Research Article, J Pulm Med Vol: 2 Issue: 1
Lung Compliance and Resistance Following Bronchial Thermoplasty in Severe Persistent Asthma: A Pilot Study and Discussion of the Physiology
Ahmet Baydur1*, Melissa Koç2, Richard Barbers1 and Darren May1
1Divisions of Pulmonary, Critical Care and Sleep Medicine, Los Angeles, CA, USA
2Department of Preventive Medicine, Division of Biostatistics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
*Corresponding Author : Dr. Ahmet Baydur
Divisions of Pulmonary, Critical Care and Sleep Medicine, Los Angeles, CA, USA
Tel: 323-409-7184
Fax: 323-226-7923
E-mail: baydur@usc.edu
Received: November 16, 2016 Accepted: January 06, 2018 Published: January 11, 2018
Citation: Baydur A, Koç M, Barbers R, May D (2018) Lung Compliance and Resistance Following Bronchial Thermoplasty in Severe Persistent Asthma: A Pilot Study and Discussion of the Physiology. J Pulm Med 2:1.
Abstract
Abstract
Rationale: Bronchial thermoplasty reduces airway smooth muscle mass, bronchoconstriction and symptoms in severe, persistent asthmatics. Despite its application only to larger airways (> 3 mm), it is not known if small airways are also affected by bronchial thermoplasty.
Objective: To determine if thermoplasty also affects the smaller airways (< 3 mm in diameter) before and after bronchial thermoplasty. Methods: Lung resistance, static and dynamic lung compliance were measured using the esophageal balloon technique before and after undergoing bronchial thermoplasty in 5 patients.
Results: All patients described improved symptoms and reduced use of short-acting bronchodilators within a few weeks following completion of bronchial thermoplasty. Pre-thermoplasty, all patients exhibited significant frequency dependency of lung compliance, with dynamic lung compliance decreasing to as much as 75% of baseline static compliance value at maximum respiratory rate. In 4 of 5 patients, post-thermoplasty values for dynamic lung compliance were higher than pre-thermoplasty over the range of respiratory rates. Post-thermoplasty, static lung compliance did not change significantly from pre-thermoplasty values. Lung resistance during quiet breathing (baseline) decreased in 4 of 5 patients following thermoplasty, and decreased at higher respiratory frequencies in 4 of 5 patients.
Conclusions: Patients with severe persistent asthma exhibit increased resting lung resistance and frequency dependence of compliance, the magnitudes of which are in ameliorated following bronchial thermoplasty, and associated with variable frequency dependence of lung resistance not apparent before bronchial thermoplasty. Bronchial thermoplasty likely exerts its effects on small as well as large airways.
Keywords: Esophageal balloon technique; Regional lung in homogeneities; Frequency dependence of compliance and resistance; Small airway disease; Viscoelastic properties of lung
Commentary
Bronchial thermoplasty reduces smooth muscle mass by radiofrequency ablation in airways of patients with severe, persistent asthma. While it has been shown to improve quality of life and reduce the rate of acute exacerbations, its exact site of action is not known. Despite the major role of inflammation in small airways in the pathophysiology of asthma, it is not known if small airways are also affected by this intervention. Following bronchial thermoplasty, the magnitude of frequency dependence of compliance decreased in 4 of 5 patients. Lung resistance at rest also diminished, while its frequency dependence becomes more apparent following the intervention. These findings can be explained by a decrease in regional in homogeneities of ventilation, suggesting that peripheral airway dysfunction in severe asthmatics is also ameliorated by bronchial thermoplasty. With loss of airway wall muscle mass following BT, in most cases, lung resistance decreases as frequency increases, suggesting an alteration in lung viscoelastic properties in addition to increase in airway caliber.
Introduction
Asthma features inflammation and remodeling of the airways [1,2]. These changes are known to occur in the peripheral airways as well as in the large airways, and even in the lung parenchyma [3,4]. The distal parts of the bronchial tree have been recognized as a predominant site of airflow obstruction in many asthmatics. In fact, the inflammation at this distal site has been described as more severe when compared to the large airway inflammation, and evidence of remodeling in the lung periphery is emerging [3,5]. The need to also consider the distal lung as a target for treatment is therefore an important consideration in the management of asthma. Bronchial thermoplasty (BT) is designed to reduce airway smooth muscle mass (ASM) with the purpose of decreasing bronchoconstriction and reducing asthma symptoms [6,7]. It is generally reserved for patients who continue to experience recurrent and severe bronchoconstriction despite receiving the recommended regimen of both inhaled short and long-acting beta-2 agonist and muscarinic bronchodilators combined with inhaled (and sometimes systemic) glucocorticoids. Its effect has been shown in the airway walls (including airways larger than 3 mm in diameter) up to the immediate peribronchial region and is long lasting in effect. The mechanism is presumed to be by reducing the mass of peribronchial ASM. It is unclear, however, if airways smaller than 3 mm in diameter may also be affected by the procedure. Peripheral airway involvement can result in regional inhomogeneities of gas distribution and exchange, and in addition, can result in air trapping with resultant dyspnea. If BT affected smaller airways, it could potentially improve gas distribution and exchange, as well as ameliorating air trapping. Reduction in hyperinflation would then place the respiratory muscles in a more mechanically efficient end-expiratory position, with resulting decrease in dyspnea during exercise.
One of the questions regarding the mechanism of BT concerns the fact that patients with severe asthma exhibit clinical improvement, although the technique is applied only to the larger airways (> 3 mm). Despite the major role of inflammation in small airways in the pathophysiology of asthma [1-5], the beneficial effects of BT suggest the contribution of only proximal airways to the clinical findings. Using a modified retrograde catheter technique in dog lungs, Macklem et al. [9] showed that at midlung volumes the greatest part of the total lung resistance (RL) is in bronchi with 3- to 8-mm internal diameter (i.d.) with the vagal nerves intact. The resistance of these airways is large because of their bronchomotor tone which also contributes to much of the resistance as lung volume is reduced. However, air trapping during bronchospasm should have a counter effect with reduction in airway resistance, thereby potentially offsetting the effects of increased bronchomotor tone and airway inflammation. Finally, these investigators also showed that the vagi constrict smooth muscle at all levels of the airway but their main effect is in the 3- to 8-mm-i.d. airways. Thus the interrelationship between smooth muscle tone, lung volume and airway conductance can be explained on a mechanical basis.
Dynamic lung compliance (Cdyn, L) does not change with breathing frequency in young healthy subjects breathing at functional residual capacity (FRC) [10], explained by the equality of time constants in lung parallel units. Macklem and Mead [11] showed that the low resistance of airways smaller than 2 mm i.d. allowed considerable inequality of time constants in units subtended by these airways without frequency dependency of compliance. However, with increase in muscle tone and airway inflammation, as occurs with asthma, there is greater variation in resistance, and consequently, greater variation in time constants amongst parallel lung units, resulting in increasing frequency dependency of compliance [11].
The main objective of this study was to determine if, in patients with severe, persistent asthma, BT also affected the smaller airways (< 3 mm in diameter) by assessment of lung mechanics using the esophageal balloon technique occurs primarily in airways > 3 mm; if frequency dependence of compliance is diminished, then the action of BT significantly affects the peripheral airways < 3 mm. To confirm or refute these hypotheses, we measured static and dynamic lung compliance and lung resistance (at different breathing frequencies) before and after BT. before and after BT. We hypothesized that if, following BT there was little or no change in the magnitude of frequency dependency of compliance, the reduction in RL.
Methods
Subjects
Stable, patients who never-smoked with severe, persistent asthma scheduled to undergo BT were recruited for obtaining measurements of lung compliance (static and dynamic) and lung resistance. All patients were using inhaled short-acting bronchodilator on as needed basis and combinations of a long-acting bronchodilator and an inhaled corticosteroid. All patients had previously experienced acute exacerbations that required systemic corticosteroids. Patients were treated with the Alair® System (Asthmatx, Inc., Mountain View, CA, USA) for BT, comprising a low-power radiofrequency (RF) generator and a basket catheter with four electrodes. The RF generator supplied power using temperature feedback control, to maintain the target treatment setting for 10 sec at each treatment site. Each patient’s BT was divided into 3 sessions spaced 3 weeks apart to optimize safety and allow patients to recover in the interim from a long procedure and to avoid adverse events. Follow up measurements were obtained 12 to 50 weeks after the last of the BTs were performed (that is, 18 and 56 weeks after the pre-BT measurements were obtained). Patients continued taking their regularly prescribed medicines throughout the BT period. The study was approved by the institutional board of the Health Sciences Center of the University of Southern California (HS-14-00289) and all patients signed an informed consent. Preliminary findings of this study were recently reported as an abstract [12].
Equipment and measurements
Patients were asked to refrain from using their inhaled bronchodilator at least 4 hours before pulmonary function testing. Spirometry was obtained using American Thoracic guidelines [13] using equipment by Medical Graphics (Elite 2, St. Paul, MN). Dead space of the mouthpiece and circuitry was 40 mL. The flow resistive properties of the Elite 2 breathing circuit were defined by the Rohrer relationship, P/V’ = K1 + K2V’, where P represents the airway pressure, V’ is flow, and K1 and K2 are constants whose values were 0.144 and 0.238, respectively. Predicted values for spirometric values of FEV1, FVC and FEV1/FVC were from [14].
Baseline measurements were obtained using the esophageal balloon technique [15] a few hours before the patient underwent the first of a series of 3 BTs spaced 3 weeks apart. Flow and pressures were recorded using the Medical Graphics Elite 2 system, with volume derived by integrating flow. Esophageal pressure was measured using a 103 cm long polyethylene catheter with an internal diameter of 1.4 mm and an external diameter of 3 mm, at the end of which was sealed a thin-walled latex balloon 5 cm long. The frequency response of the balloon-catheter system remained constant up to 22 Hz. Gastric and esophageal pressure tracings were monitored on a computer screen during placement of the esophageal catheter using its pressure transducer. Before asking the subject to begin swallowing the ballooncatheter, the catheter was emptied of air and closed with a three-way stopcock. Lidocaine gel (4%) was introduced into the subject’s nasal passage and the balloon was introduced transnasally and advanced until it reached the stomach. Swallowing the balloon-catheter was facilitated with the subject sipping water through a straw. The balloon was inflated with 0.5 ml of air. Once the balloon-catheter was inserted to a length of about 60 cm, it was slowly retracted while the patient performed a series of sniffing maneuvers. As soon as the pressure deflections became negative, indicating the balloon’s entrance into the esophagus, the catheter was withdrawn another 10 cm which placed the balloon at a distance of between 40 and 45 cm from the mouth, and secured at the nose with nonallergenic tape. The amount of gas in the balloon was periodically checked throughout the measurements. Correct placement of the balloon was confirmed by means of the occlusion test [16]. Following recording of volume, pressure and flow tracings from the Elite 2, lung compliance and resistance were measured by hand (the Elite 2 did not have the capability of automatically computing these values). Lung compliance was computed at zero flow points at each respiratory frequency from Vt/(Paw– Pes), where Paw is airway pressure and Pes is esophageal pressure (assumed to be pleural pressure); lung resistance was calculated from (Paw- Pes)/V’, where V’ is flow. Equipment resistance derived from the Rohrer relationship was subtracted from total resistance at the corresponding flow.
Breathing maneuvers
Lung mechanics measurements were preceded by a dry run in which subjects performed a series of respiratory maneuvers while watching the computer screen and without the balloon in place as follows: After 5 minutes of quiet breathing, the subjects were asked to inspire slowly to a volume of 1 to 2 L and then hold their breaths for 2-3 seconds while keeping their glottis open (for measuring static lung compliance, Cst,L). This maneuver was repeated three times, interspersed with a minute of quiet breathing in between. Then patients were instructed to breathe at increasing frequencies up to a maximum of 150 breaths per minute, while attempting to maintain their tidal volumes as constant as possible by watching the computer screen (for measuring Cdyn,L and RL). Once the subjects became accustomed to the breathing maneuvers and were able to perform them consistently, the balloon was inserted as described above, the patient was placed on the mouthpiece, and the same maneuvers were repeated. From the maneuvers, the following variables were measured: Cst,L, Cdyn,L and RL. Mean values of Cdyn,L and RL were derived from 5 to 10 breaths, depending on respiratory frequency. Three Cst,L measurements were repeated after completion of the Cdyn,L maneuvers. Care was taken to ensure that lung compliance at each frequency was measured under similar conditions.
Statistical considerations
Significant differences amongst lung mechanics variables were determined by analysis of variance of multiple measures (ANOVA) [17]. Due to small sample size, both mean and median differences of lung function measures (post - pre) were investigated accordingly via paired t-tests and Wilcoxon signed-rank tests. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Spirometry and lung compliance
Anthropometric and physiologic data of the patients and control subjects are shown in Table 1. As can be seen, pre-BT spirometry showed moderate to severe airflow limitation in 4 of 5 subjects. All 5 patients described improved symptoms and reduced use of short-acting bronchodilators within a few weeks following completion of their series of BT. Four of 5 patients (nos. 1, 2, 3, 4) exhibited improvement in FEV1 following BT. The mean change, 74%, was statistically significant (Table 2). Two patients (nos. 4 and 5) experienced upper respiratory tract viral infections several weeks after completing their BT sessions. All 5 patients completed the pre- and post-BT Cdyn,L measurements recorded at a minimum of 3 different respiratory rates. Patient 3 could only perform the post-BT testing up to a frequency of 34 breaths/min, beyond which throat discomfort necessitated removal of the esophageal balloon. Coefficients of variation for Vt within each respiratory frequency ranged from 10 to 25%, and between frequencies ranged from 16 to 44%, despite encouraging patients to maintain their Vt as best as they could by watching the computer screen. In every patient, Vt tended to diminish at higher respiratory frequencies.
| Subject no. | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | 33 | 20 | 66 | 62 | 66 | |||||
| Gender | M | F | F | M | F | |||||
| BMI | 41.4 | 39.7 | 34 | 23.5 | 29.3 | |||||
| ACT score† | 5 | 7 | 5 | 5 | 5 | |||||
| Pre-BT | Post-BT | Pre-BT | Post-BT | Pre-BT | Post-BT | Pre-BT | Post-BT | Pre-BT | Post-BT | |
| Interval betweenpre/post testing | -- | 21 wk | -- | 22 wk | -- | 56 wk | -- | 18 wk | -- | 30 wk |
| FVC, L | 3.5 | 4.9 | 2.3 | 2.7 | 1.7 | 2.2 | 1.7 | 3.0 | 2.6 | 2.2 |
| FVC, % pred. | 68 | 98 | 69 | 80 | 48 | 63 | 34 | 64 | 84 | 71 |
| FEV1, L | 2.5 | 3.3 | 2.1 | 2.4 | 1.2 | 1.8 | 1.0 | 1.7 | 1.4 | 1.2 |
| FEV1, % pred. | 58 | 84 | 71 | 81 | 44 | 70 | 26 | 51 | 59 | 48 |
| FEV1/FVC | 0.71 | 0.67 | 0.91 | 0.89 | 0.71 | 0.82 | 0.59 | 0.56 | 0.54 | 0.55 |
| Cst,L | 0.14 | 0.16 | 0.15 | 0.11 | 0.09 | 0.09 | 0.17 | 0.16 | 0.16 | 0.12 |
| RL | 12.8 | 6.7 | 7.1 | 5.9 | 12.1 | 8.4 | 5.2 | 4.5 | 8.8 | 15.4 |
†Before bronchial thermoplasty.
Table 1: Anthropometric and Physiologic Data in Patients Pre- and Post-Bronchial Thermoplasty.
| Lung Mechanics Measure | Mean Δ ± SD | P-valuea | Median Δ (IQR) | P-valueb |
|---|---|---|---|---|
| FVC, L | 0.62 ± 0.73 | 0.13 | 0.50 (1.10) | 0.19 |
| FVC, % pred. | 14.40 ± 21.71 | 0.21 | 15.00 (29.00) | 0.19 |
| FEV1, L | 0.56 ± 0.50 | 0.07 | 0.70 (1.30) | 0.13 |
| FEV1, % pred. | 74.31 ± 16.78 | <0.001 | 26.00 (1.00) | 0.13 |
| FEV1/FVC | 0.07 ± 0.15 | 0.44 | -0.01 (0.14) | 0.81 |
| Cst, L | -0.01 ± 0.03 | 0.30 | -0.01 (0.04) | 0.38 |
| RL | -2.14 ± 6.04 | 0.47 | 0.44 |
b P-value obtained by Wilcoxon signed rank test.
Table 2: Lung Mechanics Pre- and Post-Bronchial Thermoplasty.
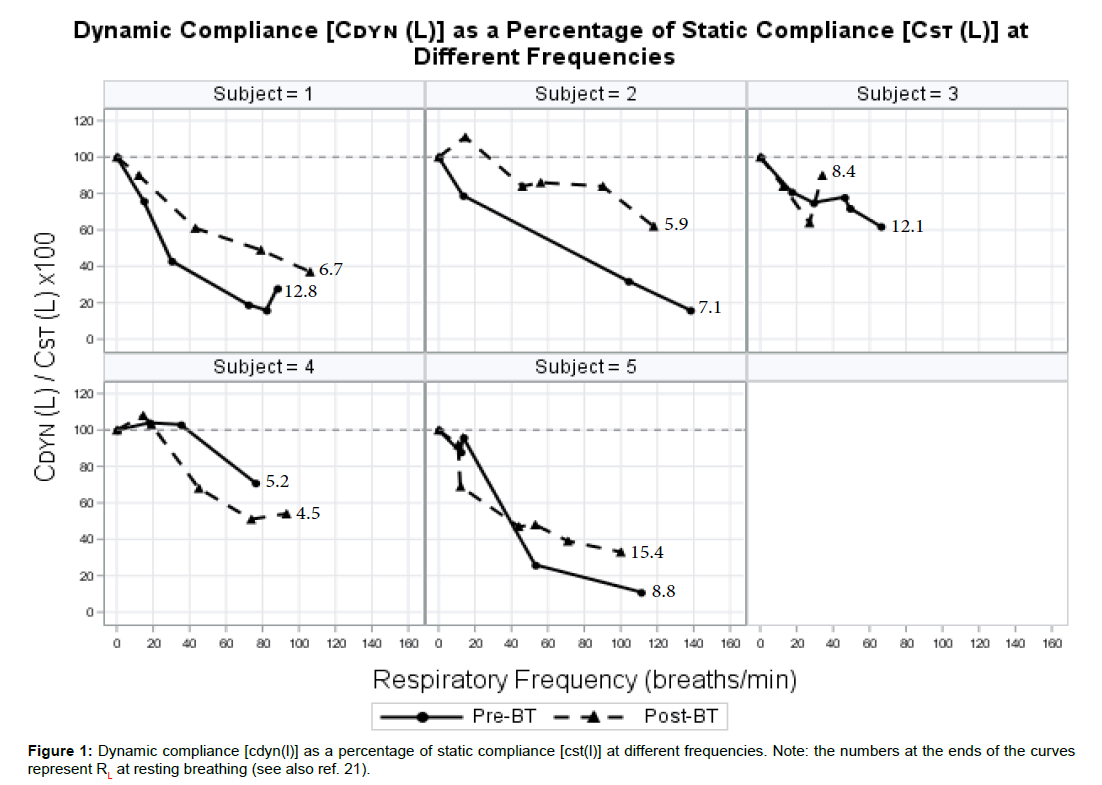
The Cst, L in all patients was within normal range of previously reported historic controls employing a similar esophageal balloon- catheter assembly [18]. As can be seen from (Figure 1), prior to undergoing BT, all patients exhibited significant frequency dependency of compliance, with Cdyn,L decreasing by as much as 75% of the baseline Cst,L value at the maximal respiratory rate (patients 1, 2 and 5).
Following BT, Cst,L did not change significantly from pre-BT (Tables 1 and 2). By contrast, in 4 of 5 patients, post-BT values for Cdyn,L were higher than pre-BT over the corresponding range of respiratory rates (Figure 1). In patient no. 4, post-BT Cdyn,L was lower over the range of respiratory frequencies; in patient no. 5, it was lower at slower frequencies but increased above pre-BT values at frequencies above 40 breaths/min. Both patients had experienced upper respiratory infections several weeks following completion of BT.
Lung resistance
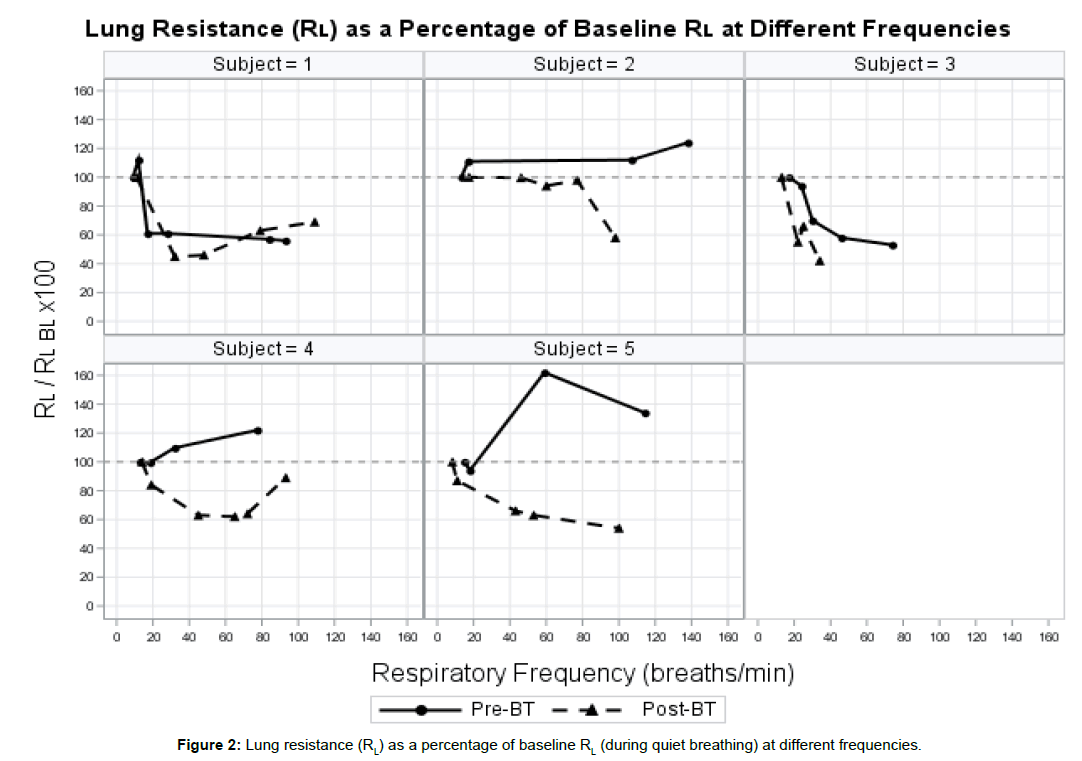
Lung resistance (RL) was 4-7 times higher than that reported in healthy subjects [18,26] measured during quiet breathing (baseline) and decreased in 4 of 5 patients following BT (Table 1). Patient no. 5 exhibited a near doubling of resting RL following BT and was one of 2 patients who had experienced an upper respiratory infection following BT. Before BT, patient 5 also exhibited a 60% increase in the RL ratio at 60 breaths/min; yet this same patient exhibited frequency dependence of resistance (i.e., decrease in the RL ratio with increasing frequency) following BT despite the increase in resting RL. In patients 2, 3 and 5, RL decreased at corresponding higher respiratory frequencies post-BT (Figure 2). In patients 1 and 4, RL initially diminished then increased at frequencies above 30 and 70 breaths/min, respectively.
Discussion
To our knowledge this is the first study that has evaluated lung resistance and compliance using the esophageal balloon technique in patients before and after undergoing bronchial thermoplasty. The main findings in this study are (a) patients with severe, persistent asthma exhibit frequency dependence of compliance, the magnitude of which is ameliorated in most patients following BT; (b) a decrease in resting lung resistance following BT, associated with frequency dependence of RL, not consistently present before BT.
Bronchial thermoplasty has been shown to impair responsiveness to methacholine inhalation [19] by attenuating airway narrowing [20]. High resolution computed tomography has shown that BTtreated airways exhibit increased diameter with any dose of inhaled methacholine [21]. These same authors later showed that a decrease in the amount of smooth muscle of canine airways 2.5-15 mm in diameter with BT resulted in increased airway dimensions and distensibility for up to 5 weeks following treatment, regardless of exposure to methacholine [22], a finding that can be attributed to loss or alteration of smooth muscle mass. These findings, however, again apply to relatively large airways accessible to the BT probe. The effect of BT on airways < 3 mm in diameter can be inferred, however, by measurement of dynamic compliance and lung resistance at different breathing frequencies.
Frequency dependence of compliance and lung resistance; discussion of physiologic mechanisms
Woolcock et al. [23] showed that, in contrast to bronchitic patients, lung compliance in asthmatic patients became less frequency dependent following bronchodilator administration, suggesting improvement in inequality of time constants and ventilation distribution. Our findings suggest that BT acts by similarly reducing variations in time constants between different lung units, thereby decreasing phase differences between them, probably by reducing small airway tone and increasing their diameter. This concept is consistent with the findings of Brown et al [22]. Indeed, a recent study with helium 3 magnetic resonance imaging and CT showed a decrease in ventilation defects that were a function of time after the final BT treatment [24].
Lung resistance during resting breathing decreased following BT in 4 of 5 patients, consistent with dilatation of both large and peripheral airways. In addition, in 4 of 5 patients lung resistance was decreased compared to pre-BT values over a corresponding range of frequencies following BT, suggesting reversal of small airway dysfunction. What would account for the changes in frequency dependence of compliance and resistance between before and after BT? A two-compartment resistance-compliance (RC) model described by Otis et al [25] was originally used to explain the heterogeneity of ventilation that could develop at different breathing frequencies. Based on its ability to detect lung heterogeneity, frequency dependence of compliance (and resistance) was thought to be an early indicator of lung disease [23,26-31]. Mead [31] stated that the compliance (including static) and resistance of airways in healthy subjects are much smaller than that for the air spaces. By modifying the compliance, resistance and time constant components in equations proposed by Otis et al [25], Mead [31] showed that RL in patients with chronic obstructive pulmonary disease can be up to twice as high as predicted values for healthy subjects over a range of respiratory frequencies up to 150 bpm.
Despite the elegant model proposed by Otis et al [25], however, reasons why compliance and resistance might be frequency dependent in normal and diseased lungs go beyond the concept of just two RC compartments. Subsequent models analyzed frequency dependence of lung compliance with respect to viscoelastic properties of the lung tissue and surface, and established the basis for later models of lung impedance [32-37]. Although such studies were more mathematically complex than that of Otis et al. [25], they were mainly theoretical in concept as there were no practical models to which the data could be applied. Impedance measurements can be performed noninvasively with a simple mouthpiece-breathing assembly and a device (such as a woofer) that can produce forced oscillations. Several models evaluating respiratory impedance, defined as the ratio of transpulmonary pressure to flow, have attempted to explain the observed changes, but with periodic signals (Eg: breathing), the variables turn out to be complex numbers dependent on magnitude and phase shifts.
Most measurements of frequency dependency of resistance, regardless of the method used (forced oscillatory, flow interruptor, esophageal balloon, alveolar capsule technique) have similarly shown a decrease in RL with increasing frequency [26,32,39,40]. This finding has also been attributed mainly to change in tissue resistance (Rtiss) and viscoelastic properties of the lung [26,35-40]. However, nonlinearities associated with the volume and frequency dependence of these properties have led to differing conclusions. An important consideration is the relative contributions of the airways and the dynamic properties of the lung and chest wall tissue to total lung resistance. Meanwhile, in a dog model Brown et al. [21] showed by computed tomography (CT) imaging relative increases in airway diameter following BT, more so in animals pretreated with methacholine, less so with atropine. Such changes have been attributed to loss or alteration of airway wall muscle, a finding that was demonstrated histologically by [19]. A loss or alteration of airway muscle mass would be expected to increase airway caliber as well as change the viscoelastic properties of the lung as a whole, and as a result, change the frequency dependence properties of lung resistance [32,33], as occurred in patients 2, 3, 4, and 5. This concept would explain why, following BT, RL was lower at rest and exhibited less variable frequency dependence than pre-BT.
Frequency dependence of compliance following BT decreased uniformly across all frequencies in the 2 most obese patients (nos.1 and 2). Lower lung zone alveolar atelectasis with reduction in airway diameters [41] and increased airway hyperresponsiveness independent of lung volume occur in obese animal models of asthma [42], findings that may be reversed with reduction in airway smooth muscle with BT. In the case of patient no. 5, the 60% increase in the RL ratio at a frequency of 60 breaths/min coupled with marked frequency dependency of compliance up to 40 breaths/min prior to BT would indicate mild-to-moderate heterogeneous airway constriction with a few highly constricted (large and small) or nearly closed small airways scattered throughout the periphery [43]. The respiratory infection that followed completion of BT would account for the near-doubling of resting RL, yet the now apparent frequency dependence of RL suggests that airway resistance originated from more uniformly involved peripheral airways with minimal contribution from larger airways. Our findings are, in general, similar to those recently reported by Farah et al. [44] who studied patients with refractory asthma using forced and impulse oscillometry techniques.
Limitations
There are some limitations to this study. First, of course, is the small number of patients tested. However, this was a pilot study, using a technique that requires some skill and is uncomfortable for patients to perform. From the standpoint of a physiologic explanation of its findings, it is intended to be hypothesis-generating. The general finding of frequency dependence of compliance and resistance attests to its reliability in its application to the question at hand. Secondly, we did not perform body plethysmography to measure airway resistance, which when separated from RL, would have provided Rtiss, a useful means of evaluating effects of BT on the viscoelastic properties of the lung. Third, patients were unable to maintain a constant Vt over the range of breathing frequencies, despite encouragement to do so. Variability in Vt can contribute to changes in Cdyn,L and RL [27,28]. However, the reduction in Vt observed with increasing frequencies exhibited a fairly consistent pattern so that its effects would be similar in all patients under similar circumstances. Another concern is that of the variable time elapsing between the pre- and post-BT measurements, which depended on when patients were able to return. However, the relative change in compliance and resistance values were not influenced by the time elapsed between measurements. More patients studied may enable the correlation of the time elapsed with changes in lung mechanics. Finally, we did not compare our patients to healthy control subjects or to asthmatic patients who underwent sham treatment with just bronchoscopy. It would be a challenge for the IRB to accept the application of sham bronchoscopy and esophageal balloon measurements in patients who would not be undergoing BT. Instead, since the main objective of the study was to assess the effects of BT on lung mechanics, patients served as their own control.
Conclusions
In this pilot study, individuals with severe, persistent asthma exhibit frequency dependence of lung compliance and resistance, which are ameliorated following BT in most patients. These findings strongly suggest that BT exerts its effects on the small airways as well as large airways.
References
- Barbers R, Papanikolaou IC, Koss MN, Ashish Patel, Elton Katagihara, et al. (2012) Near fatal asthma: Clinical and airway biopsy characteristics. Pulm Med.
- Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, et al. (2005) Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 116: 544-549.
- Hamid Q (2012) Pathogenesis of small airways in asthma. Respiration 84: 4-11.
- Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T (1992) Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol 72: 1016-1023.
- Ohrui T, Sekizawa K, Yanai M, Morikawa M, Jin Y, et al. (1992) Partitioning of pulmonary responses to inhaled methacholine in subjects with asymptomatic asthma. Am J Respir Crit Care Med.
- Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, et al. (2010) Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled study clinical trial. Am J Respir Crit Care Med 181: 116-124.
- Pavord ID, Cox G, Thomson NC, Rubin ASD, Corris PA, et al. (2007) Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 176: 1185-1191.
- Kraft M (1999) The distal airways: are they important in asthma? Eur Respir J 14: 1403-1417.
- Macklem PT, Woolcock AJ, Hogg JC, Nadel JA, Wilson NJ, et al. (1969) Partitioning of pulmonary resistance in the dog. J Appl Physiol 26: 798-805.
- Otis AB, McKerrow CB, Bartlett RA, Mead J, McIlroy MB, et al. (1956) Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 8: 427-443.
- Macklem PT, Mead JM (1967) Resistance of central and peripheral airways measured by a retrograde catheter. J Appl Physiol 22: 395-401.
- Baydur A, Virdi RR, Barbers R (2017) Lung compliance and resistance in patients with severe persistent asthma before and after undergoing bronchial thermoplasty: A pilot study. Am Rev Respir Crit Care Med 195: A6496.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, et al. (2005) Interpretative strategies for lung function tests. Eur Respir J 26: 948-968.
- Crapo RO, Morris AH, Gardner RM (1981) Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 123: 659-664.
- Milic-Emili J (1984) Measurement of pressures in respiratory physiology: techniques in the life sciences. Shannon, Ireland: Elsevier Scientific 1-22.
- Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788-791.
- Dixon WJ, Massey FJ (1983) Introduction to statistical analysis. (4th edtn), McGraw-Hill, New York.
- Baydur A, Sassoon CSH, Carlson M (1996) Measurement of lung mechanics at different lung volumes and esophageal levels in normal subjects: Effect of posture change. Lung 174: 139-151.
- Danek CJ, Lombard CM, Dungworth DL, Cox GP, Miller JD, et al. (2004) Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 97: 1946-1953.
- Cox PG, Miller J, Mitzner W, Leff AR (2004) Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 24: 659-663.
- Brown R, Wizeman W, Danek C, Mintzner W (2005) In vivo evaluation of the effectiveness of bronchial thermoplasty with computed tomography. J Appl Physiol 98: 1603-1606.
- Brown RH, Wizeman W, Danek C, Mitzner W (2005) Effect of bronchial thermoplasty on airway distensibility. Eur Respir J 26: 277-282.
- Woolcock AJ, Vincent NJ, Macklem PT (1969) Frequency dependence of compliance as a test for obstruction in the small airways. J Clin Invest 48: 1097-1106.
- Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, et al. (2015) Regional ventilation changes in severe asthma after bronchial thermoplasty with He-3 MR imaging and CT. Radiology 274: 250-259.
- Otis AB, McKerrow CB, Bartlett RA, Mead J, McIlroy MB, et al. (1956) Radford EP Jr. Mechanical factors in distribution of pulmonary ventilation. J Appl Physiol 8: 427-443.
- Grimby G, Takishima T, Graham W, Macklem P, Mead J (1968) Mead J. Frequency dependence of flow resistance in patients with obstructive lung disease. J Clin Invest 47: 1455-1465.
- Ingram RH, O’Cain CF (1971) Frequency dependence of compliance in apparently healthy smokers versus non-smokers. Bull Physiopathol Respir 7: 195-212.
- Levine G, Housley E, MacLeod P, Macklem P, Macklem PT (1970) Gas exchange abnormalities in mild bronchitis and asymptomatic asthma. N Engl J Med 282: 1277-1282.
- Seaton A, Lapp NL, Morgan WK (1972) Lung mechanics and frequency dependence of compliance in coal miners. J Clin Invest 51: 1203-1211.
- Wanner A, Zarzecki S, Atkins N, Zapata A, Sackner MA (1974) Relationship between frequency dependence of lung compliance and distribution of ventilation. J Clin Invest 54: 1200-1213.
- Mead J (1956) Contribution of compliance of airways to frequency-dependent behavior of lungs. J Appl Physiol 8: 427-443.
- D’Angelo E, Robatto FM, Calderini E, Tavola M, Bono D, et al. (1991) Pulmonary and chest wall mechanics in anesthetized paralyzed humans. J Appl Physiol 70: 2602-2610.
- Shardonofsky FR, Sato J, Eidelman DH (1994) Frequency dependence of pulmonary and chest wall mechanics in young and adult cats. J Appl Physiol 76: 2037-2046.
- Horie T, Hildebrandt J (1971) Dynamic compliance, limit cycles, and static equilibria of excised cat lung. J Appl Physiol 31: 423-430.
- Jackson AC, Watson JW, Kotlikoff MI (1984) Respiratory system, lung and chest wall impedances in anesthetized dogs. J Appl Physiol 57: 34-39.
- Kappos AD, Rodarte JR, Lai-Fook SJ (1981) Frequency dependence and partitioning of respiratory impedance in dogs. J Appl Physiol 51: 621-629.
- Peslin R, Duvivier C, Hannhart B (1984) Respiratory mechanical impedances. Methodology and interpretation. Biorrheology 1: 183-191.
- Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, et al. (1983) Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol 55: 1733.
- Hantos Z, Daroczy B, Suki S, Nagy S, Fredberg JJ (1992) Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168-178.
- Brusasco V, Warner DO, Beck KC, Rodarte JR, Rehder K (1989) Partitioning of pulmonary resistance in dogs: effect of tidal volume and frequency. J Appl Physiol 66: 1190-1196.
- Saraiva SA, Silva AL, Xisto DG, Abreu SC, Silva JD, et al. (2011) Impact of obesity on airway and lung parenchyma remodeling in experimental chronic allergic asthma. Respir Physiol Neurobiol 177: 141-148.
- Shore SA (2007) Obesity and asthma: lessons from animal models. J Appl Physiol 102: 516-528.
- Lutchen KR, Gillis H (1997) Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol 83: 1192-1201.
- Farah C, Langton D, Pierucci P, Ing A (2017) Changes in lung function and forced oscillatory technique (fot) parameters following bronchial thermoplasty (bt) in patients with refractory asthma. Am J Respir Crit Care Med 195: A6497.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi