Research Article, J Regen Med Vol: 11 Issue: 3
Minimum One-Year Follow up in Patients Undergoing Intra articular Autologous Bone Marrow Concentrate Injection for Osteoarthritic Knee Pain Therapy
Austin Yeargan1*, Meredith Thomas1, Sealy Hambright2, Thos Evans2
1Department of Orthopedic surgery, Carolina Joint and Arthritis Clinic, 5725 Oleander Drive E4, Wilmington, North Carolina 28409
2Department of Orthopedic surgery, The Steadman Clinic, 181 West Meadow Drive, Vail, Colorado 81657
*Corresponding Author: Austin Yeargan
Department of Orthopedic surgery, Carolina Joint and Arthritis Clinic, 5725 Oleander Drive E4, Wilmington, North Carolina 28409
E-mail: austinyeargan@outlook.com
Received: 24-Mar-2022, Manuscript No. JRGM-22-58289;
Editor assigned: 28-Mar-2022, PreQC No. JRGM-22-58289(PQ);
Reviewed: 11-Apr-2022, QC No. JRGM-22-58289;
Revised: 15-Apr-2022, Manuscript No. JRGM-22-58289(R);
Published: 22-Apr-2022, DOI:10.4172/2325-9620.1000215
Citation: Yeargan A, Thomas M, Hambright S, Evans T (2022) Minimum One-Year Follow up in Patients Undergoing Intra-articular Autologous Bone Marrow Concentrate Injection for Osteoarthritic Knee Pain. J Regen Med 11:3.
Abstract
Objective: Autologous Bone Marrow (ABMAC) is a promising alternative therapy to conventional treatments for knee osteoarthritis, with the potential to mitigate inflammation and improve joint function. The aim of the study was to determine the safety and therapeutic benefit at an average of twenty months (12-30) post procedure for the treatment of knee osteoarthritis with a single autologous bone marrow concentrate treatment combined with mechanical axis deviation accomplished through off-loading and a physical therapy protocol. Study participant-reported outcomes for Lower Extremity Functional Scale (LEFS), International Knee Documentation Committee (IKDC), Short-form 12 (SF12) and Visual Analog Scale for pain were assessed prior to the procedure and at specific time intervals during the treatment period.
Methods: 42 adult patients with an average age of 75 (57-90) with Kellgren-Lawrence grade II-III varus osteoarthritis of the knee were treated at a single site by a single investigator (SAYIII) in an open label pilot study with ABMAC. Each knee in each patient was treated with 6 mL bone marrow concentrate and 4 mL Nano-filtered growth factor concentrate combined with 2 mL autologous thrombin for activation of platelet dense granules.
Results: Patient reported outcomes data revealed 42 patients with an average follow up of 20 months after receiving a single intraarticular injection of autologous bone marrow concentrate. All patients had Kellgren-Lawrence stage 2-3 OA. No serious adverse events were reported with BMC harvest or injection in any patient. There were no infections. There was meaningful improvement in mean clinical outcome metrics from baseline in all patients during the period studied. At 12 months minimum with 20 months average, mean change in visual analog scale was from 5.8 to 2.3, representing an absolute change of 3.5, exceeding the published minimum clinically important difference of 2.5. The Lower Extremity Functional Scale score mean change was +17.6 from 56.3 to 73.9 exceeding a published 9 point minimum clinically important difference in scoring. The IKDC score mean change was +19.8 from 44 to 63.8 exceeding a published 8 point minimum clinically important difference. Differences from baseline to follow up in the SF-12 demonstrated improvement in both the physical component and the mental component. The physical component improved from 33.9 to 42.9 for a mean change of +9 exceeding the minimum clinically important difference of 3.77. The mental component of the SF-12 increased from 54.8 to 57.1 just falling short of the minimum clinically important difference of 3.29. Two patients had a recurrent effusion and pain at 3 months and were offered PRP augmentation that successfully relieved their clinical symptoms for the duration of the study.
Conclusion: Safety and efficacy for autologous bone marrow aspiration, concentration and intra-articular bone marrow injections for knee arthritis and catabolic knee pain syndrome provided relief for at least an average of 20 months (range 12-30) based on validated patient reported outcomes analysis. Further study is indicated to determine the longevity and endurance of the procedure in providing pain relief using a single intra-articular BMC injection in this patient subset. Additional study is needed to determine the best approach to the concentration and delivery of orthopaedic immunobiologics in this setting.
Keywords: Autologous bone marrow concentrates; Knee osteoarthritis; Knee pain; Cell therapy
Introduction
Osteoarthritis (OA) is the most common musculoskeletal condition causing significant social and health problems worldwide [1]. Up to 35% of the world’s population over 60 suffers from symptomatic disabling OA and the majority involves the knee joint [2]. OA is a whole joint disease that begins with structural compromise of the subchondral bone and synovial and leads to destruction through sinister molecular processes where there is commitment to catabolic pathways, consistent with a ‘catabolic knee pain syndrome [3]. The clinical application of autologous anti-inflammatory and immunomodulatory protein cytokines in the setting of osteoarthritis serves to convert the destructive catabolic OA joint environment to one favouring joint anabolism, potentially serving to limit the progression of OA [4].
Musculoskeletal conditions causing disease comprise the most prevalent chronic pain conditions with arthritis accounting for the majority of disorders seen clinically [5]. Conventional treatments for catabolic knee pain associated with osteoarthritis including physical therapy, home exercise, activity modification, topical and oral nonsteroidal anti-inflammatory medications, viscosupplementation, corticosteroids and platelet rich plasma have shown benefit in reducing knee pain and improving quality of life and functionality, usually for short periods of time rather than showing evidence of long-lasting pain relief and functionality [6]. It is generally accepted that immunobiologic treatments are not structure modifying when delivered intra-particularly but do favourably modify the biochemical milieu of the joint and joint fluid in the setting of osteoarthritis, allowing for symptomatic patient relief [7]. Cell based therapies such as autologous bone marrow concentrates hold promise as a nonsurgical joint-preserving treatment approach. ABMAC has inherent advantages over other treatments commonly used for catabolic knee pain syndrome. It is a point-of-care autologous immunobiologic product that simultaneously delivers growth factors, anti-inflammatory and immunomodulatory proteins and nucleated marrow cells including signalling mesenchyme stem cells that modulate the immune response in arthritic knee joints [8].
The integration of orthopaedic immunobiologics into clinical practice has increased exponentially over the last fifteen years since we first began to take advantage of the immunomodulatory and anti-inflammatory nature of molecular treatments in orthopaedic surgery. Clinical insight into the utility of these treatment modalities has increased with basic scientific research more accurately defining both canonical and non-canonical inflammatory cellular signalling pathways involved in the pathogenesis of osteoarthritis [9]. Future clarification of these signalling pathways and spatiotemporal control mechanisms will lead to additional novel treatment modalities that translate into relief for patients who suffer clinically, much in the way that novel biologic and bio similar treatments have dramatically changed the landscape in inflammatory arthritis [10].
Varus misalignment of the lower extremity increases the risk of incident medial cartilage damage, OA onset and progression [11- 13]. The risk of lateral compartment OA incidence and radiographic progression is increased in valgus misalignments and while these patients can be candidates for the procedure, they were excluded from this study group [14]. Point loading force concentration leads to location-dependent development of subchondral sclerosis and OA in the adult knee and to extend the duration of treatment relief, consideration should be given to off-loading devices such as unloaded bracing and heel wedge orthotics that favourably affect mechanical axes during gait. Cell-based therapies are strategic, molecular based approaches that exploit natural biochemical pathways and can provide relief in the setting of osteoarthritic pain syndromes.
Materials and Methods
A total of 42 adult patients with symptomatic varus gonarthrosis who had failed conventional multimodality conservative treatments were prospectively enrolled in the study.
We describe a cohort of patients treated by one orthopaedic surgeon using a standardized method for concentrating autologous bone marrow harvested from the ipsilateral anterior gluteal pillar in an office procedure room setting. Our cohort consisted of 42 patients with an average age of 75 (range 57-90) and an average follow up period of 20 months (range 12-36 months). Patients were included with unicompartmental osteoarthritis of the knee involving the medial compartment (varus gonarthrosis). Patients were excluded with tricompartmental OA and valgus gonarthrosis.
We sought to determine safety and therapeutic benefit using this modality by applying validated and accurate outcomes assessments including the short form-12 (SF-12), international knee documentation committee score (IKDC), lower extremity functional scale score (LEFS) and the visual analog score for pain (VAS pain) in the pre- and post-procedure setting at pre-determined time points. Patients were assessed at 2 weeks, 4 weeks, 12 weeks, 6 months, and one year and yearly thereafter. On average there was improvement in all outcomes analyses over the time-period studied. One patient had a persistent flexion contracture and required total knee replacement at two years. Two patients underwent PRP injection at three months each for recurrent effusion after ABMAC that relieved their symptoms for the remainder of the study period.
Procedure
Patients were deemed appropriate candidates for the ABMAC OA knee procedure where they had unicompartmental varus gonarthrosis that had failed standard multimodality conservative treatments and their other alternatives were pain management, total knee arthroplasty, unicompartmental knee arthroplasty, or living with their disease, which they were unwilling to do. The patients in our series did not want to undergo a surgical procedure. A subset of patients could have been appropriate for unicompartmental knee arthroplasty, but declined that option. There was an age limitation that precluded consideration of high tibial osteotomy in our study group. Patients understood that the procedure was experimental and not FDA approved for any orthopaedic surgical indication and was being used “off-label”. The Kellgren-Lawrence classification system was used to define the degree of arthritis in all cases [15, 16]. We narrowed our selection criteria to patients with varus, unicompartmental disease so that the affected medial compartment in this setting could be offloaded with medial unloaded bracing and lateral heel wedge orthotics that were custom constructed in point-of-care fashion by a physical therapist with expertise in gait analysis.
Patients gave informed consent for bone marrow aspiration from the ispilateral anterior gluteal pillar with concentration and intra-articular injection in isolation although for the last five years we have favoured a combination approach with medial tibial subchondroplasty and intra-articular injection. Patients were advised that the procedure was considered off-label, experimental and not FDA approved for any orthopaedic indication to date.
All patients were placed into the supine position in preparation for the BMC procedure. Each patient was admitted to the clinic with their driver. One hour prior to arrival, the patient self-dosed with 5 mg oxycodone, 0.5 mg Xanax and 150 mg Clindamycin and written instructions were provided for the pre- and post-procedure setting. The patient performed Chlorhexidine 3% scrub at home the evening before the procedure and this morning, to the harvest and target sites in addition to carefully scrubbing the feet and lower legs.
The patient was given oversized paper shorts to wear to the clinic and presented accordingly. We positively identified the ipsilateral anterior gluteal pillar as the harvest site and affected knee joints as the primary target surgical site. In bilateral cases both knees were marked. Indelible marker was used to mark the targets with the MDs initials. The patient was prescribed an Ossur® cartilage rebound medial unloaded brace and LHW orthotics to wear for a minimum of three weeks prior to their procedure. Patients used the MUB for six weeks continuously while gravity dependent after the procedure and then for an additional six weeks during impact activities. LHW orthotic wear was prescribed for lifetime use.
The patient’s vitals were taken and found to be stable and within normal range, consistent with the prior clinical visit metrics. Once the patient was deemed appropriate to proceed for preparation, they were transported to the procedural suite via wheelchair and helped onto the procedure table into a seated position. A ‘time-out’ assessment was then called and the op site was confirmed by the attendant staff and surgeon. Patient confirmed allergy status.
The patient was then placed into the supine position, with the head supported by clean, paper covered pillows. Vital signs monitor was stationed in the procedural suite. The patient was carefully positioned for the marrow aspiration procedure. Surgical scrub was accomplished using 3% chlorhexidine by the patient prior to presentation. Full sterile isolation barriers were used during the procedure following the prep. In each setting, DuraPrep® sponges were used to paint the intended harvest and target sites followed by application of sterile sticky drapes. The anterior gluteal pillar of the right hemi-pelvis was marked four fingerbreadths back (7 cm) from the ASIS at the thickest part of the crest. Carefully, 10 ml of local anesthetic containing 10 ml
Lidocaine 1% plain and marcaine 0.25% with epinephrine mixed 1:1 was used to infiltrate the skin, subcutaneous tissue and periosteum about the right anterior iliac crest at the level of the gluteal pillar. We let the local anaesthetic take full effect so that it could be obviously demonstrated before proceeding. The average VAS pain score for aspiration was graded at 4 (range 2-8) at conclusion. The appropriate entry site for the patient was identified and marked, coinciding with the thickest portion of the anterior crest at the gluteal pillar. Skin was retracted cephalad and a 3 mm shallow stab incision was made using a #11 blade. A Jamshidi needle was carefully advanced to the subcutaneous iliac crest that was easily palpable at the gluteal pillar with care taken to avoid the LFCN and cluneal nerves.
The Jamshidi was aimed towards the femoral neck. Using a 260 gram mallet, the Jamshidi was gently and carefully advanced approximately 2.0-3.0 cm across the cortical iliac bone and into the core of the anterior pillar. (Figure 1) The obturator was removed revealing the expected slow, but immediate egress of bone marrow. Six 9-10 ml syringes were used to aspirate bone marrow in sequential fashion with care taken to reposition the needle tip with rotation and/or advancement using a fanning technique after each 9-10 ml aspiration to ensure the most efficient mesenchymal cell capture from the marrow. 10 mL of marrow aspirate was extracted last for Arthrex® thrombinator processing to extract autologous thrombin for scaffold activation and platelet degranulation. Following completion of the bone marrow aspiration the Jamshidi obturator was replaced and the Jamshidi was removed uneventfully.
Pressure was held for 2-3 minutes followed by application of a sterile pressure dressing with 2% Bactroban ointment on a flexible fabric band-aid. The wound was checked prior to discharge of the patient from the clinic and noted to be dry in each case. The syringes totaling approximately 60 ml autologous marrow were sequentially handed off as drawn to the assistant who immediately injected them through the clot/particulate filter into the sterile Arthrex® Angel cell processor in preparation for isopycnic centrifugation (Arthrex Corporation, Naples, FL). The cell separator component of the procedure was activated for approximately 25 minutes to achieve three distinct layers of product put out by the Angel®. The Angel® separator produces BMC in isolation from platelet poor plasma containing growth factors that were then concentrated. Upon completion of final centrifugation, following removal of the 6 mL cellular component, the platelet poor plasma fraction was then complexed with Euflexa® Hyaluronic acid 2.5 mL to capture and activate TSG-6. Next the PPP was actively passage through the A2M nanofilter (Minntech®, Minneapolis, MN) to capture and concentrate the GFC into a total of 4 mL ensuring elimination by size of sub 65 kD pro-inflammatory nanomolecules. Next the 6 mL of BMC was transferred to the syringe containing 4 mL GFC. The autologous thrombin was kept in a separate syringe for intra-articular injection.
A superolateral injection site was used in each case with ultrasound confirmation. BMC and GFC fractions were co-administered followed by 2 ml Thrombin injection and platelet activation.
The knee was then placed through a range of motion including flexion, extension and patellofemoral mobilization. Patients were all ambulatory after their procedure and the medial unloaded brace was applied prior to their leaving the clinic.
Patients all underwent a physical therapy protocol that we have published elsewhere previously [17].
Results
We evaluated the outcomes data including validated, accurate patient reported outcome measures for a subset of patients with varus gonarthrosis consisting of 42 patients with an average follow up of 20 months after receiving a single intra-articular injection of autologous bone marrow concentrate for osteoarthritic knee pain and catabolic knee pain syndrome. Average patient age was 75. Eight patients had bilateral symptoms for a total of 46 knees available for study analysis.
All patients had Kellgren-Lawrence stage 2-3 OA on plain film radiographs corresponding to definite mild to moderate osteophytes, definite narrowing of joint space and some sclerosis of bone ends, particularly on the concave side of the joint (tibia).
No serious adverse events were reported with treatment in any patient. There was meaningful improvement in mean clinical outcome metrics from baseline in all patients during the time period studied. Mean change in visual analog scale was from 5.8 to 2.3, representing an absolute change of 3.5, exceeding the published minimum clinically important difference of 2.5.
The Lower Extremity Functional Scale score mean change was +17.6 from 56.3 to 73.9 exceeding a published 9 point minimum clinically important difference in scoring. The IKDC score mean change was +19.8 from 44 to 63.8 exceeding a published 8 point minimum clinically important difference. Differences from baseline to follow up in the SF-12 demonstrated improvement in both the physical component and the mental component. The physical component improved from 33.9 to 42.9 for a mean change of +9 exceeding the minimum clinically important difference of 3.77. The mental component improved from 54.8 to 57.1 which did not exceed the minimum clinically important difference of 3.29 [18].
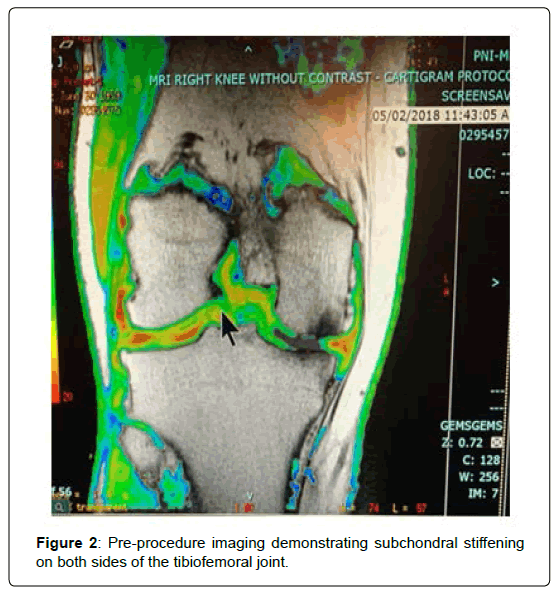
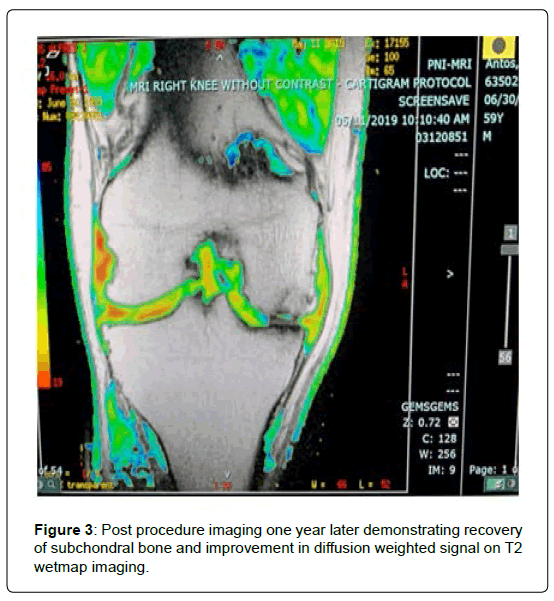
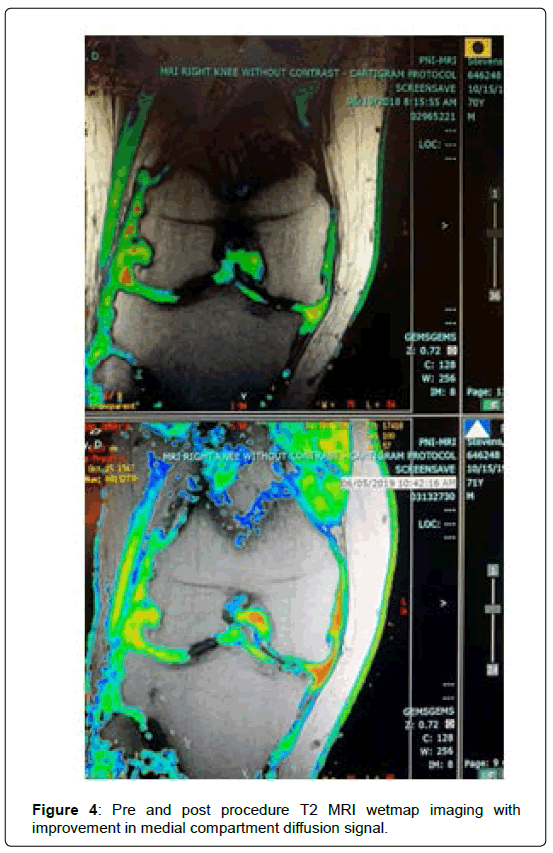
T2 wet map MRI sequences were obtained pre procedure and at 1 year with notable improvement in subchondral signal suggesting improvement in marrow pathology. Diffusion signal was improved in some patients undergoing the intra-articular procedure (Figures 2-4).
Discussion
With aging and an increasingly obese population, OA is more prevalent now than ever and novel treatment strategies are being sought to meet the growing demand for treatment in these patients. In addition to orthopaedic immunobiologics, there has been growing interest in ant-inhibitors in recent years as the drugs move into phase three trials [19, 20]. We avoid indicating patients with medial and lateral compartment disease and have found that patients with patella femoral disease in isolation or combination with medial and/ or lateral compartment disease do not typically exhibit long periods of symptomatic relief (typically two years or less).
Autologous bone marrow concentrates have been introduced clinically over the last two decades and recent literature has supported their use for the treatment of osteoarthritic knee pain [21-23]. The catabolic mechanisms underlying knee osteoarthritic pathways are becoming better understood and novel, diseasemodifying systemic drugs are being developed for the treatment of OA [24, 25]. These molecules target the molecular pathways that lead to joint destruction, potentially limiting the progression of OA. Autologous Bone Marrow Aspirate Concentrates (ABMAC) are a natural source of small molecules that direct anti-inflammatory and immunomodulatory responses at the molecular level. Autologous cell therapy treatments consist of a cellular component and a plasma protein component that play distinct roles in combatting catabolic joint osteoarthritis and auto-inflammation [26]. Nucleated signalling cells from the harvested bone marrow function through cell receptor mediated signalling mechanisms that often involve a second messenger to activate anti-inflammatory and immunomodulatory pro-anabolic genes favourable to joint health [27]. Hyaluronic acid complexes platelet poor plasma concentrates can be filtered through size exclusion using 55-65 kD Nano pore filaments to eliminate proinflammatory molecules and water, and retain anti-inflammatory and immunomodulatory molecules like TSG-6, TGF-B, IRAP and A2M (Minnetech®, Minneapolis, MN) in a growth factor concentrate. An autologous growth factor concentrate (GFC) matrix can be elaborated from the platelet poor plasma to function as nucleated autologous bone marrow concentrate cell scaffolding for knee osteoarthritis when mixed with autologous thrombin to activate platelet dense granules, which can be particularly useful when subchondral injections are contemplated in the setting of subchondral sclerosis and bone marrow edema [28]. (Thrombinator®, Arthrex®, Naples, FL).
In the osteoarthritic joint, catabolic complexes dominate the synovial fluid and lead to a continuous cycle of load-based joint destruction [29]. Pro-inflammatory protein molecules found in catabolic knee pain syndrome include interleukin one, tumor necrosis factor alpha, interleukin 6, interleukin 8, interleukin 17, interleukin 18, leukemia inhibitory factor, oncostatin M (OSM), Matrix Metalloproteinase 13 (MMP-13), ADAMTS and some prostaglandins [30,31]. These molecules lead to cartilage matrix and cellular degradation from mechanical overload of the knee joint compartment affected [32]. Inflammatory cells make up approximately fifteen to twenty percent of the synovial lining of the knee joint and include immune signalling cells that function through adaptive autocrine and paracrine signalling mechanisms [33]. Other nucleated cells that play a role in combatting osteoarthritis include hematopoetic and mesenchymal stem cells, endothelial cells and pericytes functioning as a unit [34].
Osteoarthritis emanates from the dysfunction of the entire joint, especially affecting synovium, cartilage and subchondral bone as well as tissues with close mechanical and molecular biological interactions [35, 36]. Understanding the biochemical and biophysical signaling underlying the genesis of osteoarthritis will continue to lead to additional discovery of novel treatment strategies for symptomatic knee arthritis and likely have wide application to other joint arthritides like the hip or shoulder joint [37]. Autologous cell-based therapies combat destructive intra-articular immune complexes using a cellular and molecular based approach including mesenchymal signaling cells.
To date there is no therapy that has been shown to effectively halt the structural deterioration of cartilage and bone or is able to successfully and consistently reverse any existing structural defects in the setting of knee osteoarthritis [38]. However, many treatment options exist for the treatment of the symptomatic catabolic pain syndrome associated with arthritis of the knee. While conventional treatments including physical therapy, CBD preparations, topical and oral NSAIDs, steroids and viscosupplementation have been used extensively and, with the exclusion of endocannabinoids, have a known track record, there is some question regarding the treatment safety, efficacy and endurance of orthopedic immunobiologics like platelet rich plasma and autologous bone marrow concentrates. This study used validated, accurate patient reported outcomes to clarify the role for bone marrow concentrates in the setting of knee osteoarthritis to determine safety and efficacy at an average of 20 months (12-30 months). While all the patients in our study were treated with a single intra-articular injection of bone marrow concentrate, we currently favour a combined autologous bone marrow concentrate intraosseous and intra-articular injection in the setting of varus gonarthrosis that has failed conventional treatment modalities, particularly when subchondral edema (marrow pathology) is observed on MRI images [39]. We have noted that subchondral edema typically begins on the concave side of the join, where loss of Young’s modulus of elasticity results in a stiff subchondral plate. Once the tibial bone is stiff, the load is reflected to the convex (femoral condylar) side of the joint that follows a similar pattern. By this time, the cartilage matrix and cells are subjected to supraphysiologic loading and disintegrate into the synovial fluid, prompting an insidious inflammatory response that catabolically auto-regulates until the joint fails and patients are appropriately indicated for arthroplasty.
What is not known is the best way to process these autologous cell products or how to recapitulate the critical spatiotemporal relationships between the cell signaling and their relationship to available growth factors to achieve the best results. The continued development of immunobiologics targeted against cartilage osteoarthritis will require an understanding of the condition of joint tissues at the time of intervention that may rely on synovial biomarkers currently in the development and design stage [40]. It becomes less likely that interventions will be successful if they are not applied at the early stages of disease before considerable structural and functional deterioration of the joint develop. Currently, immunobiologics are not typically sought or offered until patients present having failed all other conventional multimodality conservative interventions. Often, these patients have been given the choice between living with their disease and total knee arthroplasty. Understandably, patients can be hesitant to undergo joint replacement when only one compartment of the knee is involved and there is a chance for successful management that could last for 2-5 years in spite of out-of-pocket expenses.
There is some controversy as to whether introducing serine proteases like autologous thrombin counter the anti-autoinflammatory effects of the autologous bone marrow product. Some authors have recommended lysing platelet dense granules to release growth factors using calcium chloride as part of the cell processing rather than administering thrombin to avoid this issue. With intraarticular injection this makes sense. However, with intraosseous injection, thrombin use provides a superior handling product that can be expected to stay where it is injected in the subchondral bone.
Conclusion
Limitations of the study include the relatively short follow up period of twenty months and a limited number of participants studied. There is significant patient bias any time an out-of-pocket procedure is contemplated. Patients are more likely to be educated and have access to more disposable income in this setting and as such, significant bias is introduced into any study where patients are paying personally for these procedures. Continued, limited-bias, high-quality studies are necessary to determine the clinical utility of bone marrow concentrate in the setting of knee osteoarthritis. In particular, standardization is needed regarding optimal patient selection, autologous cell product processing, and sample testing and reporting of both functional and imaging-based outcomes. Future studies should focus on these endpoints.
References
- Cui A, Li H, Wang D, Zhong J, Chen Y, et al. (2020) Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. E Clinical Medicine, 29-30: 100587.
- Grassel S, Zaucke F, Madry H (2021) Osteoarthritis: Novel Molecular Mechanisms Increase Our Understanding of the Disease Pathology. J Clin Med, 10(9): 1938.
- Kon E, Ronga M, Filardo G, Farr J, Madry H, et al. (2016) Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc, 24(6): 1797-1814.
- Khella CM, Asgarian R, Horvath JM, Rolauffs B, Hart ML (2021) An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int J Mol Sci, 22(4): 1996.
- Williams A, Kamper SJ, Wiggers JH, Kate MB, Hopin Lee, et al. (2018) Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med, 16(1): 1-167.
- Wasserman A, Matthewson G, MacDonald P (2018) Platelet-Rich Plasma and the Knee-Applications in Orthopedic Surgery. Curr Rev Musculoskelet Med, 11(4): 607-615.
- Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT (2019) Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol, 15(2): 77-90.
- Fortier LA, Strauss EJ, Shepard DO, Becktell L, Kennedy JG (2019) Biological Effects of Bone Marrow Concentrate in Knee Pathologies. J Knee Surg, 32(1): 2-8.
- Mobasheri A (2011) Applications of proteomics to osteoarthritis, a musculoskeletal disease characterized by aging. Front Physiol, 2: 1-108.
- Lucena F, McDougall JJ (2021) Protease Activated Receptors and Arthritis. Int J Mol Sci, 22(17): 9352.
- Sharma L, Chmiel JS, Almagor O, Felson D, Guermazi A, et al. (2013) The role of varus and valgus alignment in the initial development of knee cartilage damage by MRI: The MOST study. Ann Rheum Dis, 72: 235-240.
- Sharma L, Song J, Dunlop D, Felson D, Lewis CE, et al. (2010) Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis, 69: 1940-1945.
- Bastick AN, Belo JN, Runhaar J, Bierma-Zeinstra SM (2015) What Are the Prognostic Factors for Radiographic Progression of Knee Osteoarthritis? A Meta-analysis. Clin Orthop Relat Res, 473: 2969-2989.
- Felson DT, Niu J, Gross KD, Englund M, Sharma L, et al. (2013) Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: Findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheumm, 65(2): 355-362.
- Kellgren J, Lawrence J (1957) Radiological Assessment of Osteo-Arthrosis. Ann Rheum Dis, 16(4): 494-502.
- Schiphof D, Boers M, Bierma-Zeinstra S (2008) Differences in Descriptions of Kellgren and Lawrence Grades of Knee Osteoarthritis. Ann Rheum Dis, 67(7): 1034-1036.
- Yeargan A, Bennett L, Isear J, Brien M, Montgomery B (2020) A Physical Therapy Algorithm in the Setting of Orthopedic Immunobiologics for Unicompartmental Knee Osteoarthritis. EC Orthopedics.
- Diaz-Arribas MJ, Fernandez-Serrano M, Royuela A, Kovacs FM, Gallego-Izquierdo T, et al. (2017) Minimal Clinically Important Difference in Quality of Life for Patients With Low Back Pain. Spine, 42(24): 1908-1916.
- Latourte A, Kloppenburg M, Richette P (2020) Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol, 16(12): 673-688.
- Lories RJ, Monteagudo S (2020) Review Article: Is Wnt Signaling an Attractive Target for the Treatment of Osteoarthritis?. Rheumatol Ther, 7(2): 259-270.
- Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD (2015) A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet Disord, 16: 258.
- Garay-Mendoza D, Villarreal-Martinez L, Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, et al. (2018) The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis, 21(1): 140-147.
- Doyle EC, Wragg NM, Wilson SL (2020) Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc, 28(12): 3827-3842.
- Wang MN, Liu L, Zhao LP, Yuan F, Fu YB, et al. (2020) Research of inflammatory factors and signaling pathways in knee osteoarthritis. Zhongguo Gu Shang, 33(4): 388-92.
- Hambright WS, Yeargan A, Hellwinkel JE, Whitney KE, Montgomery BE, et al. (2021) Potential Disease Modifying Treatment Strategies Targeting Senescence in the Setting of Knee Osteoarthritis. Orthopedics and Rheumatology, 17(5): 1-9.
- Manchikanti L, Centeno CJ, Atluri S, Albers SL, Shapiro S, et al. (2020) Bone Marrow Concentrate (BMC) Therapy in Musculoskeletal Disorders: Evidence-Based Policy Position Statement of American Society of Interventional Pain Physicians (ASIPP). Pain Physician, 23(2): 85-131.
- Antebi YE, Nandagopal N, Elowitz MB (2017) An operational view of intercellular signaling pathways. Curr Opin Syst Biol, 1: 16-24.
- De Candia E (2012) Mechanisms of platelet activation by thrombin: a short history. Thromb Res, 129(3): 250-256.
- Blasioli DJ, Kaplan DL (2014) The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev, 20(4): 355-363.
- Kokebie R, Aggarwal R, Lidder S, Hakimiyan AA, Rueger DC, et al. (2011) The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther, 13(2): 1-50.
- Sun ZM, Ling M, Liu M, Zhang YG (2009) Expression of interleukin-1beta and tumor necrosis factor-alpha in the synovium and synovial fluid of patients with Kashin-Beck disease and osteoarthritis. Nan Fang Yi Ke Da Xue Xue Bao, 29(1): 5-8.
- Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, et al. (2019) Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol, 165: 49-65.
- Croft AP, Campos J, Jansen K, Turner JD, Marshall J, et al. (2019) Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature, 570(7760): 246-251.
- Zhu Y, Wang Y, Zhao B, Niu X, Hu B, et al. (2017) Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther, 8(1): 64.
- Grassel S, Muschter D (2020) Recent advances in the treatment of osteoarthritis. F1000Res Faculty Rev, 4(9): 325.
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum, 64(6): 1697-707.
- Whitney KE, Briggs KK, Chamness C, Bolia IK, Huard J, et al. (2020) Bone Marrow Concentrate Injection Treatment Improves Short-term Outcomes in Symptomatic Hip Osteoarthritis Patients: A Pilot Study. Orthop J Sports Med, 8(12): 2325967120966162.
- Bay-Jensen AC, Hoegh-Madsen S, Dam E, Henriksen K, Sondergaard BC, et al. (2010) Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis?. Rheumatol Int, 30(4): 435-442.
- Sheinkop M, Langhenry M, Curran-Everett D, Sand TT (2020) Clinical Outcomes and Safety of a Combined Autologous Bone Marrow Concentrate Intraosseous and Intraarticular Injection for Knee Osteoarthritis at 12 months. J Regen Med, 9(3): 1-7.
- Ravalli S, Szychlinska MA, Lauretta G, Di Rosa M, Musumeci G (2020) Investigating lubricin and known cartilage-based biomarkers of osteoarthritis. Expert Rev Mol Diagn, 20(4): 443-452.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi