Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res 7: 219–29.
- Baidoshvili A, Krijnen P, Kupreishvili K, Ciurana C, Bleeker W, et al. (2006) Ne- (Carboxymethyl)lysine Depositions in Intramyocardial Blood Vessels in Human and Rat Acute Myocardial Infarction: A Predictor or Reflection of Infarction? Arteriosclerosis, thrombosis, and vascular biology. Am Heart Assoc 26: 2497–503.
- Krijnen P, Meischl C, Hack C, Meijer C, Visser C, et al. (2003) Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol. BMJ Publishing Group Ltd and Association of Clinical Pathologists 56: 194-9.
- Lassègue B, San Martin A, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circulation research. Am Heart Assoc 110:1364–90.
- Zhou H, Shi C, Hu S, Zhu H, Ren J (2018) BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. Springer 1–17.
- Van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, et al. (2008) Diastolic Stiffness of the Failing Diabetic Heart Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation Am Heart Assoc 117: 43-51.
- Hartog JW, Voors AA, Schalkwijk CG, Scheijen J, Smilde TD, et al. (2007) Clinical and prognostic value of advanced glycation end-products in chronic heart failure. Eur Heart J Oxford University Press 28: 2879–85.
- Willemsen S, Hartog JW, van Veldhuisen DJ, van der Meer P, Roze JF, et al. (2012) The role of advanced glycation end-products and their receptor on outcome in heart failure patients with preserved and reduced ejection fraction. Am Heart J 164: 742–9.
- Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, et al. (2014) Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J Am College of Cardio 63: 2734-41.
- Karantalis V, Hare JM (2015) Use of mesenchymal stem cells for therapy of cardiac disease. Circulation research. Am Heart Assoc 116: 1413–30.
- Peng Y, Pan W, Ou Y, Xu W, Kaelber S, et al. (2016) Extracardiac-Lodged mesenchymal stromal cells propel an inflammatory response against myocardial infarction via paracrine effects. Cell transplantation. SAGE Publications Sage CA 25: 929–35.
- Yang D, Wang W, Li L, Peng Y, Chen P, et al. (2013) The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. Public Library Sci 8: 59020.
- Basta G, Schmidt AM, De Caterina R (2004) Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res 63: 582–92.
- Sirker A, Murdoch CE, Protti A, Sawyer GJ, Santos CX, et al. (2016) Cell-specific effects of Nox2 on the acute and chronic response to myocardial infarction. J Molec Cellular Cardio 98:11–7.
- Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circulation research. Am Heart Assoc 107: 1058–70.
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, et al. (2002) Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation Am Heart Assoc 105: 816–22.
- Bodi V, Sanchis J, Nunez J, Mainar L, Minana G, et al. (2008) Uncontrolled immune response in acute myocardial infarction: unraveling the thread. Am Heart J 156: 1065–73.
- Wang QD, Sj?quist PO (2006) Myocardial regeneration with stem cells: pharmacological possibilities for efficacy enhancement. Pharmacolo Res 53: 331–40.
- Richardson JD, Psaltis PJ, Frost L, Paton S, Carbone A, et al. (2014) Incremental benefits of repeated mesenchymal stromal cell administration compared with solitary intervention after myocardial infarction. Cytotherapy 16: 460–70.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi