Case Report, J Clin Exp Oncol Vol: 6 Issue: 7
Mantle Cell Lymphoma Misdiagnosed as Chronic Lymphocytic Leukemia: Optimization of Diagnostic Approach
Numan Fateh*
Department of Hematology and Oncology, Larkin Community Hospital, South Miami, Florida, USA
*Corresponding Author : Numan Fateh
Department of Hematology and Oncology, Larkin Community Hospital, 7000 SW 62nd Avenue, Suite 401, South Miami, Florida, 33143, USA
Tel: 305-284-7761
Fax: 305-284-7706
E-mail: numanaf@yahoo.com
Received: October 16, 2017 Accepted: November 06, 2017 Published: November 13, 2017
Citation: Fateh N (2017) Mantle Cell Lymphoma Misdiagnosed as Chronic Lymphocytic Leukemia: Optimization of Diagnostic Approach. J Clin Exp Oncol 6:6. doi: 10.4172/2324-9110.1000200
Abstract
Mantle cell lymphoma (MCL) is a mature B-cell non-Hodgkin lymphoma that is relatively uncommon. MCL is an aggressive lymphoma and at times can share many features with chronic lymphocytic leukemia (CLL). CLL is positive for CD5+ (in 80% of cases), CD20+ (95%) and CD23+ (85%). MCL is also positive for CD5+ (80%) and CD20+ (94%), but generally negative for CD23-. However, there are cases of CD23 positive MCL which can lead to misdiagnosis. Cyclin D1 is more specific than CD markers, but is positive in approximately 85-90% of cases. Thus for 15% of cases this test is also not reliable to diagnose MCL. For example, there are reports of Cyclin D2 and Cyclin D3 involvement instead of the more commonly known Cyclin D1. The same is true for t (11;14) studies by Fluorescence in situ hybridization (FISH) for MCL. Though it is a rare entity, there are known cases of t (11;14) negative MCL. In cases such as these, it may be difficult to correctly diagnose MCL. Therefore, it is important to have an understanding of the similarities and differences of these two diseases and to be aware of the less commonly used tests to help differentiate between MCL and CLL.
Keywords: Mantle Cell Lymphoma; Chronic lymphocytic leukemia; MCL; CLL
Introduction
Mantle cell lymphoma (MCL) is a mature B-cell non-Hodgkin lymphoma involving the small to medium sized B cells. Pathologically, MCL can be diffuse or nodular in architecture expressing CD5, CD20, and CD43, but usually lacking CD10 and CD23. Interestingly, overexpression of Cyclin D1 is more specific for MCL and therefore important in the diagnosis as well [1]. The most common presenting symptom is lymphadenopathy with an estimated â…“ of patients presenting with B symptoms such as fever, night sweats, and unintentional weight loss at presentation. Unfortunately, it has been found that ~ 70% of patients have stage IV disease at diagnosis. Hence it is imperative to develop methods for identifying and differentiating MCL from other forms of cancer [1].
Prior to flow cytometry, cytogenetics and molecular assays, the diagnosis of chronic lymphocytic leukemia (CLL) vs. MCL could be misdiagnosed in atypical cases. For the medical hematology/ oncology clinician, this has significant implications on the decisions of monitoring and management of the disease. It is also important to note that not all facilities internationally use these more modern tests routinely.
We present a case of a patient who was initially diagnosed with CLL in her native country. She remained untreated for many years until she immigrated to the US. She was suspected of having progression of her CLL requiring treatment. A repeat work up including peripheral flow cytometry and bone marrow biopsy was initially interpreted as consistent with CLL. However, a more insightful examination using immunohistochemistry and molecular studies revealed that she in fact had MCL and not CLL as she was originally told for many years. Therefore, it is important to have an understanding of the similarities and differences of these two diseases and to be aware of the less commonly used tests to help differentiate between MCL and CLL a matter highlighted in recent case.
Case Report
A 64 year old Cuban female with a past medical history of hypertension, hyperlipidemia and hypothyroidism was previously diagnosed with chronic lymphocytic leukemia (CLL). The patient reported those 7 years prior to our consultation, in another facility overseas; she had an elevated white blood cell count and was diagnosed with CLL. She states that she was asymptomatic at that time and did not need treatment. Per the records she provided, she had a lymphocyte predominant leukocytosis which was CD5+, CD23+, but CD10-. In 2013, she moved to Florida and saw a hematologist to establish care in the USA. She was told that she instead had MCL. The patient states she was nonetheless told she did not require treatment. Apparently a repeat bone marrow biopsy was not performed at that time. Her leukocytosis was monitored and values available showed steadily increasing WBC count. Specifically her WBC had been trending upward from 02/2014 WBC 67.8×109/L,06/2014 WBC 72.4×109/L, 03/2015 WBC 87.9×109/L, 10/2015 WBC 85.4×109/L, 12/04/2015 WBC 98.4×109/L with 89% lymphocytes. The Hgb, Hct and Plt values were 12.1g/dl, 38.7% 173,000 /mcL, respectively. She visited our service for consultation in 12/2015. At that time she complained of night sweats and fatigue. She was also noted to have splenomegaly on exam as well as a 2cm right axillary lymph node which was nontender. In order to clarify her diagnosis, further workup was performed including repeat bone marrow biopsy and excisional lymph node biopsy.
Serum Protein Electrophoresis (SPEP) showed a decreased gamma globulin 0.4 g/dl. The B2 microglobulin was elevated at 3.30 mg/L. Peripheral flow cytometry was interpreted with a diagnosis of CLL. The clonal B cells detected CD5+ and CD23+ and surface kappa light chain lymphocytes, CD20 was dim and CD38 was expressed in 0.3% of CD19+CD5+ B cells. PET-CT showed multiple focal regions of increased Fluoro-deoxyglucose (FDG) uptake identified within the neck and axillary regions bilaterally corresponding to multiple prominent lymph nodes. There was also diffuse uptake within the spleen which measured at 13.8cm corresponding to a borderline enlargement. The patient underwent a bone marrow biopsy which was initially interpreted as a B cell lymphoproliferative disorder with CD5+, CD20+ and CD23+, but monoclonal B cells felt to be consistent with CLL. In addition, immunohistochemistry testing for Cyclin D1 was found to be positive raising concern for MCL. Therefore a FISH study for t(11;14) was requested which turned out to be positive for as well as P53 and 13q14 deletions.
The patient was started on a Bendamustine 90 mg/m2 and Rituximab 375 mg/m2 regimen. Her course was complicated by severe neutropenia requiring decreased Bendamustine dosing by 25%. Her B symptoms resolved with the treatment. She underwent a repeat bone marrow biopsy which showed maturing trilineage hematopoiesis and no immunophenotypic evidence of lymphoma. Bone marrow flow cytometry did not demonstrate a monoclonal B cell population suggesting that she was responding to the current treatment .
Discussion
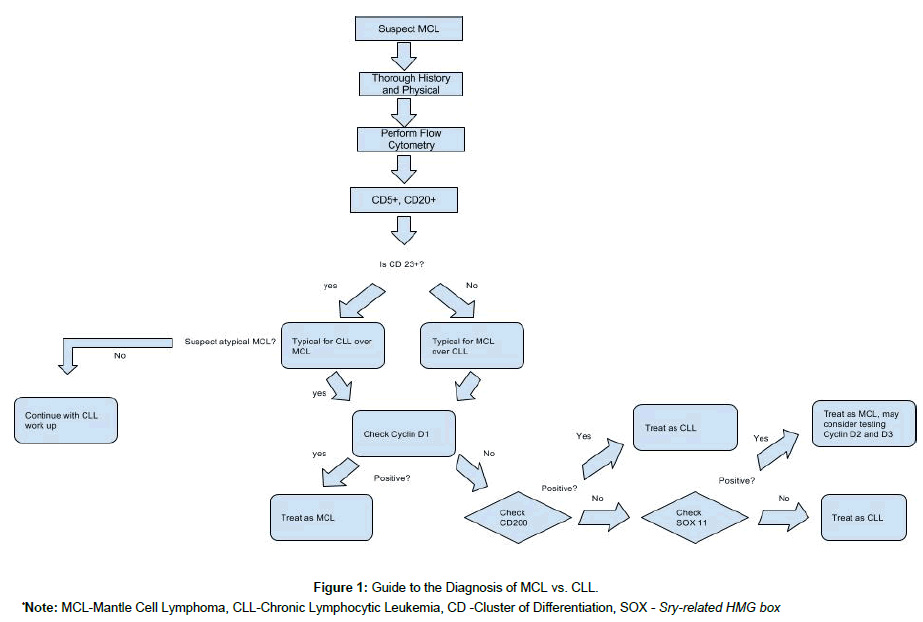
Both MCL and CLL are considered malignancies of the small to medium-sized lymphocytes with some similarities by immunohistochemistry. Normally, CLL can be differentiated from MCL-based on morphology. CLL is known to generally infiltrate the lymph node in a diffuse pattern with pseudo nodules. In contrast, MCL can infiltrate lymph nodes in a diffuse, nodular or mantle zone type pattern [5]. Generally, flow cytometry was employed to aid in the diagnosis of either of these two diseases. Typically, CLL is positive for CD5+ (in 80% of cases), CD20+ (95%) and CD23+ (85%). MCL is also positive for CD5+ (80%) and CD20+ (94%), but generally negative for CD23-. However, there are cases of CD23 positive MCL which can lead to misdiagnosis. One test that can help differentiate CLL from MCL is immunohistochemistry for Cyclin D1 (Table 1). In addition, FISH study for t(11;14) is also important in the diagnosis of MCL as this translocation is not typical of CLL [6]. However there are studies suggesting that not all MCL is Cyclin D1 positive. Cyclin D2 and Cyclin D3 have also been implicated in cases which appear morphologically consistent with MCL [5]. Further complicating distinguishing between these two diseases is a case in which a patient had morphological lymph node characteristics of CLL, but Cyclin D1 positivity. The FISH for t(11;14) was negative in this case [7].
| Type of Malignancy | CD5 | CD 20 | CD 23 | CD 43 | CD 200 | Cyclin D1 | t(11:14) |
|---|---|---|---|---|---|---|---|
| Mantle Cell Lymphoma | + | + | - | + | - | + | + |
| Chronic Lymphocytic Leukemia | + | + | + | +/- | + | - | - |
Table 1: Common Features of MCL vs. CLL.
Recently, the transcription factor SOX11 was found to be significantly upregulated in cases of MCL [8]. Classical MCL is SOX11 positive suggesting that the lymphoma has not entered the follicular germinal center. SOX11 negative MCL is a known variant but is less common than SOX11 positive type. SOX11 is not generally upregulated in CLL and testing for this by immunohistochemistry may help differentiate between the two diseases (Figure 1).There is also a study testing unique methylation profiles of these two diseases. In particular, the degree of methylation of homeobox genes was noted to be higher in MCL as compared to CLL. In the future, this may serve as an alternate means to differentiate these two diseases as well [9,10].
In regards to treatment, approximately 10-15% of MCL patients have an indolent course for which a watch and wait approach is appropriate. However, the majority of patients have disseminated disease at diagnosis. In contrast, it is known that a larger percentage of patients with CLL are asymptomatic and found due to incidental leukocytosis and/or lymphocytosis. In those patients with MCL requiring treatment, there is some overlap with commonly accepted CLL regimens. Frequently used regimens include R-CHOP, R-CVP or BR. HyperCVAD is a more aggressive approach however this regimen is more difficult to tolerate. For those patients who relapse, bortezomib, lenalidomide and ibrutinib are examples of FDA approved treatments.1There is no single standard regimen that is accepted in patients who are not transplant candidates [2].
Conclusion
This case demonstrates the difficulties in distinguishing MCL and CLL. It also demonstrates the need to remain vigilant when a patient presents with a previous diagnosis as they may have been misdiagnosed. In this case the patients’ initial work up with peripheral flow cytometry as well as initial bone marrow biopsy analysis suggested the incorrect diagnosis of CLL.
It is important to keep in mind those patients who were diagnosed many years prior and whose clinical picture is inconsistent may need to undergo repeat work up to confirm their diagnosis. Also those patients diagnosed in countries which more modern studies are not as readily available can potentially mistake CLL and MCL. Though testing for cyclin D1 or t(11;14) translocation helped to make the correct diagnosis in this case, these may not be detected in all cases. SOX11 is an additional test that can help distinguish between these two disease entities. In the future, testing for unique methylation profiles may help to differentiate these two diseases as well.
References
- DeVita VT, Lawrence TS, & Rosenberg SA (2015) Cancer: Principles & practice of oncology (10th edtn), Wolters Kluwer Health, Philadelphia.
- Campo E, Rule S (2015) Mantle cell lymphoma: evolving management strategies. Blood 125: 48-55.
- Ferrer A, Salaverria I, Bosch F, Villamor N, Rozman M (2007) Leukemic involvement is a common feature in mantle cell lymphoma. Cancer 109: 2473-2480.
- Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD (1997) Mantle Cell Lymphoma: A Clinicopathologic Study of 80 Cases. Blood 89: 2067-2078.
- Zhao XF (2009) Pitfalls in Diagnostic Hematopathology: Part I. Int J Clin Exp Pathol 2: 11-20.
- Barna G, Reiniger L, Tátrai P, Kopper L, Matolcsy A (2008) The cut-off levels of CD23 expression in the differential diagnosis of MCL and CLL. Hematological Oncology 26: 167-170.
- O'Malley DP, Vance GH, Orazi A (2005) Chronic lymphocytic leukemia/small lymphocytic lymphoma with trisomy 12 and focal cyclin d1 expression: a potential diagnostic pitfall. Arch Pathol Lab Med 129: 92-95.
- Wasik AM, Priebe V, Lord M, Jeppsson-Ahlberg A, Christensson B (2015) Flow cytometric analysis of SOX11: a new diagnostic method for distinguishing B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma from mantle cell lymphoma. Leuk Lymphoma 56: 1425-1431.
- Halldórsdóttir, Anna Margrét (2012) Mantle Cell Lymphoma Displays a Homogenous Methylation Profile: A Comparative Analysis with Chronic Lymphocytic Leukemia. Am J Hematol87: 361-367.
- Hamad N, McIlroy K, Ward C, Armytage T (2014) Primary Cutaneous Mantle-Cell Lymphoma: A Case Report and Literature Review. J Clin Oncol 33.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi