Research Article, Res Rep Metals Vol: 2 Issue: 1
Metallothermic Reactions - Past, Present and Future
Fathi Habashi*
Department of Mining, Metallurgical, and Materials Engineering, Laval University, Quebec City, Canada
*Corresponding Author : Fathi Habashi
Department of Mining, Metallurgical, and Materials Engineering , Laval University, Quebec City, Canada
E-mail: fathi.habashi.1@ulaval.ca
Received: January 11, 2017 Accepted: January 26, 2018 Published: February 02, 2018
Citation: Habashi F (2018) Metallothermic Reactions - Past, Present and Future. Res Rep Metals 2:1.
Abstract
Metallothermic reactions are batch processes in which both reactants are solids. There are a few exceptions, however, where at the reaction temperature the reducing metal is a liquid and the other reactant is a gas. In both cases numerous steps are involved and that is why it has been challenged in the future by fused salt electrolysis.
Keywords: Alkali metals; Aluminium, Humphry Davy; Sainte-Claire Deville; Uranium; Thermit; Fused salt electrolysis
Introduction
The displacement of a metal by another metal from aqueous solution is an old technology known to the alchemists. Thus copper was precipitated from a copper sulfate solution by metallic iron was practiced already in the middle ages. The displacement of another metal from its solid compounds by another metal by thermal methods was possible only after the discovery and isolation of the alkali metals by Humphry Davy (1778-1809) (Figure 1). The technique was expanded further after the discovery and isolation of aluminum by Hans Christian Ørsted (1777-1871) (Figure 2) in 1826 and Friedrich Wöhler (1800-1882) (Figure 3) in 1827-45. The fact that it was difficult to liberate the alkali metals and aluminum from their “earths” suggested that these metals can themselves be used to liberate other metals difficult to isolate from their compounds [1].
Figure 2: Hans Christian Ørsted (1777-1871) Danish medical doctor who became professor of physics at the University of Co- penhagen in 1806. He laid the foundation for electromagnetism and was the first to isolate alu- minum in 1825. He was ap- pointed director of the Polytech- nical College in Copenhagen when founded in 1829.
Figure 3: Friedrich Wöhler (1800-1882) was a student of Berzelius in Stockholm. On his way home he stopped in Copen- hagen to visit Ørsted and know about his discovery of alumi- num. He accidentally synthe- sized urea in 1828 which was the first organic synthesis that shat- tered the vitalism theory.
Historical Background
This idea of displacement of a metal from its solid compounds by another metal by thermal methods was first applied in 1808 by the French chemists Joseph Louis Gay-Lussac (1778–1850) (Figure 4) and Louis Jacques Thénard (1777–1857) (Figure 5) and independently by Davy who was the first to isolate boron by reacting boric acid with potassium. In 1811, Gay-Lussac and Thénard passed gaseous silicon tetra- fluoride over heated potassium; although they did not identify the brown mass they obtained, it was believed to be an impure amorphous silicon. Silicon tetrafluoride was already prepared in 1771 by the Swedish chemist Carl Wilhelm Scheele (1742-1786) by heating a mixture of sand and fluorspar with concentrated sulfuric acid. It was another Swedish chemist Jöns Jakob Berzelius (1779-1848) (Figure 6), however, who developed the technique further and prepared few other metals for the first time by this method. He prepared zirconium in 1824, titanium in 1825, thorium in 1828, and vanadium in 1831. Other chemists followed suit later [2].
Figure 4: Joseph Louis Gay- Lussac (1778–1850), Professor of Chemistry at the École Poly- technique (1806), then Professor of Physics in the Sorbonne (1809), then Professor of Chem- istry in the Jardin des Plantes (1832). In 1802 he showed that different gases expanded by equal amounts with a rise in temperature. He isolated boron in 1808.
Figure 6: Jöns Jakob Berzelius (1779–1848) was professor at the Karolinska Institutet in Stock- holm. In 1809 he began his long association with the Swedish Academy of Sciences. He dis- covered cerium, selenium, and thorium. Students working in his laboratory also discovered lith- ium, vanadium, and several rare earths. He determined the atomic weights of nearly all the ele- ments then known and created the system of symbols of ele- ments used today. He wrote Lärbok i Kemien in 1808 which was widely translated.
Industrial Application
The first industrial application of metallothermic reactions was in 1854 by Henri Sainte-Claire Deville (1818–1881) (Figure 7) who produced metallic aluminium by reduction of the molten double chloride, AlCl3·NaCl, with sodium. The endothermic reaction was conducted in a reverberatory furnace (Figure 8).
Figure 7: Henri Sainte- Claire Deville (1818–1881) was appointed in 1851 a re- searcher in the École normale supérieure in Paris where he started his work on the pro- duction of aluminum. In 1853 he moved to the Sor- bonne and in 1859, wrote De l’aluminium, ses propriétés, sa fabrication etses applications.
AlCl3·NaCl + 3Na → Al + 4NaCl
When in 1888 Charles Martin Hall (1863-1914) (Figure 9) in USA and Paul Louis Toussaint Héroult (1863-1914) (Figure 10) in France invented the electrolytic process for the production of aluminum, simultaneously and independently, their process immediately displaced Sainte-Claire Deville’s process. Thus an electrochemical process displaced a metallothermic process.
Development of the Process
Metallothermic reactions are, therefore, concerned with the preparation of metals and alloys by reduction of their oxides or halides with a metal. These reactions can be expressed in general by the equation:
AX + B → A + BX
Where X is oxygen, chlorine, or fluorine, and A and B are two metals. It is characterized by the fact that the reducing metal is converted to a solid or a liquid product and not to a gas as in other reduction processes, e.g., by carbon or hydrogen where CO + CO2 and H2O are formed, respectively. In fact this method is used when reduction by carbon and hydrogen or by electro winning from aqueous solutions is not possible (Table 1).
| Metal | Year | Researchers | Reaction | Purity |
|---|---|---|---|---|
| B | 1808 1895 |
Gay-Lussac, Thénard, Davy Moissan | HBO3 + K B2O3 + Mg | |
| Si | 1822 | Berzelius | SiF4 + K | |
| Zr | 1824 1914 |
Berzelius Leyle and Hamburger |
ZrCl4 + Na K2ZrF6 + K or Na ZrCl4 + Na | |
| Th | 1828 1914 |
Berzelius Leyle and Hamburger |
ThO2 + Na | Pure |
| Ti | 1825 1887 1910 1937 |
Berzelius Nilson and Pettersson Hunter Kroll |
K2TiF6 + K TiCl4 + Na TiCl4 + Na TiCl4 + Mg or Ca |
Impure 95% 99.9% |
| Be | 1828 1828 |
Wöhler Bussy | BeCl2 + K BeF2 + Na | Impure |
| Y | 1828 | Wöhler | YCl3 + Na | |
| Mg | 1830 | Bussy | MgCl2 + K vapour | |
| 1940 | Pidgeon | MgO + FeSi | ||
| V | 1831 | Berzelius | ||
| 1927 | Marden and Rich | V2O5 + Ca | ||
| La | 1840 | Mosander | LaCl3 + K | |
| U | 1841 | Péligot | UCl4 + K | |
| 1856 | Péligot | UF4 + Mg | ||
| Al | 1825 | Ørsted | AlCl3 + K(Hg) | Tiny particles of aluminum |
| 1827, | Wöhler | AlCl3 + K | Large particles of aluminum | |
| 1845 | Sainte-Claire Deville | AlCl3 + Na | The first bar of aluminum | |
| 1854 | Rose | Na3AlF6 + Na | ||
| Ta | 1866 | Marignac | K2TaF9 + Na | |
| 1903 | von Bolton | |||
| Nb | 1866 | Marignac | K2NbF6 + Na | |
| 1906 | von Bolton | NB2O5 + Al | ||
| 1929 | Balke | NbCl5 + Mg | Pure | |
| Cs | 1905 | Hackspill | CsCl + Ca | Vacuum |
| Rb | 1905 | Hackspîll | RbCl + Ca | Vacuum |
| Ba | 1906 | Guntz | BaO + Al | |
| 1913 | Matignon | BaO + Al | Vacuum | |
| Hf | 1924 | Coster and von Hevesy | HfCl4 + Mg | Impure |
| 1925 | van Arkel and de Boer | Decomposition of HfI4 | Pure | |
| Pu | 1943 | Researchers at Manhattan Project | PuF4 + Ba vapor | Microgram amount under vacuum |
| 1944 | Researchers at Los Alamos | PuF3 + Ca | Gram amounts in a bomb reactor | |
| Ca | 1951 | Hackspill | CaCl2 + Al |
Table 1: Metals first isolated by metallothermic reaction.
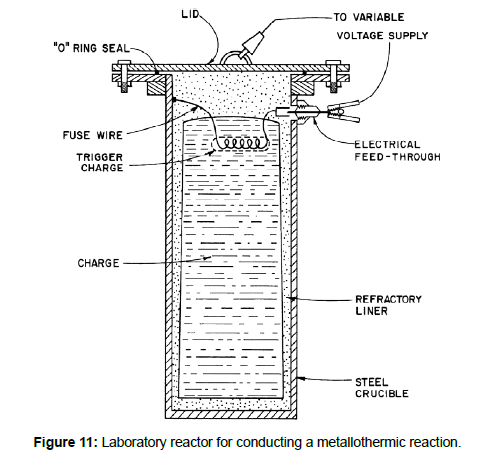
The most commonly used reducing metals are aluminum, calcium, ferrosilicon, magnesium, and sodium. The choice of a reducing metal is based on many factors [3]. In general, the metal must have a strong affinity for the compound to be reduced, it should be cheap, have a high boiling point, low vapour pressure, produce a slag that can be easily melted or leached away, does not form intermetallic compounds with the metal produced, and can be easily handled. Metallothermic reactions may be exothermic or endothermic. In the exothermic system, the reaction takes place in such a way that when the mixture is ignited at one place, the reaction continues to go on spontaneously usually with the formation of a fluid slag, and the reduced metal is obtained as a compact uniform regulus [4,5](Figures 11-13).
Thermit Reaction and the Incendiary Bomb
The reduction of Fe2O3 by aluminium is so highly exothermic that molten iron and molten alumina are obtained:
Fe2O3 + 2Al → 2Fe + Al2O3
This reaction is the basis of Thermit process introduced by the German chemist Hans Goldschmidt (1861–1923) (Figure 14) in 1899 to weld rails (Figure 15). The same reaction was also used to make incendiary bombs. In this case, the mixture is enclosed in a magnesium alloy which ignites rapidly when the above reaction takes place thus extensive heat is generated.
Figure 14: Hans Goldschmidt (1861–1923) studied chemistry at Berlin University then joined the management of the family firm Chemische Fabrik Theodor Goldschmidt. In 1899 he developed the Thermit process originally meant for the preparation of carbon-free metals became the standard method for welding railroad and streetcar rails.
Boron
After the isolation of elemental boron by Gay-Lussac, Thénard, and Davy in 1808 crystalline boron was obtained in 1856 by the German chemist Friedrich Wöhler (1800-1882) by dissolving amorphous boron in molten aluminum. On cooling crystalline boron separates out and recovered by dissolving aluminum in sodium hydroxide. The French chemist Henri Moissan (1852-1907) (Figure 16) first obtained in 1895 considerable quantities of boron of 86% purity, by reduction of boric oxide with magnesium.
Silicon
In 1811 Gay-Lussac and Thénard prepared impure amorphous silicon by heating silicon tetrafluoride with potassium but they did not purify and characterize the product. In 1824 Berzelius prepared amorphous silicon using approximately the same method, but purifying the product to a brown powder by repeatedly washing it. Silicon in its more common crystalline form was prepared in 1854 by Sainte Claire Deville by electrolyzing impure sodium-aluminum chloride containing around 10% silicon.
Zirconium
The mineral zircon was not known to contain a new element until 1789 when German chemist Martin Heinrich Klaproth (1743- 1817) analyzed a sample from the island of Ceylon (now Sri Lanka). He named the new element Zirkonerde. Zirconium metal was first obtained in an impure form in 1824 by Berzelius by heating a mixture of potassium and potassium zirconium fluoride in an iron tube. The iodide process was the first industrial process for the commercial production of metallic zirconium. The process involves the formation and subsequent thermal decomposition of zirconium iodide. This method was superseded in 1945 by the Kroll process developed by in which zirconium tetrachloride was reduced by magnesium.
Thorium
Berzelius analyzed a mineral from the Falun district in Sweden in 1828 and determined that it contained a new element, which he named thorium after Thor, the Norse god of thunder. He prepared the impure metal by heating a mixture of potassium thorium fluoride with potassium in a glass tube. The metal had no uses until Carl Auer von Welsbach invented the gas mantle in 1885. Thorium was first observed to be radioactive in 1898, independently by Marie Curie in Paris and G. C. Schmidt at the University of Mün- ster in Germany. In 1914 two researchers in Germany D. Leyle and L. Hamburger obtained the metal by reduction of ThO2 with sodium.
Titanium
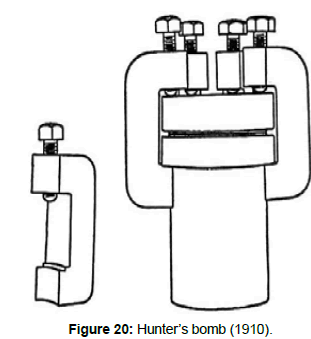
Titanium was first isolated by Berzelius in 1825 by reduction of K2TiF6 with potassium. In 1887 Lars Nilson (1840–1890) (Figure 17) and Sven Otto Pettersson (1848-1941) (Figure 18) in Stockholm isolated the metal in 95% purity by reduction of TiCl4 with sodium. In 1910 M. A. Hunter (Figure 19) in USA succeeded in preparing 99.9% pure titanium metal by reducing TiCl4 with sodium in a steel bomb (Figure 20). In 1922 Anton Eduard van Arkel (1893-1976) in the Netherlands produced pure ductile titanium in the laboratory by the iodide process. In 1938 Wilhelm J. Kroll (1889-1973) (Figure 21) in Luxemburg produced pure titanium by reducing TiCl4 with magnesium. Under Kroll’s direction the US Bureau of Mines operated in 1942 a pilot plant in Boulder City, Nevada, for the production of titanium based on his process.
Figure 19: Matthew A. Hunter (1878–1961) was born in Auckland, New Zealand, studied at Uni- versity College in London, then came to USA to accept an employment with the research laborato- ries of General Electric Company. In 1908 he joined Rensselaer Polytechnic Institute in the Elec- trical Engineering Department.
Figure 21: Wilhelm J. Kroll (1889–1973) had to flee Luxemburg to the United States in 1940 when Nazi troops were advancing in Europe. He joined US Bureau of Mines in Albany, Oregon where he de- veloped further his research on the production of zirconium and titanium. In the 1950s, he produced tita-nium on commercial scale. On his retirement in 1960, he donated generously to Colorado School of Mines, where the Kroll Institute for Extractive Metallurgy was founded in 1973.
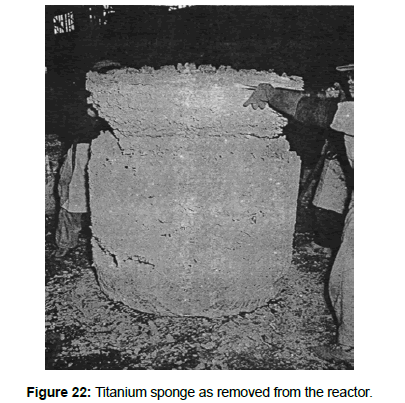
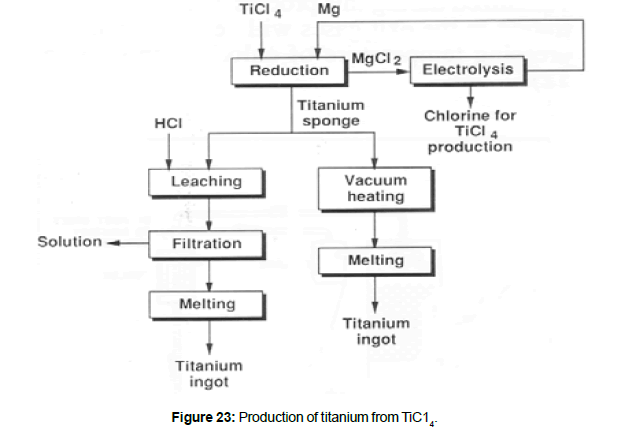
1948 E. I. du Pont de Nemours & Company produced the first commercial titanium (10 tonnes/year). In 1952 Imperial Chemical Industries in England produced titanium on a commercial scale (5 tonnes/year) by the Hunter Process. In 1954 Osaka Titanium Company in Japan produced titanium on a commercial scale (12 tons/year) by the Kroll Process. At about the same time, titanium production also started in the former USSR. In 1990 world titanium metal production was about 120 000 tonnes. (Figure 22) shows a titanium sponge as removed from the reactor. Metallic titanium is produced by the reduction of pure TiCl4 by metallic magnesium or sodium. Reduction by magnesium accounts for 80 % of world production (Figure 23). The metal produced is in form of a sponge which is then purified and then melted. When magnesium is used, the reaction can be rep- resented by:
TiC14 + 2Mg → Ti + 2MgC12
Titanium tetrachloride which is liquid at room temperature is injected into an evacuated air-tight heated reactor containing the reducing metal in the molten state. Titanium tetrachloride immediately evaporates and the vapors interact with the molten metal to deposit titanium crystals on the walls of the reactor at the liquid interface. These crystals form the nuclei for the further deposition of the metal in the form of a sponge. Liquid slag (MgC12) obtained in the reaction sinks to the bottom below the molten metal because of differences in the specific gravities. Enough TiC14 is admitted into the reactor so that the molten metal is completely consumed. The bulk of slag is then forced out of the reactor from the discharge pipe by means of argon. (Figure 24) show typical reactor used for the reduction of TiC14 . Magnesium chloride formed in the reduction step is treated in an electro winning plant in the fused state to produce the magnesium necessary for reduction and the chlorine necessary for the formation of TiC14.
Beryllium
Beryllium was discovered by the French chemist Nicolas-Louis Vauquelin (1763-1829) in 1797 and was prepared in 1828 in minute amounts by Friedrich Wöhler and independently by Antoine Bussy by the action of potassium on beryllium chloride. The French chemist Paul Lebeau (1886-1959) prepared the pure metal in 1898 by electrolysis of a fused beryllium fluoride-potassium fluoride mixture. In 1932 Alfred Stock (1876-1946) (Figure 25) and Hans Goldschmidt in Germany devised the first commercial process, in which a mixture of the fluorides of beryllium and barium are electrolyzed.
Yttrium
Yttrium was discovered in 1794 by the Finnish chemist Johan Gadolin (1760-1852) in Ytterby mine near Stockholm in Sweden. It was first isolated by Wöhler in 1828 by reacting YCl3 with sodium.
Magnesium
Magnesium was discovered and isolated by Davy in 1808 by fused salt electrolysis of MgCl2. Antoine Alexandre Brutus Bussy (1794–1882) (Figure 26) prepared a sample in 1831 in a coherent form by heating magnesium chloride and potassium in a glass tube. When he washed out the potassium chloride, small, shining globules of magnesium remained. The first industrial production of magnesium by electrolysis of molten carnallite from Stassfurt deposits began in 1886 in Hemelingen near Bremen in Germany. The quantity was small but sufficient for the need in the world at that time. Few years later an- other operation started by Chemische Fabrik Griesheim Elktron in Bitterfeld near Leipzig. In 1915 Dow Chemical Company started producing magnesium in Midland, Michigan from naturally-occurring subterranean brine. The German chemical companies were amalgamated in 1916 and became known as IG Farbenindustrie in 1925. In the 1930s production increased owing to the introduction of the light, corrosionresistant alloy Electron containing aluminum, magnesium, and zinc. The use of magnesium in the aircraft industry in World War II resulted in greater demand and large-scale production methods had to be found. In 1941, the Dow Chemical Company produced at Freeport, Texas an ingot of magnesium which was the first metal ever to be taken from sea water. During World War II, Lloyd M. Pidgeon (1903–1999) (Figure 27) succeeded in producing the metal by metallothermic reduction of MgO using ferrosilicon. Later on, however, electrolytic processes were developed that resulted in sharing nearly half of the production.
Vanadium
Vanadium was discovered twice: in 1801 by the Spanish mineralogist Manuel del Rio (1764-1849) at the School of Mines in Mexico City and in 1830 by the Swedish chemist Nils Sefström ((1787-1854) at the School of Mines in Falun. It was the Swedish chemist Jöns Jacob Berzelius who confirmed that both metals were the same. The metal was then isolated in powder form in England by Henry Roscoe (1833-1915) in 1867 by reduction of vanadium dichloride with hydrogen. In 1927, J.W. Marden and M.N. Rich of the research staff of the Westinghouse Lamp Company in USA obtained ductile vanadium 99.9% pure by reducing vanadium pent oxide with calcium.
Lanthanum
Lanthanum was discovered in 1828 by the Swedish chemist Carl Gustav Mosander (1797–1858) (Figure 28) and he isolated the pure metal by heating LaCl3 with potassium.
Uranium
When the French chemist Eugène Péligot (1811–1890) (Figure 29) realized that the substance consid- ered by Martin Heinrich Klaproth (1743-1817) to be metallic uranium was in reality an oxide, he obtained the metal in 1841 by reduction of the tetrachloride with sodium. During World War II, uranium was prepared for the first time on large scale by the reduction of UF4 by magnesium. This was in connection with the Manhattan Project for the manufacture of an atomic bomb. The work was conducted at Iowa State University in Ames, Iowa, under the direction of Harley A. Wilhelm (1900–1995) (Figure 30). With his process, still in use today, Ames Laboratory produced over 1000 tons of high-purity uranium for the Manhattan Project.
Figure 29: Eugène Péligot (1811–1890) professor of chemistry at the École centrale des Arts et des Manufactures in Paris. He was the first to isolate uranium by reduction of UCl4 by potassium and UF4 by magnesium and the first to use an organic solvent for the extraction and purification of metal ions. He extracted uranyl nitrate solution by ether in 1842 to separate it from other constituents of pitchblende. About one hundred years later, the Mallinckrodt Chemical Works operated a uranium refinery in the USA based on this principle. Extraction of metal ions from aqueous solutions by organic solvents in now wide spread. Péligot was employed at the Mint in Paris for forty years.
Niobium and Tantalum
Jean Charles Galissard de Marignac (1817-1894) (Figure 31) in Geneva was the first to isolate niobium and tantalum in 1866 by crystallization of the double fluorides, K2NbF6 and K2TaF6 then their reduction with sodium. Werner von Bolton (1868-1912) (Figure 32) in Berlin prepared pure tantalum in 1903 and pure niobium in 1906 by reacting the oxides with aluminum powder. Clarence William Balke (1880-1948) Professor of Inorganic Chemistry at the University of Illinois (1907-1916) then director of Fan- steel Products in North Chicago in 1916 prepared high-purity niobium in 1929 by reducing NbCl5 with magnesium.
Cesium, Rubidium, and Calcium
Cesium and rubidium were discovered in 1860 and 1861, respectively, by Robert Bunsen (1811-1899) in the mineral water of Durkheim using the spectroscope he recently invented with Gustav Kirchhoff. Bunsen also isolated salts of the metals in 1863 by evaporating large volumes of the mineral water then purifying the crystals obtained by alcohol extraction and precipitating the lesssoluble cesium and rubidium hexachloroplatinate, (Cs,Rb)2PtCl6. Cesium was isolated in 1883 by his student Carl Setterberg by electrolysis of the cyanide. The pure metals were prepared by Louis Jean Henri Hackspill (1880-1963) by reduction of their chlorides with calcium while he was assistant to Henri Moissan in Paris from 1903– 1907. When he became Director of the École nationale supérieure de chimie he prepared calcium in 1951 by reduction of calcium chloride with aluminum.
Barium
Davy isolated the metal barium in 1808 by means of the voltaic pile. Although the mineral barite in which this element was first recognized has a high specific gravity, the metal itself is very light. John William Mallet (1832-1912) (Figure 33) Professor of Chemistry at the University of Virginia described in 1878 the preparation of barium by the reduction of barium oxide with aluminum at 1100ºC in an evacuated retort. Barium vapor is collected in the condenser. Antoine Guntz (1859-1935) Professor of Chemistry in Nancy was the first to produce a considerable amount of the metal in 1970 by this process.
Hafnium
Georg von Hevesy (1885–1966) (Figure 34) together with Dirk Coster (1889-1950) (Figure 35), pursuing a suggestion of Niels Bohr, discovered hafnium among ores of zirconium in 1923 and isolated the metal by reducing HfCl4 with magnesium.
Figure 34: Georg von Hevesy (1885–1966) was born in Budapest, educated at the Technische Hochschule in Berlin and the University of Freiburg. In 1911 he worked at the University of Manchester under Ernest Rutherford on the chemical separation of radium, then he explored the use of radioactive isotopes as tracers. He joined Friedrich Paneth at Vienna (1912) and made significant progress in tracer studies. Invited to Copenha- gen (1920). Fleeing from the Nazis (1943), Hevesy became professor at the Institute of Organic Chemistry, Stockholm. He earned the 1943 Nobel prize for chemistry.
Plutonium
After the discovery of plutonium by Glenn T. Seaborg (1912- 1999) and his co-workers, reports from the University of Chicago laboratories showed widely varying densities of these almost invisible beads of metal. The small amount of plutonium in Los Alamos at the end of 1944 served for all chemical and metallurgical development work. When there was enough material for one bomb, the entire efforts were directed at shaping the whole available supply into the final form as quickly as possible. On July 15, 1945, the plutonium core in the sub-assembly on the test site at Alamogordo was placed. (Figure 36) shows one of the first 10-gram reductions in a “bomb” after a number of improvements. Shortly thereafter electrolysis from a fused-salt bath was used. Today, a large supply of plutonium (hundreds of tonnes) is stored under strictly-controlled conditions at Rocky Flats in Colorado.
References
- F. Habashi (editor) ( 1994) A History of Metallurgy, Métallurgie Extractive Québec, Québec City,distributed by Laval University Bookstore “Zone”, Canada.
- F. Habashi (2002) Textbook of Pyrometallurgy, Métallurgie Extractive Québec, Québec City, distributed by Laval University Bookstore “Zone”, Canada.
- F. Habashi (2006) Readings in Historical Metallurgy, Changing Technology in Extractive Metallurgy, Métal- lurgie Extractive Québec, Québec City, distributed by Laval University Bookstore “Zone”, Canada.
- F. Habashi (2015) The Story of Metals, 2 volumes, Métallurgie Extractive Québec, Québec City, distrib- uted by Laval University Bookstore “Zone” Canada.
- M.E. Weeks (1960) Discovery of the Elements, published by Journal of Chemical Education, Easton, Pennsylvania.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi