Case Report, Jceog Vol: 9 Issue: 3
Iron Overload in the Setting of H63D Gene Mutation and Inrahepatic Cholangiocarcinoma
Fateh Numan1, Roy Kondepati2, Pamela2, Wang Yichen2 and Limgala Prasanthi2
1Geisinger Community Medical Center, Scranton, USA.
2Wright Center for Graduate Medical Education, Scranton, USA.
*Corresponding Author: Numan Fateh
Department of Geisinger Community Medical Center 1800 Mulberry Ave, Scranton, PA 18510, USA
Tel: + 5183641197
E-mail: numanaf@yahoo.com
Received: May 25, 2020 Accepted: June 10, 2020 Published: June 17, 2020
Citation: Numan F, Kondepati R, Pamela, Yichen W, Prasanthi L (2020) Iron Overload in the Setting of H63D Gene Mutation and Inrahepatic Cholangiocarcinoma. J Clin Exp Oncol 9:3. doi: 10.37532/jceog.2020.9(3).244
Abstract
Hereditary hemochromatosis (HH) is characterized by progressive iron deposition and tissue injury secondary to inappropriate intestinal iron absorption. The H63D mutation is a relatively rare cause of symptomatic HH. Heterozygous H63D patients rarely show disease symptoms. The risk of hepatic malignancy as a consequence of HH is largely prevalent in the setting of cirrhosis. Cholangiocarcinoma is the second most common hepatic malignancy. Intrahepatic Cholangiocarcinoma (ICC) secondary to heterozygous H63D HH in the setting of secondary iron overload is a very rare occurrence. This patient was found to have iron overload with H63D heterozygous mutation and cirrhosis. This was a suspicious finding given this usually benign mutation. A CT of the abdomen revealed intrahepatic cholangiocarcinoma. We present a case of this patient with H63D heterozygous`mutation who developed iron overload as the first sign of cholangiocarcinoma. Treatment of the malignancy lead to improvement in the ferritin level.
Keywords: H63D; Intrahepatic Cholangiocarcinoma; Iron overload
Abbreviations
HH, Hereditary Hemochromatosis; ICC, Intrahepatic Cholangiocarcinoma; HCC, Hepatocellular Carcinoma.
Case report
Patient is a 69-year-old male referred from his primary care physician’s office to the Hematology Clinic for evaluation and management of elevated iron/ferritin. His past medical history is significant for alcohol dependence, anemia, asthma, chronic obstructive pulmonary disease, esophageal reflux and tobacco abuse disorder. His family history included colon cancer in his mother at age 64, chronic obstructive pulmonary disease in his father and muscular dystrophy in 1 of his 2 sons. His social history included a smoking history of 30 pack-year, an alcohol consumption history of six to seven beers per day, and an occupational history as a retired transport company worker.
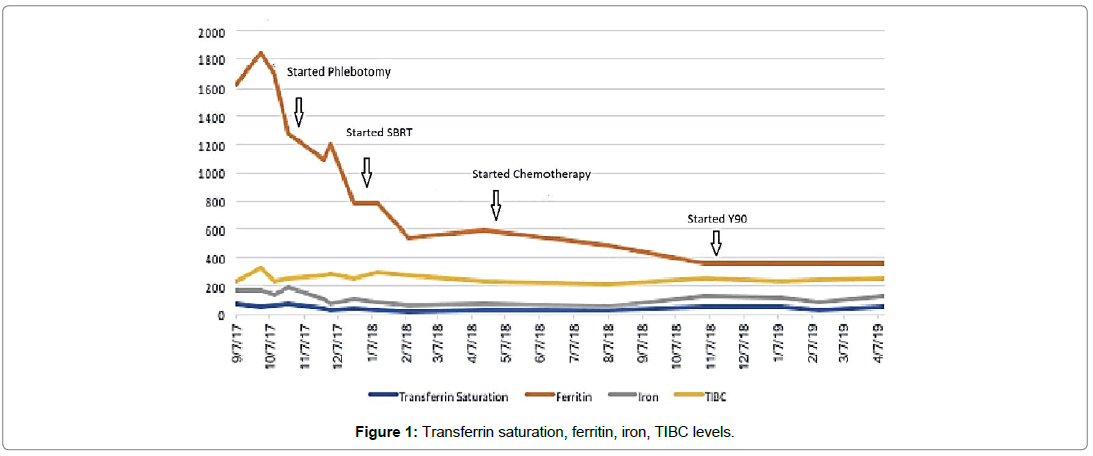
The patient’s ferritin level was 1625 ng/mL, serum iron was iron 170 ug/mL and iron saturation 73%. On recheck the same month the iron saturation was 50% with rising ferritin at 1839 ng/ mL. Acute hepatitis panel was negative, and alpha fetal protein was within normal range. Patient also reported a history of leukopenia and thrombocytopenia for the past 20 years. Per patient this has been worked up, but all workup had been negative. Hemochromatosis gene testing revealed the patient was heterozygous for H63D mutation. He was started on therapeutic phlebotomy. MRI of the liver revealed a 4.8 cm mass in the posterior right lobe of the liver. PET-CT showed a 5.2 by 4.0 centimeter ill-defined mass in the right posterior lobe of the liver with mild fluorodeoxyglucose (FDG) avidity, no metastatic disease was seen. After multidisciplinary tumor board discussion, the patient went on to complete stereotactic radiation therapy to the liver mass (total radiation absorbed dose: 4500 centigray). At this point portal vein embolization was held and decision to reassess in 2 months was made. After about a 6-8 weeks recovery period, the possibility of surgical resection of the tumor was evaluated by surgical oncology. However, due to surrounding cirrhosis, it was felt that resection was not safe. The patient instead underwent needle biopsy of segment VII intrahepatic cholangiocarcinoma with microwave ablation of segment eight. The biopsies again showed adenocarcinoma in the background of cirrhosis. Patient went on to complete chemotherapy with cisplatin and gemcitabine for six cycles. He then underwent yttrium-90 radioembolization to segment VII of right hepatic lobe. With treatment of the patients underlying malignancy we noted improvement in his iron studies (Figure 1).
Discussion
Common causes of iron overload include hemochromatosis, sideroblastic anemia, and patients with medical conditions that require repeated red blood cell transfusions. We believe there are three contributing factors to the patient’s iron overload status in our case: excessive enteral iron ingestion, heterozygous H63D gene mutation, and chronic liver disease. To our knowledge, none of these factors by itself would typically cause significant ferritin elevation and iron-induced tissue parenchymal damage, therefore the cause of the clinical scenario is likely multifactorial.
The daily requirement for iron in male adults without iron absorption disorder or active blood loss is 0.9 mg/day. Our patient ingests ferrous sulfate 325 mg daily, which consists of 65 mg of elemental iron, far exceeding the daily iron requirement [1]. Over 20 years, he ingested 475 grams of inorganic iron as supplements. Excessive iron supplementation can lead to increased iron storage, which is evident by studies of professional road cyclists, a population among which the use of iron supplements to boost competition performance is common [2,3]. Iron overload caused by excessive enteral iron ingestion in patients without underlying genetic iron metabolism disorder is rare but has been reported. In a case series, J. Barton et. al reported three cases of iron overload and prolonged ingestions of iron supplements without C282Y, H63D, or S65C gene mutations [4]. A few additional case reports had been reported in pre- HFE era (before the year of 1996); however, gene status in these cases were unknown [5-9].
A heterozygous H63D gene mutation is generally not considered a cause of a clinically significant iron accumulation disease. For example, in a cross-sectional study of 35,372 U.K. women aged 35- 69 in 1995, H63D heterozygotes, as well as H63D homozygotes have similar serum ferritin concentrations to wild type [10,11]. However, this has not taken alcohol abuse into consideration. Excessive alcohol consumption has long been recognized as a major contributor to symptomatic hemochromatosis in patients with HFE gene mutations [12].The mechanism of this is believed to be a cumulative effect of the hepatic oxidative effect of iron and alcohol [13]. In our case, the patient had been consuming more than five drinks a day for many years, which in combination with excessive iron ingestion, can potentially contributed to his iron overload.
Although tests pertinent to liver function were within normal limits in our patient, a liver biopsy confirmed the diagnosis of cirrhosis which is a known cause of iron overload disorder. The pathophysiology of cirrhosis leading to iron overload is comparable to that of hemochromatosis, in which a decreased synthesis of hepcidin leads to increased enteral iron absorption. However, liver cirrhosis by itself generally does not cause elevated ferritin levels to over 1400 ng/ml as seen in our patient. For example, in a study of 192 patients with stable decompensated cirrhosis, T. Oikonomou et al found a median ferritin level is 161 ng/ml, a level significantly lower than levels in our case [14]. It is reasonable to speculate that in a patient with compensated cirrhosis, ferritin level should be less severely elevated, unless there are other factors contributing to malfunction of iron metabolisms, such as excessive oral iron ingestion and H63D gene mutation.
Patients with hemochromatosis inappropriately absorb iron despite adequate iron stores. HFE mutation causes low hepcidin levels which increases the absorption of iron. Iron gets deposited in various organs including the heart, liver and causes tissue damage by generation of free radicals. Indirect effects of iron overload induce formation of reactive oxygen species, lipid peroxidation and fibrogenesis. Iron is an essential substrate for cellular proliferation. Excess amount of iron leads to direct and indirect carcinogenesis. Mechanism of iron as a carcinogen involves 1) Iron autoxidation involving only Fe(2+)+O2 in oxidant formation in biological system and its PH dependency, 2) Activation of oxidative response transcription factors and proinflammatory cytokines, and 3) Iron induced hypoxia signaling. Iron binding to DNA causes inactivation of tumor suppressor gene p53. Ko et al reported the carcinogenic role of iron for hepatocellular carcinoma after reviewing 5224 patients with end-stage liver disease. This patient developed intrahepatic cholangiocarcinoma in the setting of iron overload and liver cirrhosis. The iron overload improved with treatment of the underlying malignancy (Table 1).
| Date | 09/28/17 | 11/07/2017 | 11/24/17 | 12/21/17 | 12/22/17 | 04/18/18 | 08/29/18 | 11/01/18 | 11/13/18 | 01/09/19 |
| Ferritin (ng/ml) | 1839 | 1099 | 788 | 593 | 355 | 362 | ||||
| Treatment | Started Phlebatomy | Started SBRT | Started Cis/Gem | completed Cis/Gem | Y90 |
Table 1: The iron overload improved with treatment of the underlying malignancy.
Our literature review shows no definite association of H63D gene mutation with intrahepatic cholangiocarcinoma. In contrast, HCC does appear to show increased incidence in the setting of HFE mutation likely due to the carcinogenic effect of increased iron stores.
Pathogenesis of ICC is unclear in our patient. It is a rare and unusual presentation in a patient with heterozygous H63D gene and iron overload to develop intrahepatic cholangiocarcinoma. Further studies are recommended in patients with heterozygous H63D gene mutation in the setting of malignancy.
Conclusion
This case demonstrates the importance of assessing alternate causes of iron overload even in the setting of HFE mutation. Not all the patients with H63D mutation develop iron overload but the risk is increased when associated with risk factors such as hepatitis B, hepatitis C, cirrhosis or alcohol abuse. Our patient was heterozygous for H63D allele of HFE gene. This mutation along with over supplementation of oral iron and liver disease induced by chronic alcohol use were initial considerations for the abnormal iron studies. In this case an unexpectedly elevated ferritin served as an early sign of underlying malignancy and warranted further work up. In our case the ferritin level improved with treatment of the underlying malignancy.
References
- V. Vallenhoven, Adatrechtbundles, 17 Adatrechthing 17 (1919) 4.
- J. Wenas, Minahasa history, and culture, manado: north sulawesi art institute culture, Academic historians, (2007).
- L. Samovar, Communication Between culture, edtn 7, Jakarta, Salemba Humanika (2010).
- R. Bogdan and SJ Taylor, Introduction to qualitative research methods, New York: John Wiley, 1 (1977).
- J. P. Spradley, Culture and Cognation: Rules, Map, and Planes, San Francisco: Calendar Publishing Company, (1972).
- D. Richard, Cap tikus as drinks typical of the minahasa people, Faculty Theology, Christian University of Tomohon, (1998).
- F. Salesman, S. R. Juraman, A. Lette, G. D. Gobang and M.P. Erika, The Controversy between the Indonesian government policy and manggarai's culture values about a " Sopi " liquor, J Drug Alcohol Res, 7 (2018), 1- 6.
- M. H. Pratiknjo and R. Mambo, The cultural value of the minahasa people about liquor "Cap Tikus", J Drug Alcohol Res, 8 (2019), 1-4.
- K. Koentjaraningrat, Pengantar Antropologi, Jakarta: Dian Rakyat, (1979).
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi