Editorial, J Clin Exp Oncol Vol: 7 Issue: 2
Mfsd2b, a Novel Sphingosine-1-Phosphate Transporter: Implication in Cancer Therapeutics
Ashok Kumar* and Neha Arya
Department of Biochemistry, All India Institute of Medical Sciences Bhopal, Saket Nagar, Bhopal, India
*Corresponding Author : Ashok Kumar
Department of Biochemistry, All India Institute of Medical Sciences Bhopal, Saket Nagar, Bhopal, 462 020 India
Tel: +91-755-2672319
E-mail: ashok.biochemistry@aiimsbhopal.edu.in
Received: April 2, 2018 Accepted: April 5, 2018 Published: April 12, 2018
Citation: Kumar A, Arya N (2018) Mfsd2b, a Novel Sphingosine-1-Phosphate Transporter: Implication in Cancer Therapeutics. J Clin Exp Oncol 7:2. doi: 10.4172/2324-9110.1000e113
Introduction
Sphingosine-1-Phosphate (S1P) is a potent sphingolipid metabolite that regulates physiological functions including cell proliferation, survival, migration, angiogenesis, lymphocyte trafficking, mitochondrial functions as well as carcinogenesis [1]. Growing evidences suggest that S1P promotes tumor growth while inhibiting apoptosis and conferring chemo- and radiation resistance to cancer cells [2]. S1P is secreted in the extracellular environment and mediates its actions by binding to a family of G-protein-coupled receptors known as S1P receptors (S1PRs) in an autocrine as well as paracrine fashion [1]. S1P concentration is more in the circulatory fluid (blood and lymph) compared to lymphoid organs and tissue interstitial fluid. In blood, most of the plasma S1P is transported in bound state to highdensity lipoprotein (HDL) and albumin. On HDL, S1P remains attached to Apolipoprotein M (Apo M) where latter may protect S1P from degradation and facilitates its presentation to receptors [3]. The major sources of plasma S1P are endothelial cells, platelets and RBCs, while lymphatic S1P is produced by lymphatic endothelial cells [3].
S1P is generated by the phosphorylation of sphingosine, a step catalyzed by sphingosine kinase 1 and 2 (Sphk1 and -2), and can be dephosphorylated by various lipid phosphatases to regenerate sphingosine. Additionally, S1P is irreversibly degraded by S1P lyase (SPL), which cleaves S1P, yielding two products, ethanolamine phosphate and trans-2-hexadecenal [1,4]. Therefore, S1P levels are tightly regulated by Sphk, lipid phosphatases and SPL. After synthesis, S1P can be exported outside the cell; once in the extracellular space, S1P can bind and activate the S1PRs present on neighboring cells (paracrine action) or on the same cell (autocrine action). The latter mechanism is also known as “inside-out signaling” [1].
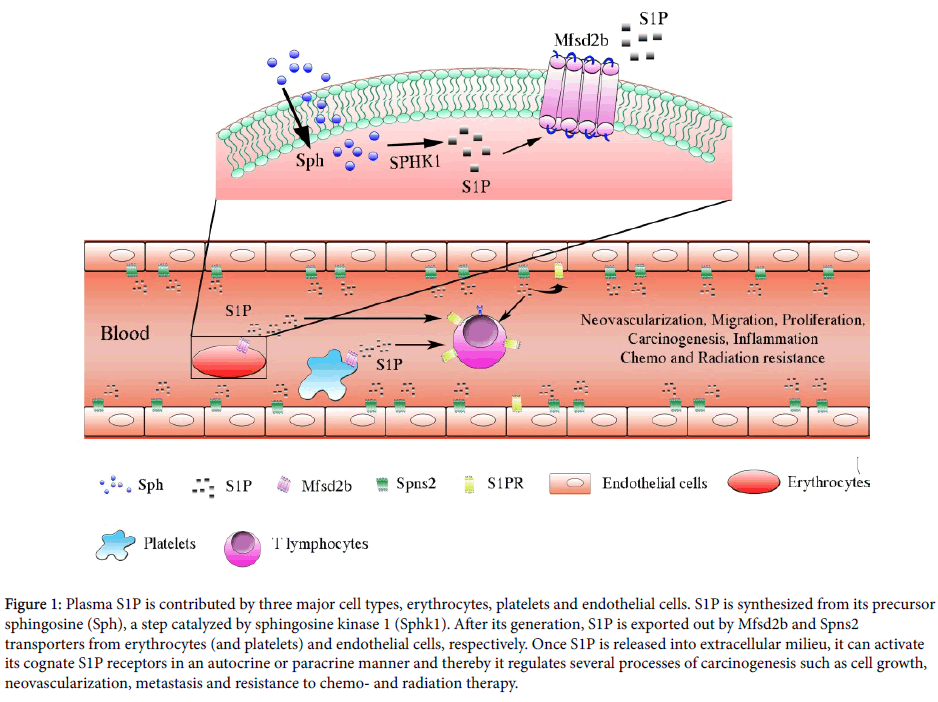
S1P is an amphipathic molecule that requires facilitated export. Previously, ATP-binding cassette (ABC) family of transporters including ABCA1, ABCC1, and ABCG2 has been shown to export S1P and its analog. However, exogenously expressing ABC transporters in CHO/SphK1 cells do not export S1P and ABCA1, ABCA7 or ABCC1 knock out (KO) mice show no change in plasma S1P levels and its related functions, potentially indicating the existence of compensatory mechanisms with other transporters [5]. In this context, an important regulator of extracellular S1P, Spns2, has been shown to control S1P levels in the plasma and lymph by regulating S1P release from endothelial and lymph endothelial cells [6,7]. They demonstrated that plasma S1P levels decreased to 60% in Spns2 KO mice compared to wild type mice [6]. However, disruption in Spns2 gene does not affect the S1P release from erythrocytes or platelets [6]. Therefore, exact characterization of S1P transporter in RBCs and platelets remained elusive, until, Kobayashi et al. demonstrated S1P export by erythrocytes in an ATP-dependent manner and its suppression by glyburide [8]. Although, S1P transport was being explored, the gene for S1P exporter in erythrocytes was not known. Two separate groups, Vu et al. and Kobayashi et al. identified Mfsd2b as an S1P transporter from erythrocytes and platelets [8,9]. Mfsd2b, which belongs to MFS transporter family, contains 12 transmembrane domains and is detected in bone marrow, spleen and prominently in plasma membrane of RBCs and platelets [9]. Kobayashi et al. characterized Mfsd2b as an S1P transporter in MEDEP-E14, a mouse erythroid cell line similar to erythrocytes [8]. However, Mfsd2a, an Mfsd2b homologue does not export S1P from erythroid cells [8]. Additionally, Vu et al. demonstrated that RBCs isolated from Mfsd2b-knockout mice accumulated S1P, suggesting the role of Mfsd2b as an exporter of S1P, and not as an importer [9]. Consequently, mice lacking Mfsd2b transporter exhibited approximately 50% reduction in the plasma S1P levels, which is critical for immune cell trafficking. However, Mfsd2b- KO mice did not exhibit significant lymphopenia indicating that 50% reduction in plasma S1P is not sufficient enough to impair lymphocyte egress and trafficking [9]. They also showed that Mfsd2b was required for S1P export in platelets under basal as well as activated conditions [9]. In Mfsd2b-KO mice, circulatory S1P maintained the integrity of endothelium; however, the mice were more susceptible to anaphylactic shock. Indeed, Mfsd2b-KO mice treated with platelet activating factor showed decreased survival, suggesting the protective role of Msdf2breleased S1P in anaphylaxis [9]. Based on these studies and previous reports, it can be concluded that plasma S1P is transported by two types of S1P transporters (Figure 1). A) Spns2 that exports S1P from endothelial cells, and B) Mfsd2b that exports S1P from RBCs and platelets.
Figure 1: Plasma S1P is contributed by three major cell types, erythrocytes, platelets and endothelial cells. S1P is synthesized from its precursor sphingosine (Sph), a step catalyzed by sphingosine kinase 1 (Sphk1). After its generation, S1P is exported out by Mfsd2b and Spns2 transporters from erythrocytes (and platelets) and endothelial cells, respectively. Once S1P is released into the extracellular milieu, it can activate its cognate S1P receptors in an autocrine or paracrine manner and thereby regulating several processes of carcinogenesis such as cell growth, neovascularization, metastasis and resistance to chemo-and radiation therapy.
Recent studies have provided mechanistic details of the roles of S1P and their downstream targets in the regulation of tumour growth and response to chemotherapy, radiotherapy and/or immunotherapy. S1P generated inside the cells is exported outside into the tumor microenvironment (TME) where it stimulates specific S1P receptors on the cell surface. This “inside-out” signaling of S1P is considered to play a fundamental role in cancer progression [10]. Cancer cells and other cell types in the TME, such as inflammatory cells and endothelial cells, express different combinations of S1P receptors that contribute to diverse cellular functions regulated by S1P. Earlier studies using mouse models of cancer and patient samples suggested that the tumours themselves, in which SPHK1 is upregulated, may be a key source of S1P. However, a recent study suggested that local tumour growth is regulated by both S1P from the tumour and systemic S1P, whereas lung colonization and metastasis is selectively controlled via systemic S1P [11]. However, the exact role of systemic S1P in the inflammation and cancer is not clear. Sphk1, a key enzyme which catalyzes S1P generation is overexpressed in the tumors tissues of variety of cancers [2].
Selective inhibition of Sphk1 using PF-543 failed to inhibit tumor growth in animal models; on the other hand systemic inhibition of S1P has shown promising results. Neutralization of systemic S1P with a specific monoclonal antibody (known as sphingomab) slowed tumor growth in mouse models of renal cell carcinoma [12]. Mice bearing tumors that had developed resistance to sunitinib treatment also exhibit tumor growth suppression with sphingomab. Furthermore, sphingomab suppresses lung metastasis, which suggests a new therapeutic strategy to prevent cancer metastasis. Sonepcizumab, the humanized version of sphingomab, has recently completed Phase II clinical trials in renal cell carcinoma patients [13].
In another study, Weyden et al. identified Spns2 as major regulator of pulmonary metastasis since Spns2 deficient mice showed largest reduction in pulmonary metastasis with increased effector T cells and natural killer cells in the lung microenvironment [14]. These findings have implications in targeting the S1P axis towards the development of better therapeutics. Additionally, several investigators have explored the potential of plasma S1P to serve as a biomarker for cancer detection. As an example, ovarian cancer patients demonstrated twice as high plasma S1P levels as to healthy controls. In a separate study, elevated plasma S1 levels have been associated with the increased risk of developing lung cancer [15]. Thus, targeting S1P production in the tumour and the host would help reduce both growth and metastasis.
References
- Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397-407.
- Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10: 489-503.
- Ksiazek M, Chacińska M, Chabowski A, Baranowski M, et al. (2015) Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res 56: 1271-1281.
- Kumar A, Zamora-Pineda J, Degagne E, Saba JD (2017) S1P Lyase Regulation of Thymic Egress and Oncogenic Inflammatory Signaling. Mediators Inflamm 2017: 7685142.
- Takabe K, Spiegel S (2014) Export of sphingosine-1-phosphate and cancer progression. J Lipid Res 55: 1839-1846.
- Hisano Y, Kobayashi N, Yamaguchi A, Nishi T (2012) Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7: e38941.
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, et al. (2012) The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest 122: 1416-1426.
- Kobayashi N, Kawasaki-Nishi S, Otsuka M, Hisano Y, Yamaguchi A, et al. (2018) MFSD2B is a sphingosine 1-phosphate transporter in erythroid cells. Sci Rep 8: 4969.
- Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, et al. (2017) Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550: 524-528.
- Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, et al. (2016) Sphingosine-1 phosphate: A new modulator of immune plasticity in the tumor microenvironment. Front Oncol 6: 218.
- Ponnusamy S, Selvam SP, Mehrotra S, Kawamori T, Snider AJ, et al. (2012) Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol Med 4: 761-775.
- Zhang L, Wang X, Bullock AJ, Callea M, Shah H, et al. (2015) Anti-S1P antibody as a novel therapeutic strategy for VEGFR TKI-resistant renal cancer. Clin Cancer Res 21: 1925-1934.
- Pal SK, Drabkin HA, Reeves JA, Hainsworth JD, Hazel SE, et al. (2017) A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer 123: 576-582.
- van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, et al. (2017) Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 541: 233-236.
- Nema R, Vishwakarma S, Agarwal R, Panday RK, Kumar A (2016) Emerging role of sphingosine-1-phosphate signaling in head and neck squamous cell carcinoma. Onco Targets Ther 9: 3269-80.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi