Research Article, J Virol Antivir Res Vol: 10 Issue: 2
In-vitro Antiviral Activity of ZingiVir-H, a Novel Herbomineral Drug against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Hareendran Nair J and Shan Sasidharan*
Department of Research and Development, Pankajakasthuri Ayurveda Medical College Campus, Kerala, India.
*Corresponding Author:Sasidharan S
Department of Research and Development, Pankajakasthuri Ayurveda Medical College Campus, Kerala, India.
E-mail: drshansasidharan@yahoo.co.in
Received date: 21 February, 2022; Manuscript No. JVA-22-55130;
Editor assigned date: 22 February, 2022; PreQC No JVA-22-55130(PQ);
Reviewed date: 04 March, 2022; QC No JVA-22-55130;
Revised date: 16 March, 2022; Manuscript No. JVA-22-55130(R);
Published date: 23 March, 2022; DOI: 2324-8955/jva.1000651
Citation: Sasidharan S (2022) In-vitro Antiviral Activity of ZingiVir-H, a Novel Herbomineral Drug against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J Virol Antivir Res 11:2.
Keywords: Antiviral activity; Covid-19; Herbomineral drug; SARS-CoV-2; ZingiVir-H
Keywords
Antiviral activity; Covid-19; Herbomineral drug; SARS-CoV-2; ZingiVir-H
Introduction
A case of pneumonia caused by a viral infection was diagnosed in Wuhan, China, at the end of 2019 [1]. The pathogen was identified as a novel enveloped RNA betacoronavirus2, now known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which shares a phylogenetic relationship with SARS-CoV. Since then, the Coronavirus SARS-CoV-2, also known as Covid-19, has caused a global pandemic [2,3]. The Coronavirus Disease 2019 (Covid-19) has led to more than 100 million infections and over 2.1 million deaths worldwide, and more than 25 million infections and over 4,29,000 deaths in the US alone as of 28 January in the year, rendering it one of the most life-threatening infectious disease outbreaks in human history.
Covid-19 infection causes severe pneumonia, characterized by symptoms such as fever, a persistent cough, and progressive breathing failure associated with respiratory complications. The high hospitalization rate, risk of mortality, and lack of a specific established treatment made it critical to develop an effective Covid-19 therapy [4]. Since there is no specific Covid-19 medication available in clinics, supportive care and on occasion, combination therapy with wide-spectrum antiviral drugs and corticosteroids remains the mainstay of standard clinical treatment to manage this illness [5]. Hence, it's far urgent to identify more effective therapeutic options in response to the quick propagation of SARS-CoV-2. As future coronavirus outbreak is highly possible, it is desired to develop broad-spectrum antivirals suitable for the prevention and treatment of current circulating CoVs and future emerging CoVs. As a result, it is critical to find novel medications, particularly antivirals that can effectively treat this prevalent SARS-CoV-2 outbreak [6].
Natural — derived metabolites constantly become a worthy therapeutic alternative against several upcoming ailments, including viral infections, because they are innately better tolerated in the human body. According to a study, natural products or their derivatives accounted for 49% of all small molecules approved by the US Food and Drug Administration (FDA) between 1940 and 2014 [7]. Recently Pankajakasthuri herbals India has formulated ZingiVir-H drug. Furthermore, a pilot clinical trial of ZingiVir-H demonstrated significant efficacy and safety in hospitalized adults diagnosed with viral infection [8]. Two different toxicity studies on ZingiVir-H and its ingredients revealed that the drug is safe and free from any toxic effects [9,10]. The ingredients used for the formulation of ZingiVir-H are provided in [Table 1].
| Sl. No | Scientific name | Form used | Quantity/ |
|---|---|---|---|
| 500 mg | |||
| 1 | Eugenia caryophyllus | Dried clove bud powder | 55 mg |
| 2 | Zingiber officinale | Fresh rhizome juice and aqueous extract | 200 mg |
| 3 | Cyperus rotundus | Dried roots and rhizomes powder | 35 mg |
| 4 | Hedyotis corymbosa | Dried whole plant powder | 30 mg |
| 5 | Trachyspermum ammi | Dried fruit powder | 60 mg |
| 6 | HgS (Mercuric sulphide) | Purified and processed as per texts. | 20 mg |
| 7 | As2S3 (Arsenic trisulphide) | Purified and processed as per texts | 10 mg |
| 8 | Starch | - | 90 mg |
Table 1: Composition of ZingiVir-H tablets.
In this context, we sought to assess the in vitro antiviral activity of ZingiVir-H against SARS-CoV-2 and, on top of that, could be readily available for further pre-clinical and clinical evaluation.
Materials and Methods
Materials
The known antiviral drug, remdesivir was purchased from Med-Chem Express and dissolved in DMSO before use. All other reagents and solvents used in this study were of the highest quality available. Milli-Q water was used in all experiments.
ZingiVir-H-study drug
The study drug is ZingiVir-H, a herbomineral Ayurvedic preparation in tablet form. ZingiVir-H (500 mg) tablets are produced at a Good Manufacturing Practices (GMP) approved production line at Pankajakasthuri Herbals India Pvt. Ltd. situated at Poovachal, Thiruvananthapuram, and Kerala, India. The ingredients used for formulating ZingiVir-H were provided in Table 1.
Virus and cell collection
The clinically isolated SARS-CoV-2 strain was maintained in production in Vero E6 cells (American type culture collection ATCC CRL-1586.) in Dulbecco’s Modified Eagle’s Medium (DMEM) with 4% of fetal bovine serum and 1% glutamine (complete medium). Vero cells were used to inoculate the virus against the SARS-CoV-2 strain. Cells were seeded in a 12-well microplate at a density of 5 cells/well × 104 cells/well and cultured in DMEM (Gibco, USA) containing 10% foetal bovine serum (Gibco, USA), 1% penicillin-streptomycin (Gibco, USA), and 1% amphotericin-B (Gibco, USA) (Gibco, USA). Cells were cultured for 24 hours in a CO2 incubator at 37°C in a humidified atmosphere of 5% CO2 in order to achieve 80%-90% confluence.
Preparation of ZingiVir-H solution for the study
Each tablet containing active drugs was triturated and mixed until homogenous. Approximately 50 mg equivalent mass of drugs was weighed and added to dimethyl sulfoxide to solubilize the drugs. The suspension was sonicated for 15 minutes in a water bath before being added to Rosewell Park Memorial Institute (RPMI) media, sonicated again, and vortexed to mix until homogeneous. Under aseptic conditions, the suspension was filtered through a polycarbonate membrane with a pore size of 0.45 m and then a pore size of 0.22 m. The filtrate was vortexed with 10% foetal bovine serum and penicillin-streptomycin to create a homogeneous mixture for use as a stock solution. The samples were made by diluting each drug's stock solution with RPMI complete media at an appropriate level of dilution to produce a determined concentration.
Cytotoxicity assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) colorimetric assay is a reliable measure of cell viability, was used to determine the cytotoxicity of the test drug. The basic idea behind this assay is that mitochondrial succinate dehydrogenase reduces yellow MTT dye to an insoluble, coloured (dark purple) formazan product. The purple formazan-containing cells are then solubilized in an organic solvent such as DMSO, releasing the solubilized formazan reagent, which is then measured using spectrophotometry. The assay was carried out based on the following protocol. In brief, ten-fold serial dilutions (1000 μg/ml to 50 μg/ml) of the ZingiVir-H were made with the maintenance. Monolayers of Vero cells were seeded into each well of a 96-well microplate and treated with various concentrations of ZingiVir-H. Plates were incubated at 37°C in 5% CO2 humidified incubator for 72 h. The plates were then examined under a microscope for signs of cell death Cyto Pathic Effect (CPE). The maximum non-toxic concentration was defined as the lowest dilution of ZingiVir-H that had no toxic effect on cells. Following the incubation period, the old medium was removed and 25 µl of MTT solution (2 mg/ml) was added to each well before incubating for 2 hours at 37°C. After that, the MTT solution was removed from the wells and DMSO (75 μl) was added to dissolve formazan crystal. Optical Density (OD) was measured spectrophotometrically at 490 nm. The experiment was conducted in a triplicate set and the 50% Cytotoxic Concentration (CC50) was calculated based on the reading obtained.
Antiviral activity assay of ZingiVir-H
The antiviral activity assay was performed as per the protocol routinely followed for determining antiviral activity against SARS-CoV-2. A known inhibitor of SARS-CoV-2 (Remdesivir) was used as a positive control in the assay. To check the antiviral activity, Vero E6 cells were seeded in 96-well plates at 80% confluency and then infected with the SARS-CoV-2 isolate at a Multiplicity of Infection (MOI) of 0.1 h for 2 h. Subsequently, the inoculum was aspirated and fresh media containing different concentrations of the ZingiVir-H (25 µg/ml, 50 µg/ml, 100 µg/ml, 200 µg/ml, 300 µg/ml and 400 µg/ml) was added to the cells. Each concentration was assayed in triplicates. ZingiVir-H showing more than 50% anti-SARS-CoV2 activity was considered for EC50 determination. At 24 h post-infection, the supernatant and cells were subjected to viral RNA isolation followed by qRT-PCR to determine the SARS-CoV-2 viral load in the cells (cell associated) and culture supernatants (released virus particles), qRT-PCR was performed using primers specific for the viral spike, nucleocapsid and ORF1a for both released and cell-associated viruses.
Statistical Analysis
All experiments were performed in triplicate sets. Data are shown as mean ± S.D. all the statistical analysis for this study was conducted using Graph Pad Prism Software 8.0 (Graph Pad Software Inc., La Jolla, CA, USA).
Results
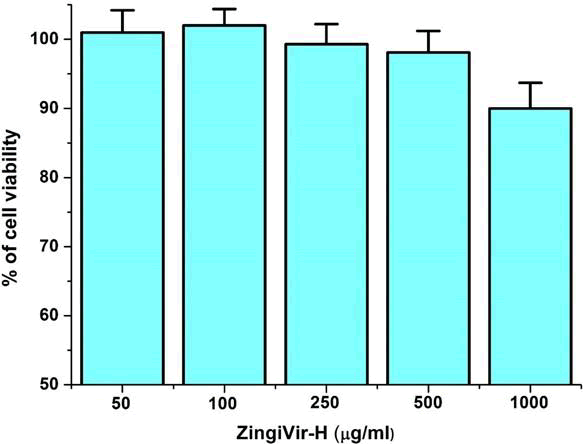
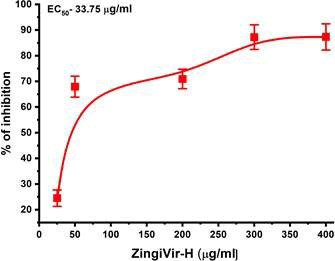
Before conducting the antiviral assay, we first conduct the cytotoxicity of ZingiVir-H in Vero cell lines by MTT assay. The ZingiVir-H was not toxic to the cells at concentrations upto at least 500 µg/ml. The cell viability at this concentration was 98.1% ± 3.1% relative to untreated cells (Figure 1). In our study ZingiVir-H produced dose-dependent inhibition of viral replication, with an EC50 (concentration producing 50% inhibition of viral replication) of about 33.75 µg/ml) (Figure 2).
Discussion
The Covid-19 pandemic has gravely illustrated the need for counter measures against emerging epidemic and pandemic CoVs. Broad-spectrum antiviral drugs, antibodies, and vaccines are needed to combat the current pandemic and those that will emerge in the future. Given the magnitude and devastation of the current Covid-19 outbreak, as well as the ongoing threat of CoVs causing human disease, there is an urgent need to develop effective and safe therapies to treat these patients. There are currently no approved therapies for CoVs, including SARS-CoV-2. The experimental therapies used in conjunction with known antiviral agents have either limited efficacy (remdesivir) or high systemic toxicity (hydroxychloroquine), limiting their usefulness [11]. Finding new therapies that are both effective and safe is critical.
Currently, no specific therapy such as relevant antiviral drugs is available for COVID-19. A variety of traditional medicinal plants and herbs have been shown to have antiviral activity against various viruses. Herbs can provide valuable sources of components with immune modulatory, anti-inflammatory, anti-oxidative, and antiviral properties, resulting in positive effects on virus-affected systems [12].
Ginger one of the major components of ZingiVir-H displays direct antiviral effects and can have a protective role against ARDS [13,14], which is the major cause of mortality in patients with severe COVID-19. Thus, ginger can have several advantageous effects on many organs that are known to affect by the COVID-19 virus. S. Aromaticum another ingredient used for formulating ZingiVir-H is rich with eugeniin. At a concentration of 5 g/mL, this compound was identified as an anti-herpes simplex virus compound. Eugeniin inhibits viral DNA synthesis by acting as a selective inhibitor of the HSV1 DNA polymerase, and eugenol inhibits viral replication and infection by acting as a selective inhibitor of the HSV1 DNA polymerase [15,16].
A report on the modulation of CYP3A4 enzyme by the rhizome fractions of C. Rotundus suggests its safe consumption concerning drug metabolism and efficacy. The study also provided the basis for the hepatoprotective and Hepatitis B Virus (HBV) inhibitory activity of C. Rotundus [17]. C. Rotundus was rich in β-amyrin and stigmasta-5, 22-dien-3-ol. β-amyrin and stigmasta-5, 22-dien-3-ol-exhibited the best binding interactions and stability. Finally, Absorption Distribution Metabolism Excretion and Toxicity (ADMET) studies were carried out to better understand the compounds' pharmacokinetic properties and safety profile. Overall, the results show that phytochemicals derived from Cyperus rotundus Linn, specifically amyrin and stigmasta-5, 22-dien-3-ol, can be tested as potential inhibitors of SARS-CoV-2 Mpro [18].
Trachyspermum ammi, another ingredient inhibited viral protease enzymes in Hepatitis C Virus (HCV) infection. In addition to this Roy et al. also reported the antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. From all these, it is clear that potent herbs with strong antiviral principles formulated ZingiVir-H. The results of our also clearly revealed that ZingiVir-H has strong in-vitro antiviral activity.
Conclusion
To summarize our findings, we demonstrated ZingiVir-antiviral H's activity against SARS-CoV-2. More research is being conducted to isolate and elucidate the bioactive components responsible for ZingiVir-antiviral H's activity, as well as to determine their mechanism of action. To the best of our knowledge, this is the first report of antiviral activity of ZingiVir-H, a herb mineral ayurvedic drug, and it suggests that ZingiVir-H may have some benefits in the treatment of infectious diseases, warranting further research.
Acknowledgement
The authors express their gratitude to "Aadhyathma Chinthalayesan" of Chinthalaya Ashram in Pothencode, Trivandrum, Kerala, India, for his kindness and blessings. We are grateful to Pankajakasthuri Herbal Research Foundation, Kattakada, Thiruvananthapuram, Kerala, India, and Pankajakasthuri Herbals India Pvt. Ltd Poovachal, Kattakada, Thiruvananthapuram, Kerala, India, for assisting us with the research. We also want to express our gratitude to all of the directors and employees of Pankajakasthuri Herbal Research Foundation and Pankajakasthuri Herbals India Pvt. Ltd for their assistance in completing this project. The authors sincerely thank The Director, Institute of Life Sciences (ILS), Bhubaneswar Odisha, and India for providing the necessary infrastructure for carrying the antiviral studies.
References
- Chaolin H, Yeming W, Xingwang L, Lili R, Jianping Z, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506. [Cross Ref][Google Scholar][Indexed]
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395: 565-574. [Cross Ref][Google Scholar][Indexed]
- Na Z, Dingyu Z, Wenling W, Xingwang L, Bo Y, et al. (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733. [Cross Ref][Google Scholar][Indexed]
- Purwati, Andang M, Nasronudin, Eryk H, Deya K, et al. (2021) An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia. PLoS One 16: e0252302. [Cross Ref][Google Scholar][Indexed]
- Michelle LH, Chas DB, Scott L, Kathy HL, John W, et al (2020) First case of 2019 novel coronavirus in the United States. N Engl J Med 382: 929-936. [Cross Ref][Google Scholar][Indexed]
- Marco C, Michael R, Abdul A, Scott CD, Raffaela DN (2022) Features, evaluation, and treatment of coronavirus (COVID-19). In: Stat Treasure Island (FL): Stat Pearls Publishing. [Cross Ref][Google Scholar][Indexed]
- Namita AS, Pradeep K, Jyoti, Naresh Kumar (2021) Spices and herbs: Potential antiviral preventives and immunity boosters during Covid‐19. Phytother Res 29 : 10. [Cross Ref][Google Scholar][Indexed]
- Hareendran NJ, Rajani AN, Sreekumar GS, Sheeja C, Parvathy SP, et al. (2021) A pilot clinical study to evaluate the safety and efficacy of zingivir-h, herbo-mineral drug in patients with viral fever. Curr Res Complement Altern Med 5: 144. [Cross Ref][Google Scholar][Indexed]
- Xia X, Conghui W, De Chang, Ying W, Xiaojing D, et al. (2020) Identification of potent and safe antiviral therapeutic candidates Against SARS-CoV-2. Front Immunol 11: 586572. [Cross Ref][Google Scholar][Indexed]
- Ahmad A, Rehman MU, Alkharfy KM (2020) An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. Eur Rev Med Pharmacol Sci 24: 4030-4034. [Cross Ref][Google Scholar][Indexed]
- Vahdat SZ, Mokhtari M, Taleban FA, Alavi F, Mohamad HSS, et al (2013) Effect of enteral feeding with ginger extract in acute respiratory distress syndrome. J Crit Care 28: 217.e1-6. [Cross Ref][Google Scholar][Indexed]
- Xie X, Sun S, Zhong W, Soromou LW, Mohamad HSS, et.al. (2014) Zingerone attenuates lipopolysaccharide induced acute lung injury in mice. Int Immunopharm 19: 103-109. [Cross Ref][Google Scholar][Indexed]
- Kurokawa M, Hozumi T, Basnet P, Nakano M, Kadota S, et.al. (1988) Purification and characterization of eugeniin as an antiherpesvirus compound from Geum japonicum and Syzygium aromaticum. J Pharmacol Exp Ther 284: 728-735. [Cross Ref][Google Scholar][Indexed]
- Reichling J, Schnitzler P, Suschke U, Saller R (2009) Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties–an overview. Forsch Komplement Med 16: 79–90. [Cross Ref][Google Scholar][Indexed]
- Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (Covid-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. J Am Med Assoc 323: 1239-1242. [Cross Ref][Google Scholar][Indexed]
- Kumar SB, Krishna S, Pradeep S, Mathews DE, Ramya P, et.al. (2021) Screening of natural compounds from Cyperus rotundus Linn against SARS-CoV-2 main protease (Mpro): An integrated computational approach. Comput Biol Med 134: 104524. [Cross Ref][Google Scholar][Indexed]
- Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, et.al. (2000) Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res 14: 510–516. [Cross Ref][Google Scholar][Indexed]
- Roy S, Chaurvedi P, Chowdhary A (2015) Evaluation of antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. Phcog Res 7: 263-7. [Cross Ref][Google Scholar][Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi