Research Article, J Spine Neurosurg Vol: 6 Issue: 5
Isolation and Purification of Schwann Cells from Human Peripheral Nerves via the Cold Jet Technique
Namuun Khentii1, Wei-Hong Hei1,2, Bo-Han Li3, Vitaly Ryu4, Joo-Young Lee6, Sung-Ho Lee1,8, NaRi Seo1, Bong-Ju Kim7, Soung-Min Kim1,8, Jeong-Won Jahng8 and Lee Jong-Ho1,8*
1Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital, Korea
2The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, China.
3Bin Zhou Medical College, School of Stomatology, Yantai, China
4Department of Biology, Georgia State University, Atlanta, GA, USA
5Center for Obesity Reversal, Georgia State University, Atlanta, GA, USA
6Division of DNA Analysis, Scientific Investigation Laboratory, Criminal Investigation Command, Ministry of National Defense, Seoul, Korea
7Dental Life Science Research Institute, Clinical Translational Research Center for Dental Science, Seoul National University Dental Hospital, Seoul, Korea
8Dental Research Institute, Seoul National University, Seoul, Korea
*Corresponding Author : Dr. Jong-Ho Lee
03080, 275-1, Yeongun-Dong, Jongro-Gu, Seoul
Tel: +82-2-2072-2630
Fax: +82-2-766-4948
E-mail: leejongh@snu.ac.kr
Received: August 28, 2017 Accepted: December 11, 2017 Published: December 20, 2017
Citation: Khentii N, Hei W, Li B, Ryu V, Lee J, et al. (2017) Isolation and Purification of Schwann Cells from Human Peripheral Nerves via the Cold Jet Technique. J Spine Neurosurg 6:5. doi: 10.4172/2325-9701.1000287
Abstract
Schwann cells (SCs) play an important role in peripheral nerve regeneration. However, established methods for SC preparation have limitations in that they are non-specific and time-consuming. The purpose of this study was to assess the amount and purity of SCs isolated from human peripheral nerve tissue using the cold jet technique. The cold jet technique is an SC isolation and purification method that exploits the different responses of the SCs and fibroblasts to temperature shock. Specifically, the temperature shock causes specific detachment of SCs from fibroblasts. A total of 32 human peripheral nerve tissues (sensory and motor) were obtained from reconstruction procedures for oral cancer. After the length and weight of nerve segments were measured, the nerve fascicles were digested and SCs were isolated and purified by the cold jet technique. The purity of SCs was confirmed by morphology, RT-PCR, immunohistochemistry and flow cytometry. Phasecontrast photomicrographs, S100 mRNA expression rates and immunohistochemical staining show that the SC purification rate was greater with the cold jet technique. Flow cytometry showed the percentage (%) gated value increased from 56.10 to 95.95%. On average, 2.48 ± 2.57 × 106 SCs/g or 0.16 ± 0.18 × 106 SCs/cm were harvested efficiently from human peripheral nerve segments. The cold jet technique was an efficient method for SC isolation and purification from the human peripheral nerves. The determined amount and the level of purity of the SC harvested by this method may be helpful for tissue engineering or cell therapy
Keywords: Schwann cells; Human peripheral nerve; Cold jet technique; Purification and isolation; Flow cytometry
Introduction
Schwann cells (SCs), the glial cells of the peripheral nervous system, are the main cell type within peripheral nerve stumps. SCs play a crucial role in functional recovery of injured peripheral nerves and are responsible for the formation and maintenance of the myelin sheath around axons in peripheral nerve fibers. After peripheral nerve injury, SCs proliferate and remove axon debris along with macrophages and also produce extracellular matrix molecules, integrin, and various neurotrophic factors to promote and guide axon growth in distal nerve stumps [1-3].
Methods have been developed to enrich SCs using antimitotic treatment, a combination of antimitotic treatment and antibodymediated cytolysis employing complements, repeated explantations, differential adhesion, and immunoselection [1,4-6]. These techniques, however, have limitations. For example, antimitotic agents are able to diminish contaminated fibroblasts but are harmful to SC function and can reduce SC yield because of their non-specific antimitotic effects. Antibodies and complements are expensive for large scale preparation of SCs; thus, making it difficult to meet clinical goals that demand an economical approach. Repeated explantation and differential adhesion require comparatively complicated and time consuming procedures, which may lead to loss of SCs and possibly delay the required therapy. Immunoselection is considered a good method for achieving high purity, but again requires expensive antibodies and special facilities [1].
In the current study, the cold jet technique was applied for human SC isolation as previously reported [7]. The cold jet technique has many advantages: no need for special equipment, low cost, and no utilization of antimitogens that are clinically acceptable for a short period of SC growth. The use of artificial nerve grafts containing SCs is a promising method for peripheral nerve repair. Therefore, the production of a large number of viable SCs within a short period of time, which is essential for the application of tissue engineering techniques, is necessary for clinical application.
The purpose of this study was to assess and confirm the purity and number of SCs isolated from a specific amount (gram or centimeter) of human peripheral nerve tissue via the cold jet technique. Differences in SC yield were also evaluated according to nerve type, gender, and age.
Materials and Methods
Harvesting human nerve segments
The use of human tissues for the experiments was approved by the Seoul National University Dental School Ethics Committee. Human peripheral nerve samples were obtained from oral cancer ablation and reconstruction procedures in patients at the Department of Oral and Maxillofacial Surgery, Seoul National University, Republic of Korea. A total of 32 nerves (19 men and 13 women) were harvested from 28 patients (age range 31 to 77 years, average 63.2 years old). Sensory nerves included the cervical plexus of the neck (CP; n=4), the lingual nerve (LN; n=6), the forearm cutaneous nerve (FN; n=8), the greater auricular nerve (GAN; n=4), and the inferior alveolar nerve (IAN; n=2). The thoracodorsal nerve of the latissimus dorsi muscle served as a motor nerve (TDN; n=8).
The harvested nerves were immediately transferred to Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Pen/Strep) (Gibco, Life Technologies Co., Grand Island, NY, USA) and were stored at 4°C.
Primary culture of human SCs
After removing perineural connective tissue and blood, the nerve segments were blotted with filter paper to remove excess water, and then their length and weight were measured. Following nerve dissection into 1-cm sections, the nerve fascicles were isolated from the nerve segments by stripping off the epineurium using microscissors and Dumont (no. 5) forceps under a microscope. Isolated nerve fascicles were pre-degenerated with DMEM containing 10% FBS (Gibco, Life Technologies Co., Grand Island, NY, USA), 2 μM forskolin (FK; Calbiochem, Merck KGaA, Darmstadt, Germany), 10 ng/ml fibroblast growth factor (FGF; Invitrogen, Life Technologies Co., Carlsbad, CA, USA), 50 ng/ml glia growth factor (GGF; Reprokine Ltd., Valley Cottage, NY, USA) and 5 μg/ml bovine pituitary extract (BPE; Invitrogen, Life Technologies Co., Carlsbad, CA, USA) for 2 weeks. The culture medium was changed two or three times per week.
Enzymatic dissociation of pre-degenerated nerve fascicles was carried out as described by Haastert et al. [8]. Briefly, fascicles were minced using a sterile scalpel and were incubated in dissociation solution containing 10% FBS, 1% Pen/Strep, 0.125% collagenase (Type IV, Sigma-Aldrich Co., St. Louis, MO, USA), and 1.25 U/ ml dispase (Roche Diagnostics GmbH, Penzbeg, Germany) for 24 h at 37°C and 5% CO2. Dissociation solution and tissue residues were transferred into 15-ml tubes, and the same volume of Hank’s Balanced Salt Solution was added for quenching enzymatic activity. Tissue residues were gently triturated using a glass pipette until a homogenous solution was observed. Samples were then centrifuged at 800 rpm for 5 minutes at room temperature. The supernatant was removed and the pellet was re-suspended in DMEM containing 10% FBS. Centrifugation was repeated once more, and primary peripheral nerve cells were plated at a density of 106-107 cells per well on a laminin-ornithine-coated six-well plate (35 mm2). DMEM supplemented with 10 ng/ml heregulin (HRG; R&D Systems Inc., Minneapolis, MN, USA), 2 μM FK, 10 ng/ml FGF, 50 ng/ml GGF, and 5 μg/ml BPE served as the growth medium, and 1% bovine serum albumin (BSA) was added to enhance the seeding efficiency during overnight culture. The next day, the medium was changed using growth medium without BSA, and primary nerve cells were cultured for 2-3 days at 37°C and 5% CO2.
SC purification by the cold jet technique
For purification of SCs from primary cultures mixed with fibroblasts, the cold jet technique described by Jirsova et al. was applied [7]. In order to collect SCs of high purity, colonies of SCs were marked on the bottom of the six-well plate, the medium was aspirated, and colonies of SCs were gently rinsed with a stream of ice-cold phosphate-buffered saline (PBS) and then immediately aspirated. Detachment of SCs growing on top of a bottom layer of fibroblasts was conducted with ice-cold growth medium using a 1-ml blue pipette tip and monitored by phase-contrast microscopy. The suspension of floating cells, mainly SCs, was transferred into fresh laminin-ornithine-coated six-well plates and cultured at 37°C and 5% CO2. The cold jet technique was repeatedly applied up to three times.
SC counting
SCs were marked as phase bright, bi-, tri-, or multipolar with a small cytoplasm-to-nucleus ratio as seen under a phase-contrast microscope, while fibroblasts were identified by a much more flattened polymorphic shape with larger, rounded nuclei [9]. Total cell numbers and number of SCs were counted from six random fields (magnification 40X) with a phase-contrast microscope.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from serially purified SCs with Isol-RNA Lysis Reagent (5 PRIME Inc., Gaithersburg, MD, USA) according to the manufacturer’s instructions from two different patients (greater auricular nerve). The yield of total RNA was determined by measuring the absorption at 260 nm. Reverse transcription of 1 μg of total RNA was performed in a final volume of 20 μl using 200 units of Superscript II Reverse Transcriptase, 0.5 μg of oligo dT12-18 primer, 0.5 mM dNTP Mix, 10 mM DTT, and First Strand Buffer (Invitrogen). The mixture of oligo dT12-18 primer, dNTP Mix, total RNA, and DEPC-treated ddH2O was heated at 70°C for 10 min. Then, the other components were added and the solution was incubated at 42°C for 1 h. Subsequent incubation at 70°C for 15 min was conducted to inactivate the reverse transcriptase.
PCR amplification was performed with PCR-PreMix (AccuPower PCR PreMix, Bioneer Inc., Daejeon, Korea) in a My Cycler Thermal Cycler (Bio-Rad Laboratories Inc., Hercules, CA, USA) with a specific S100 primer set: sense 5'-TGGTTGCCCTCATTGATGTC-3', antisense 5'-TCAAAGAACTCATGGCAGGC-3' (Cosmo Genetech Co., Ltd., Seoul, Korea). The reactions were started at 94°C for 5 min and amplified for 32 cycles of 45 sec at 94°C, 45 sec at 61°C, and 1 min at 72°C. A final extension was continued for 10 min at 72°C to complete polymerization. GAPDH was used as an internal control to confirm equal loading of the samples: sense 5'-ACACCCACTCCTCCACCTTT-3', antisense 5'-TGCTGTAGCCAAATTCGTTG-3' (Cosmo Genetech, Co., Ltd.). The PCR products were separated on a 2% agarose gel, imaged using a ChemiDoc XRS molecular imaging system (Bio-Rad Laboratories Inc.), and analyzed with Multi Gauge software (version 3.0, Fuji Photo Film, Co., Ltd., Tokyo, Japan).
Immunohistochemistry
Purified cells were cultured on laminin-ornithine-coated cover slips and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 30 min at room temperature and then rinsed with PBS three times for 5 min each. Cells were then permeabilized with 0.2% Triton X-100 and 1% BSA in PBS for 10 min, blocked with 5% goat serum in PBS for 1 h, and then incubated with mouse anti-S100 (1:1000 dilution, Chemicon International Inc., Temecula, CA, USA), mouse anti-p75 (nerve growth factor receptor) (1:200 dilution, Chemicon International Inc.), or rabbit anti-glial fibrillary acidic protein (GFAP) (1:100 dilution, Chemicon International Inc.) overnight at 4°C. Cells were then rinsed in PBS three times, and the bound primary antibodies were visualized by incubating with Cy3-conjugated goat anti-mouse or anti-rabbit IgG (1:100 dilution, Jackson Immuno Research Lab, West Grove, PA, USA) or Alexa488-conjugated goat anti-mouse for 1 h at room temperature. For counterstaining, cells were incubated with DAPI solution (0.2 μg/ml, Sigma-Aldrich Co., St. Louis, MO, USA) for 10 min. Stained cells on the cover slips were washed in PBS, mounted carefully onto glass slides with Gel/Mount (Biomeda, Foster City, CA, USA), and then observed under a confocal laser scanning microscope (LSM510 META, Carl Zeiss, Jena, Germany). The photographic images were captured at 40X objective magnification. Negative controls were processed in a parallel manner omitting primary antibodies.
Fluorescence-activated cell scanning (FACS) analysis
Cells obtained by the cold jet technique were analyzed by a FACS Calibur flow cytometer (Becton, Dickinson, and Company, Franklin Lakes, NJ, USA) directly after the staining procedure. The percentage of SCs purified by the cold jet procedure was determined by measuring the fraction of S100-positive cells in the fluorescence intensity dot plot compared to the total amount of intact cells. A histogram was subsequently prepared. The percentage of putative SCs among total cells was obtained from three independent trials, although only representative data are shown. For each assay, 10,000 cells were analyzed using 488-nm excitation for FITC. The data were analyzed with a software program (Cytomics RXP, Beckman-Coulter Co., Pasadena, CA, USA).
Comparison of donor nerve types and statistical analysis
Total SCs were counted after the second cold jet treatment and expressed as SC number per mg and per mm of nerve tissue. Parameters based on the type of donor nerve from patients were analyzed by one-way analysis of variance (ANOVA), and preplanned comparisons with the controls were performed by the post hoc Fisher’s Protected Least Significant Difference (PLSD) test using StatView software (Version 5.0.1, SAS Institute, Cary, NC, USA). Significance was set at P<0.05. All values are presented as mean ± standard deviation (SD).
Results
SC purification via the cold jet technique
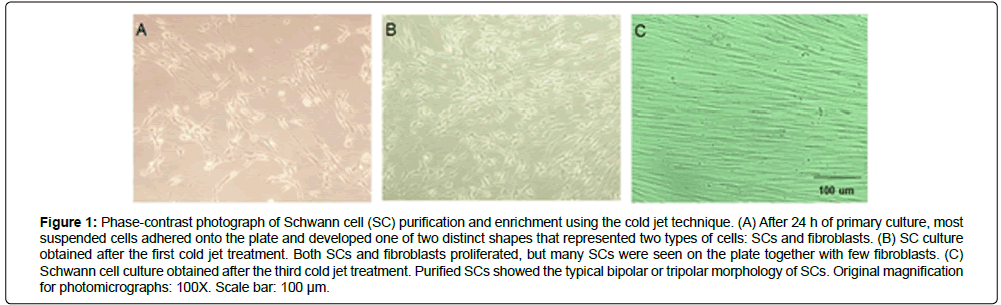
The purity of SCs isolated from human nerve segments was confirmed by morphology, immunocytochemistry, RT-PCR, and flow cytometry. Phase-contrast photomicrographs showed that the SC purification rate gradually increased by the cold jet technique. After 24 h of primary culture, two types of cells—fibroblasts and SCs—were mixed on the culture dish (Figure 1A). However, after the first and third cold jet treatments, SCs showing the typical bipolar or tripolar morphology were highly enriched, and only a few fibroblasts remained (Figure 1).
Figure 1: Phase-contrast photograph of Schwann cell (SC) purification and enrichment using the cold jet technique. (A) After 24 h of primary culture, most suspended cells adhered onto the plate and developed one of two distinct shapes that represented two types of cells: SCs and fibroblasts. (B) SC culture obtained after the first cold jet treatment. Both SCs and fibroblasts proliferated, but many SCs were seen on the plate together with few fibroblasts. (C) Schwann cell culture obtained after the third cold jet treatment. Purified SCs showed the typical bipolar or tripolar morphology of SCs. Original magnification for photomicrographs: 100X. Scale bar: 100 μm.
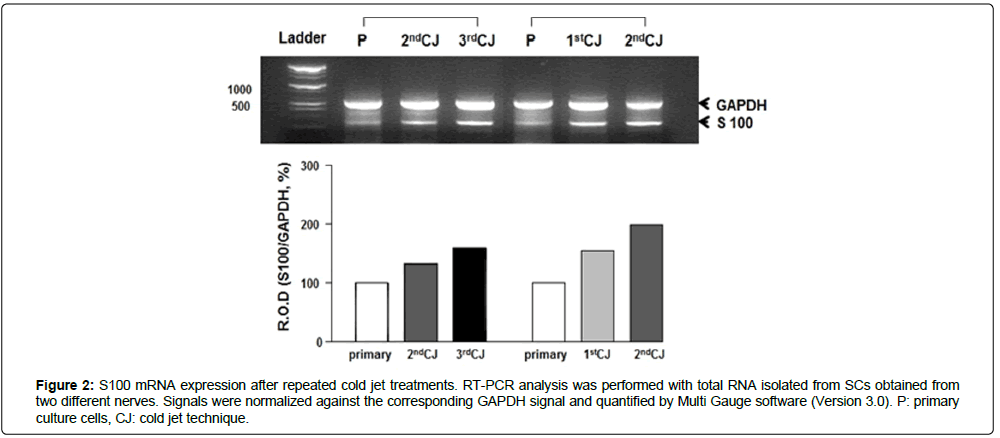
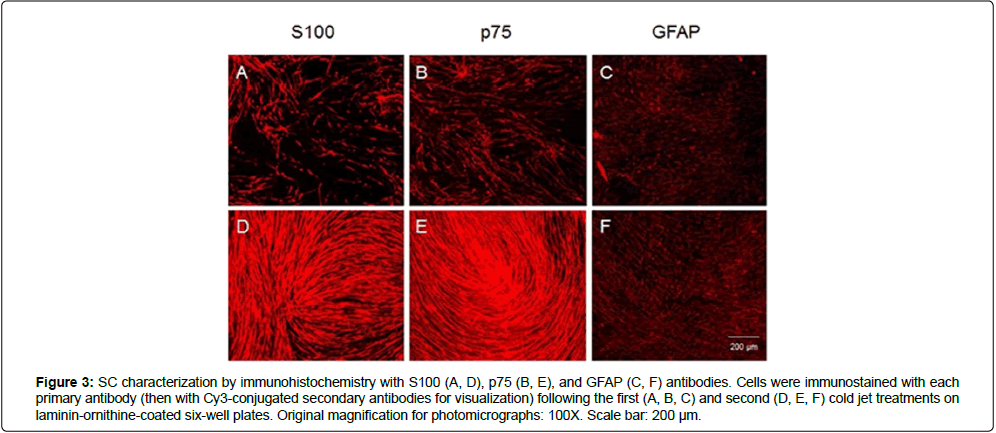
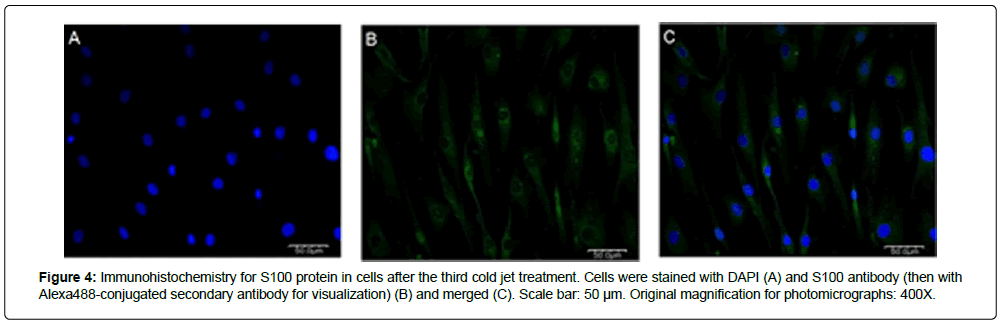
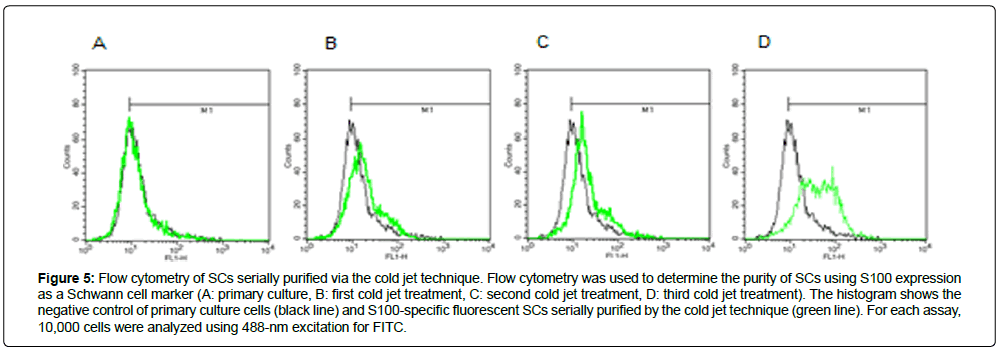
The purity of SCs was characterized at RNA and protein levels. Expression levels of S100 mRNA were investigated in SCs obtained after repeated cold jet treatments. Figure 2 shows that S100 mRNA expression rates of SC obtained from the greater auricular nerve (GAN) of two different patients gradually increased with the cold jet method; 132.19% and 160.02% after the second and third cold jet treatment for the first sample, and 154.60% and 198.79% after the first and second cold jet treatment for the second sample, compared to primary cells (100%), respectively. This result suggests that the purity of SC was improved by the cold jet technique. The purity of cells was also confirmed by immunohistochemistry with Schwann cell markers such as S100, p75, and GFAP antibodies [10-13] (Figures 3 and 4). Specifically, expression of S100 and p75 proteins was greater following cold jet treatments, and cell morphologies maintained the bipolar or tripolar shape of SCs. Flow cytometry of SCs using FACS was performed with a FITC-labeled S100 antibody to assess the purity of SCs obtained from repeated cold jet treatments [14]. S100-positive staining cells were quantified (green histogram) (Figure 5). The results showed that the fluorescence histogram of the cells was shifted to the right compared to the primary cells, and the percentage of gated cells in the population increased from 56.10 to 95.95 (Table 1). This comparison indicates that the purity of SCs was highly increased by repeated cold jet treatments.
Figure 2: S100 mRNA expression after repeated cold jet treatments. RT-PCR analysis was performed with total RNA isolated from SCs obtained from two different nerves. Signals were normalized against the corresponding GAPDH signal and quantified by Multi Gauge software (Version 3.0). P: primary culture cells, CJ: cold jet technique.
Figure 3: SC characterization by immunohistochemistry with S100 (A, D), p75 (B, E), and GFAP (C, F) antibodies. Cells were immunostained with each primary antibody (then with Cy3-conjugated secondary antibodies for visualization) following the first (A, B, C) and second (D, E, F) cold jet treatments on laminin-ornithine-coated six-well plates. Original magnification for photomicrographs: 100X. Scale bar: 200 μm.
Figure 4: Immunohistochemistry for S100 protein in cells after the third cold jet treatment. Cells were stained with DAPI (A) and S100 antibody (then with Alexa488-conjugated secondary antibody for visualization) (B) and merged (C). Scale bar: 50 μm. Original magnification for photomicrographs: 400X.
Figure 5: Flow cytometry of SCs serially purified via the cold jet technique. Flow cytometry was used to determine the purity of SCs using S100 expression as a Schwann cell marker (A: primary culture, B: first cold jet treatment, C: second cold jet treatment, D: third cold jet treatment). The histogram shows the negative control of primary culture cells (black line) and S100-specific fluorescent SCs serially purified by the cold jet technique (green line). For each assay, 10,000 cells were analyzed using 488-nm excitation for FITC.
| Primary cells | 1st cold jet | 2nd cold jet | 3rd cold jet | |
|---|---|---|---|---|
| % Gated (MI) | 56.1 | 81.57 | 90.44 | 95.95 |
| Mean (MI) | 21.32 | 25.58 | 28.07 | 55.99 |
Table 1: Flow cytometry of S100-positive SCs obtained using sequential cold jet techniques.
SC yield based on the type of donor nerve
A total of 2.48 ± 2.36 × 106 SCs per gram or 0.16 ± 0.18 × 106 per centimeter of nerve tissue were obtained. Tables 2, 3, and 4 show the difference in numbers of SCs isolated according to nerve type (sensory or motor), gender, and age. Calculation per gram of nerve tissue (Table 2) demonstrated that the number of SC isolates from sensory nerves tended to be higher than the one from motor nerves (2.86 ± 2.57 × 106 vs. 1.36 ± 1.05 × 106, P=0.122). Isolation of SCs per centimeter from sensory nerves (Table 2) also obtained more SCs compared to isolation from motor nerves (0.18 ± 0.20 × 106 vs. 0.11 ± 0.09 × 106, P=0.33). Gender-dependent analysis (Table 3) of SCs isolates per gram and per centimeter did not show significance. Age-dependent analysis (Table 4) of SC isolates showed that sensory and total SC isolations per gram from patients over 65 years old were markedly greater in comparison with ones under 65 years (4.18 ± 3.16 × 106 vs. 1.92 ± 1.59 × 106 in sensory nerves; 3.39 ± 2.84 × 106 vs. 1.68 ± 1.53 × 106 in total nerves). Sensory and total SC isolation per centimeter from patients over 65 years old was also significantly higher when compared with those under 65 years old (0.29 ± 0.27 × 106 vs. 0.11 ± 0.09 × 106 in sensory; 0.24 ± 0.24 × 106 vs. 0.10 ± 0.09 ×106 in total nerves P<0.05).
| SC Isolation (x106) | Nerve type | ||
|---|---|---|---|
| Sensery | Motor | Total | |
| SC number*(NO/g) | 2.86 ± 2.57 | 1.36 ± 1.05 | 2.48 ± 2.36 |
| SC number† (NO/cm) | 0.18 ± 0.20 | 0.11 ± 0.09 | 0.16 ± 0.18 |
*p=0.122 Sensory vs. Motor
†P=0.33
Table 2: Nerve types of Schwann cell proliferation following the cold jet technique.
| SC Isolation (x106) | Gender | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Sensery | Motor | Total | Sensery | Motor | Total | |
| SC number*(NO/g) | 2.33 ± 1.95 | 1.80 ± 1.09 | 2.19 ± 1.75 | 3.60 ± 3.22 | 0.62 ± 0.38 | 2.92 ± 3.08 |
| SC number† (NO/cm) | 0.15 ± 0.18 | 0.15 ± 0.10 | 0.15 ± 0.16 | 0.23 ± 0.24 | 0.04 ± 0.02 | 0.19 ± 0.22 |
Table 3: Gender-dependent analysis of Schwann cell proliferation following the cold jet technique.
| SC Isolation (x106) | Age | |||||
|---|---|---|---|---|---|---|
| <65 | >65 | |||||
| Sensery | Motor | Total | Sensery | Motor | Total | |
| SC number*(NO/g) | 1.92 ± 1.59 | 0.57 ± 0.29 | 1.68 ± 1.53 | 4.18* ± 3.16 | 1.83 ± 1.06 | 3.39 ± 2.84 |
| SC number† (NO/cm) | 0.11 ± 0.09 | 0.05 ± 0.03 | 0.10 ± 0.09 | 0.29* ± 0.27 | 0.14 ± 0.10 | 0.24 ± 0.24 |
*p<0.05 vs. <65 years
Table 4: Age-dependent analysis of Schwann cell proliferation following the cold jet technique.
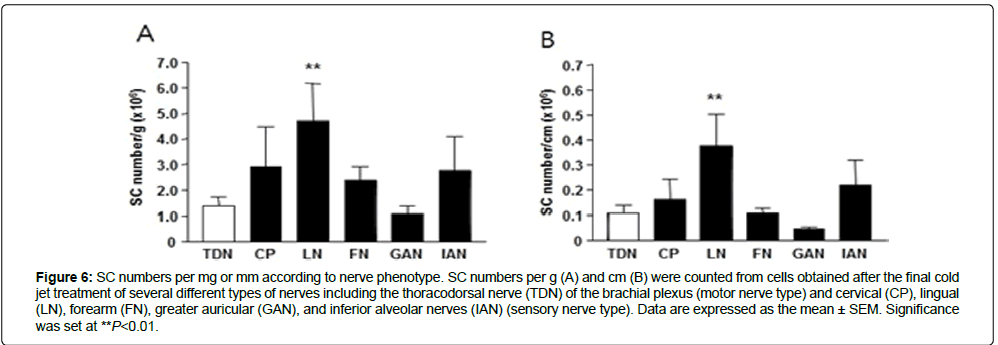
Concerning nerve phenotype-related purification of SCs, the total yield of SCs from the motor thoracodorsal nerve of the brachial ple×us (TDN) was 1.36 ± 1.05 ×106 SC/g (Figure 6A) and 0.11 ± 0.09 ×106 SC/cm (Figure 6B). The SC yield per gram from the sensory lingual nerve (LN) was significantly higher than that of the TDN, cervical nerve (CP): 2.91 ± 3.15 ×106; LN: 4.70 ± 3.66 ×106; forearm nerve (FN): 2.38 ± 1.52 ×106; greater auricular nerve (GAN): 1.07 ± 0.59 ×106; and inferior alveolar nerve (IAN): 2.75 ± 1.89 ×106). The SC number from the LN was also significantly higher per centimeter than the ones from the other nerve types (LN: 0.38 ± 0.31 ×106; FN: 0.11 ± 0.06 ×106; GAN: 0.05 ± 0.02 × 106; and IAN: 0.22 ± 0.14 ×106).
Figure 6: SC numbers per mg or mm according to nerve phenotype. SC numbers per g (A) and cm (B) were counted from cells obtained after the final cold jet treatment of several different types of nerves including the thoracodorsal nerve (TDN) of the brachial plexus (motor nerve type) and cervical (CP), lingual (LN), forearm (FN), greater auricular (GAN), and inferior alveolar nerves (IAN) (sensory nerve type). Data are expressed as the mean ± SEM. Significance was set at **P<0.01.
Discussion
SCs play a pivotal role in peripheral nerve regeneration, and highly purified SCs are essential for constructing tissue-engineered artificial nerve grafts for peripheral nerve regeneration [15,16]. However, a common problem in obtaining highly purified SCs is fibroblast contamination of SC cultures. This problem is the reason that existing results have a negative effect on engineered nerve repair by disturbing SC-mediated axonal regeneration [1]. Over the past 30 years, many efforts have been made to harvest highly enriched SCs [1,17-21]. The current methods, however, require special equipment or have complicated procedures resulting in relatively low cell yields. In contrast, clinical application of SC therapy requires high yields of SCs obtained by safe, rapid, and easy purification methods.
The protocol used in the present study was similar to procedures involving regular cell passage; therefore, it is relatively easy to perform. Additionally, it does not require special facilities or agents, except for ice-cold culture medium. More importantly, this simple procedure was very efficient and posed no harm to the cells; therefore, it is possible to apply this procedure in the clinic. In the present study, it took approximately 27-40 days to obtain the purified SCs after the third trial using the cold jet technique. The time required from the primary seeding up to the first cold jet treatment was 10.5 days on average, from the first to the second cold jet treatment was 13 days on average, and from the second to the third was 15 days on average. It took a relatively long time because the cell culture was started from a small cell number and the cold jet method was performed when the cell confluency was 100%. The purity of SCs isolated with the cold jet technique was assessed by evaluating protein and RNA levels using SCspecific RT-PCR, flow cytometry analysis, and immunohistochemical staining. As shown in Figure 2, S100 mRNA expression rates of SC obtained from the GAN of two different patients was slightly greater with the cold jet method. Flow cytometry data showed that the percentage of gated cells in the population increased from 56.10 to 95.95% after the third cold jet treatment. Collectively, these results suggest that the purity of SCs was much greater using repeated cold jet treatments, although the collection rate of SCs was lower.
A variety of different prototypes in the cells isolated from individual nerve segments were also observed. During the cell culture period, the relationship between cell phenotype and SC yield was observed. More numbers of SCs were harvested from sensory nerves than from motor nerves. Possible governing mechanisms behind this phenomenon may include differences in biochemical markers, SC phenotype, neurotrophic support, and/or nerve architecture [22,23]. Chemical and cellular differences between sensory and motor nerves may also play a relevant role [24]. SC number per gram and per centimeter of LN was higher than the ones of the other nerves. The reason might be that LN itself has relatively more SCs than compared with other nerves and has fewer peri-neural tissue, or low proportion of epi- or inter-fascicular epineurium.
It has been suggested that there are slight differences in female and male peripheral tissues [25], so gender-based analysis was performed. Nonetheless, no differences were observed herein in SC yield between male and female patients (Table 3). However, SC yield depended on age and showed significant differences in both SC numbers per gram and per centimeter. Although initially we were going to divide samples into young and old ages with the cut-off at 50 years old, there was only one nerve sample from a patient under 50 years old, thus we adjusted the cut-off to 65 years old; there were 15 nerve samples from patients over 65 years old and 17 samples from patients under 65 years old. Interestingly, the sensory and the total SCs isolations per gram and per centimeter of patients over 65 years old were significantly higher in comparison with ones from patients under 65 years old. In further SC yield studies that investigate the effects of age, it will be necessary to collect more nerve samples from younger patients, specifically those under 50 years old.
Conclusion
In conclusion, 2.48 ± 2.36 × 106 SCs/g and 0.16 ± 0.18 × 106 SCs/cm were efficiently harvested from human peripheral nerves, an important finding that might be useful in future studies on tissueengineered nerve conduits constructed with SCs for nerve regeneration. In addition, the yield of SCs isolated from the sensory lingual nerve using the cold jet technique was significantly higher than that of motor nerves, and there was no difference in the SC yield depending on gender. These findings can be used to improve the current nerve regeneration techniques by accurate calculation of nerve length or weight necessary for isolation of SCs. Thus, this investigation has implications for immediate clinical management of nerve injuries.
Conflict of Interest
None declared
Acknowledgement
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI15C1535).
Copyright transfer agreement and responsibility statements
To the Scitechnol Publishing Commission. The authors submit the original manuscript above to Scitechnol, represented by the journal’s Publishing Commission, and confirm that the manuscript herewith submitted is an original and does not violate any patent, trademark, copyright, trade secret, or other third party property rights.
The Authors also declare that, except when expressly stipulated, they hold no financial interest or agreement with any entity that may be perceived as having influence over the objectivity of the manuscript, unless such financial interest or agreement has been revealed in writing to Scitechnol, in a separate document and signed by all Authors, as per international guidelines. The Authors furthermore declare that the study, whose results are reported in the manuscript, was performed in observance of the policies in force in the institutions that the Authors are bound to, concerning human and/or animal use, and/or material derived from humans or animals (Approval in Institutional Ethics Committee).
The Authors agree to indemnify Scitechnol and to exonerate it from any allegations, costs, lawyer fees, compensations, or costs of user licenses incurred by Scitechnol as a consequence of any allegation, rights infringement, or noncompliance of the determinations of the Institutional Ethics Committee caused by the publishing, partially or totally, of the Manuscript.
References
- Jin YQ, Liu W, Hong TH, Cao Y (2008) Efficient Schwann cell purification by differential cell detachment using multiplex collagenase treatment. J Neurosci Methods170: 140-148.
- Li Q, Ping P, Jiang H, Liu K (2006) Nerve conduit filled with GDNF geneâ€Âmodified schwann cells enhances regeneration of the peripheral nerve. Microsurgery 26: 116-121.
- Mahanthappa NK, Anton ES, Matthew WD (1996) Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J Neurosci 16: 4673-483.
- Niapour A, Karamali F, Karbalaie K, Kiani A, Mardani M, et al. (2010) Novel method to obtain highly enriched cultures of adult rat Schwann cells. Biotechnology letters 32: 781-786.
- Zhu J, Qin J, Shen Z, Kretlow JD, Wang X, et al. (2012) Dispase rapidly and effectively purifies Schwann cells from newborn mice and adult rats. Neural regeneration research 7: 256.
- Pannunzio ME, Jou I-m, Long A, Wind TC, Beck G, et al. (2005) A new method of selecting Schwann cells from adult mouse sciatic nerve. Journal of neuroscience methods 149: 74-81.
- Jirsova K, Sodaar P, Mandys V, Bär P (1997) Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci Methods 78: 133-137.
- Haastert K, Mauritz C, Chaturvedi S, Grothe C (2007) Human and rat adult Schwann cell cultures: fast and efficient enrichment and highly effective non-viral transfection protocol. Nature protocols 2: 99-104.
- Morrissey TK, Kleitman N, Bunge RP (1991) Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci 11: 2433-2442.
- Raff MC, Fields KL, Hakomori S-I, Mirsky R, Pruss RM, et al. (1979) Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res 174: 283-308.
- Woodhoo A, Sommer L (2008) Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56: 1481-1490.
- Ziegler L, Grigoryan S, Yang IH, Thakor NV, Goldstein RS (2011) Efficient generation of Schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Reviews and Reports 7: 394-403.
- Bianchini D, De Martini I, Cadoni A, Zicca A, Tabaton M, et al. GFAP expression of human Schwann cells in tissue culture. Brain research 570: 209-217.
- Bao F, Bailey CS, Gurr KR, Bailey SI, Rosas-Arellano MP, et al. (2009) Increased oxidative activity in human blood neutrophils and monocytes after spinal cord injury. Experimental neurology 215: 308-316.
- Rodrigues MCO, Rodrigues AA, Glover LE, Voltarelli J, Borlongan CV (2012) Peripheral nerve repair with cultured schwann cells: getting closer to the clinics. The Scientific World Journal.
- Hood B, Levene HB, Levi AD (2009) Transplantation of autologous Schwann cells for the repair of segmental peripheral nerve defects. Neurosurgical focus 26: E4.
- Argall KG, Armati PJ, Pollard JD (1994) A method for the isolation and culture of rat peripheral nerve vascular endothelial cells. Mol Cell Neurosci 5: 413-417.
- Assouline J, Bosch EP, Lim R (1983) Purification of rat Schwann cells from cultures of peripheral nerve: an immunoselective method using surfaces coated with anti-immunoglobulin antibodies. Brain Res 277: 389-392.
- Brockes J, Fields K, Raff M (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res165: 105-118.
- Lopez T, De Vries G (1999) Isolation and serum-free culture of primary Schwann cells from human fetal peripheral nerve. Exp Neurol 158: 1-8.
- Manent J, Oguievetskaia K, Bayer J, Ratner N, Giovannini M (2003) Magnetic cell sorting for enriching Schwann cells from adult mouse peripheral nerves. J Neurosci Methods 123: 167-173.
- Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, et al. (2006) Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. The Laryngoscope 116: 1685-1692.
- Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, et al. (2004) Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol 190: 347-355.
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ (2000) Principles of neural science. McGraw-hill New York.
- Moriyama H, Hayashi S, Inoue Y, Itoh M, Otsuka N (2016) Sex differences in morphometric aspects of the peripheral nerves and related diseases. NeuroRehabilitation 39: 413-422.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi