Research Article, J Spine Neurosurg Vol: 7 Issue: 2
Multiple Nerve Root Injuries after Incidental Durotomy: Case Report with Anatomical Correlation and Review of Literature
Joseph Yazdi*
Department of Neurosurgery, SpineMore Surgical Associates, Inc., USA
*Corresponding Author : Dr. Joseph Yazdi, MD, FAANS
SpineMore Surgical Associates, Inc., 12152 Tesson Ferry Rd., Saint Louis, MO 63128, USA
Tel: (314) 849-5414
Fax: (314) 842-6972
E-mail: joeyaz21@yahoo.com
Received: April 24, 2018 Accepted: May 04, 2018 Published: May 11, 2018
Citation: Yazdi J (2018) Multiple Nerve Root Injuries after Incidental Durotomy: Case Report with Anatomical Correlation and Review of Literature. J Spine Neurosurg 7:2. doi: 10.4172/2325-9701.1000296
Abstract
CSF leak is one of the most common complications in primary spine surgery. It is even more common in revision surgery. Its causes are multifactorial. It is usually a benign process, but at times it can lead to nerve injury. There have been a number of case reports regarding diagnosis and treatment of spinal nerve injuries due to incidental durotomies. However, they involve either single nerve root or ipsilateral nerves of the cauda equina that were trapped in the durotomy. We present a case of a morbidly obese patient who underwent discectomy for recurrent lumbar disc herniation. During the revision surgery, we encountered a small incidental durotomy through which a significant number of nerve roots exited and as a result were injured. This resulted in bilateral nerve dysfunction, which is a unique aspect of our case presentation. We will discuss the anatomical and pathophysiological bases for this injury. We will also review the literature regarding outcomes after incidental durotomies in regard to spinal nerve injuries.
Keywords: Durotomy; CSF leak; Nerve injury; Cauda equine; Obesity; Vascular
Introduction
Lumbar discectomy is one of the most common spinal procedures performed. The typical risks associated with this type of surgery include nerve injury resulting in paralysis or numbness of the legs, loss of bowel and bladder function, and cerebrospinal fluid (CSF) leak associated with incidental durotomy (ID). Of these risks, CSF leak has the highest rate of occurrence at about 1 to 13% [1-6]. That risk increases dramatically to 13.2 to 17% during revision surgery [7-9]. The most common reason seems to be the more challenging dissection due to the addition of scar tissue or altered anatomy. The nerve roots may be particularly vulnerable when exposed during surgery, and especially more so in cases of ID. Some of the means of injury include transection, compression, traction, and entrapment [10-12]. Most injuries involve either single or unilateral nerve root injuries. We present a case where an ID led to multiple bilateral nerve root injuries. We will discuss the anatomical and physiological bases for this injury and review the literature regarding nerve injury in cases of CSF leak.
Case Presentation
History and examination
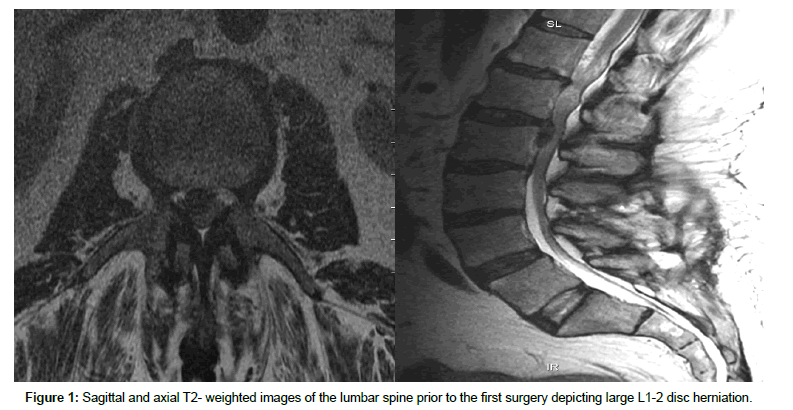
A 63-year-old morbidly obese patient presented with sudden onset of severe right hip and anterior thigh pain that started about 9 or 10 months prior to presentation. His pain had progressively worsened to the point that he had recently started using crutches. His pain was exacerbated by any movement of the leg and relieved by lying down. He had tried conservative measures without any benefit. Significant finding on examination included right iliopsoas muscle weakness at 4/5 and decreased sensation in the right anterior and lateral thigh. Straight leg raising was positive on the right side. He used his crutches as well as assist of another person to ambulate. His BMI was 45. The MRI of the lumbar spine showed a large right L1-2 disc herniation (Figures 1A and 1B).
Operative course
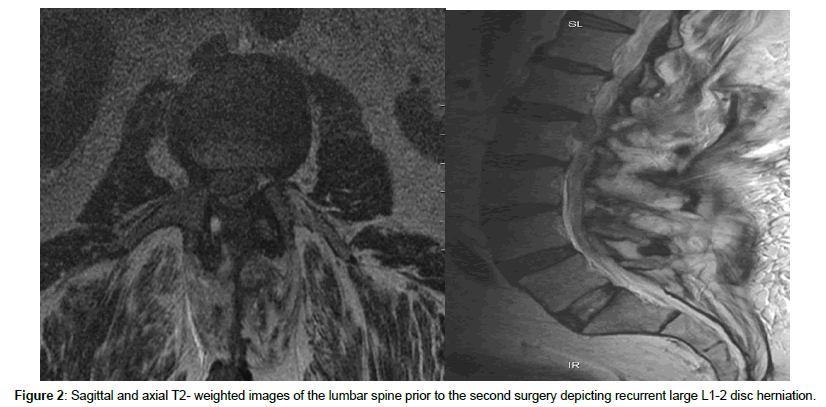
He underwent a right L1-2 laminectomy and sequesterectomy. Immediately after surgery, he had significant improvement of his symptoms. However, eleven days after surgery, he was leaning forward and felt a pop in his back and experienced an acute onset of moderate to severe pain in his lower back radiating to his right thigh. Despite physical therapy, his symptoms progressively worsened. On examination, he demonstrated weakness of the right iliopsoas and knee extension at 4/5 and significant difficulty ambulating. There was persistence of numbness in his right anterior and lateral thigh as well. A repeat MRI demonstrated recurrence of the right L1-2 disc herniation as well as granulation tissue around the surgery site (Figures 2A and 2B). Because of his significant symptoms, he chose to undergo surgery. The procedure was performed in a similar manner as the first time. He was placed on a Jackson table in order to decompress the abdominal contents as much as possible. The previous incision was made and carried down to the residual L1 and L2 lamina. The laminectomy was enlarged so that access around the dura through the lateral recess could be obtained. A large extruded fragment was then encountered and removed. Given that this patient experienced recurrent disc herniation in such a short interval, decision was made to perform a discectomy as well in order to possibly decrease the chance of a subsequent disc herniation. However while trying to expose the annulus bluntly, an incidental durotomy was encountered. Immediately, CSF and multiple nerve roots exited through this very small opening. The nerve roots were reduced back into the dural sac, and the durotomy was closed with one simple stitch. The repair was covered with DuraGen and DuraSeal.
Post-operative course
The patient experienced multiple nerve root injuries immediately after surgery. His examination demonstrated left iliopsoas and knee extension at 3/5 and trace movement distally. On the right side, the iliopsoas and knee extension were 2/5, and there was slightly more movement in the toes than on the left side. There was no movement in the rest of the muscle groups. Sensation was decreased in both feet more so than the thighs. Bowel and bladder function were intact. He was essentially bedridden at this time. Aggressive physical and occupational therapy were instituted. He also developed neuropathic pain which was treated with gabapentin. Over the next 4 months, his examination progressively improved to the point that his left leg was intact except for tibialis anterior which was 0/5 and the right leg was 0-1/5 except gastrocnemius which was 5/5. Sensation had returned to normal. Functionally, he had become modified independent with sit-to-supine and supineto- sit, rolling side to side, and slide board transfers. He needed minimal assistance with sit-to-stand and stand-to-sit activities. He was also able to ambulate up to 85 feet with a right knee brace and a walker. Over the next three months, his right proximal leg strength also improved to antigravity level.
Discussion
Neural anatomy of the conus and cauda equina
The spinal cord terminates at the conus which is usually located at about the L1 or L2 vertebrae. Throughout the length of the spinal cord, a pair of ventral and dorsal rootlets emerge from each side of the spinal cord. The respective ventral and dorsal rootlets join together forming the nerve roots. Just distal to the dorsal root ganglion, the anatomy of the spinal nerves changes to that of peripheral nerves. The nerves then exit at their respective neural foramen.
One of the unique anatomical aspects of the nerves of the cauda equina is that they share more in common with the central nervous system (CNS) structures than they do with peripheral nerves. Peripheral nerves axons are encased in endoneurium provided by Schwann cells. A bundle of axons form a fascicle which are covered by perineurium. The fascicles are grouped together to form a peripheral nerve which is covered by epineurium. On the other hand, the axons of the nerves of the cauda equina are encased by oligodendrocytes. The nerves are then covered by pia and arachnoid, bathed in CSF, and encased in dura. This transition occurs just distal to the dorsal root ganglion [13-15]. Furthermore, the nerves of the cauda equina are loosely encased by the arachnoid membrane in such a manner that allows them to maintain a very consistent orientation. The nerves that are about to transition to peripheral nerves and exit the neural foramen are situated most anterolaterally and the coccygeal nerves are situated most dorsally. All the other nerves are arranged in order between them. As each nerve approaches its respective foramen, it takes the most anterolateral position [13,16,17]. The loose arachnoid membrane as well as the redundancy in the nerves provide for a significant degree of vertical movement of the nerves. These are essential characteristics that allow for full range of motion of the spine, hips, and legs without causing stretch injury to the nerves.
Vascular anatomy of the conus and cauda equina
The arterial supply of the spinal cord is through the anterior spinal artery (ASA) and a pair of posterior spinal arteries. The ASA and its perforating branches provide the vascular supply to the anterior 66-75% of the spinal cord. The ASA is actually not a single continuous artery. Each segment is supplied by an anterior radicular artery. The main anterior radicular artery supplying the lower segment of the cord is called the artery of Adamkiewicz. This artery usually arises in the region between the dorsal branch of the left posterior ninth intercostal artery and first lumbar artery. The ASA gives rise to sulcal branches which run deep in the anterior sulcus and supply most of the grey matter. It also contributes to the vasa corona, a rich anastomotic plexus supplying the surface of the cord. In the lumbosacral section of the cord, the vasa corona gives rise to a pair of accessory anterolateral longitudinal arteries (ALA’s) which run adjacent to the rootlets as they emerge from the cord. The ALA’s give rise to the anterior proximal radicular arteries which continue as the longitudinal radicular arteries supplying the nerve rootlets. The longitudinal radicular arteries give off acute angled, coiled collateral arteries which provide blood flow to the intrafascicular arteries supplying the fascicles of the nerve bundle. The coiling in the collateral arteries is a post-natal adaptation that allows these blood vessels to “unwind” in response to the differential movement of the fascicles so that blood flow is not disrupted [14,18,19].
The posterior spinal arteries (PSA’s) run adjacent to the root entry zone. Their blood supply is derived from the segmental arteries through the posterior radicular arteries. The PSA’s are not continuous and depend on multiple small caliber posterior radicular arteries. At the conus, the ASA and the PSA’s form a cruciate anastomotic arcade [18,20]. The PSA’s also contribute to the vasa corona. The vasa corona not only provides vascular support to the posterior columns, but also to the peripheral white matter of the cord. The vasa corona also gives rise to the dorsolateral longitudinal arteries which supply the proximal aspect of the dorsal rootlets [14].
As described above, the rootlets and the proximal portion of the nerves of the cauda equina derive their blood supply from the proximal longitudinal radicular arteries. However, the distal aspect of the nerves receive their blood supply from the lumbar and sacral radicular arteries. As described by Parke et al., this opposing blood flow arrangement supplying each spinal nerve sets up a watershed zone that is very susceptible to ischemic injury [14,19]. The lack of collateral flow is especially important in cases of multiple compressions along the nerves [15].
The venous system of the spinal cord is a complex array of valveless plexi connecting the anterior and posterior spinal veins to the inferior vena cava (IVC). The spinal veins connect to the anterior and posterior radicular veins which in turn connect to the epidural venous system. The venous system connecting the epidural plexus of veins to the retroperitoneal veins was first described by Breschet but is commonly known as Batson’s plexus because of his description of the anatomy and function of the system. There are three components to Batson’s plexus, namely the internal venous plexus which is located in the epidural space, the basivertebral veins which travel through the vertebral bodies, and the external vertebral venous plexus which is located at the periphery of the spinal column [21]. The external vertebral plexus system communicates through the intercostal, lumbar and sacral veins to the azygous and hemiazygous veins which in turn terminate at the IVC [22]. There are numerous arteriovenous anastomoses between the radicular arteries and veins [14,23,24]. This system of shunts seems to be a protective mechanism allowing continued arterial flow and venous drainage in case of venous compression at one site along the nerves.
Physiologically, the venous system acts as a valveless reservoir that allows for redistribution of blood in response to certain pressure changes. This unique bidirectional plexus transmits intrathoracic and intraabdominal pressures toward the central nervous system and vice versa. This can have a profound effect on intracranial pressure (ICP) as a change in visceral cavity pressure will cause a change in ICP [21,24,25]. The ICP is further regulated by the absorption of CSF into the venous system. Studies have shown that at the spinal level, this occurs through the arachnoid villi which are situated at the level of radicular veins [26,27]. However, this process is pressure mediated, and therefore, high venous pressure leads to decreased CSF absorption [28].
Nerve damage during incidental durotomy
Intraoperative nerve damage may be categorized as direct or indirect. Direct nerve damage can occur during nerve manipulation, laceration, or entrapment. Nerve manipulation includes nerve traction which can lead to stretch injury, or nerve compression which typically occurs by an instrument. Nerve manipulation, even if not excessive, can lead to battered lumbar nerve root syndrome resulting in neuropathic pain, numbness, and weakness. Nerve laceration can occur during drilling, electrocauterization, or instrumentation [29]. Nerve entrapment is frequently encountered as a result of unrepaired dural tear allowing either a single or a few nerve roots to herniate through and get trapped at the dural opening [10-12,30-32]. Indirect injuries are usually vascular, including arterial or venous compression, hypovolemia, and hypotension [29]. Regardless of etiology, all these mechanisms of nerve injury typically present with pain, weakness, and numbness. Williams et al. evaluated over 100,000 surgical cases submitted to the Scoliosis Research Society and found that there was a three-fold increase in the rate of post-operative neurological deficit in cases involving ID compared to those without ID, which was significant. Furthermore, 64% of those patients realized only partial or no recovery of their deficits [6].
Another factor that can affect the incidence and severity of intraoperative nerve damage is the patient’s body habitus as it relates to intraoperative positioning and its effect in the ICP. Burks et al. studied the rate of ID in morbidly obese (BMI greater than 40 kg/m2), obese (BMI between 30 to 40 kg/m2), and non-obese patients. They found that in the cases of initial or revision lumbar decompression, as well as initial or revision lumbar fusion, the rate of ID was significantly increased in the morbidly obese and obese groups compared to the non-obese group [33]. One reason for this finding seems to be the increased ICP in obese patients. Roth et al. measured ICP of trauma patients with intracranial monitors in the supine and prone position. They found an increase of ICP from an average of 9.5 mm Hg in the supine position to 15.4 mm Hg in the prone position despite proper positioning [34]. This was statistically significant. Robba et al. studied 30 volunteers with an average BMI of 24.7 kg/m2 undergoing elective spinal procedures and found that by simply turning them prone, indirect measurement of ICP increased from an average value of 7.07 mm Hg to 14.83 mm Hg [35]. Again, this finding was despite proper surgical positioning. Since intraoperative abdominal decompression is so critical, a number of frames and tables have been designed for that purpose. We prefer to use the Jackson table because it offers the most ample room for abdominal decompression especially for the obese and morbidly obese patients [36,37]. However, despite that, a patient with a BMI of 45 will experience increased ICP and be more prone to ID and CSF leak.
One unique aspect of our case is the number and bilaterality of the spinal nerves that exited the small durotomy. We believe this to be multifactorial. The significantly increased ICP caused a substantial pressure gradient across the dura. Therefore, as soon as the small ID occurred, this pressure gradient forced some CSF as well as multiple spinal nerves, which were connected to each other by arachnoid membrane, out through the durotomy. This resulted in both direct and indirect nerve injuries as loops of nerves exited the small durotomy. As each nerve root was forced through the small opening, it experienced significant compression at both the exit and re-entry points by other nerves as well as the dural edge. This mechanism of injury was previously reported by Chang et al. as well as Oterdoom et al.; however, in their case report, all the cauda equine nerve herniations and strangulations were unilateral [10,32]. Direct compression of nerves results in disruption of axonal flow and damage to the myelin sheath thus resulting in nerve edema and dysfunction. Furthermore, the multiplicity of the areas of compression along each nerve decreases the chance of recovery. Indirect injury from vascular compromise also occurred due to the two separate points of compression along each herniated nerve. Parke et al. and Parke et al. point out that not only is there no collateral arterial supply to the spinal nerves, the main arterial supply is scant and highly susceptible to disruption [14,19]. This is even more prominent in cases where there are two points of arterial compression. In this case, there is complete loss of blood flow from both the proximal and distal aspects. This creates an area of nerve ischemia and possible death. As discussed above, there are also a significant number of arteriovenous fistulas along the length of the nerves. Reverse flow through these fistulas could potentially avoid or minimize ischemia in cases where there is only one point of compression. However, in our case, there were two points of compression in each nerve which completely prevented any blood flow to that segment of nerve that had exited and re-entered the dural sac. This lack of blood flow adds to the injury suffered from direct neural compression. It is for these reasons that not only in our case, but in literature in general, where recovery is limited [10,12,30].
Conclusion
Our case adds to the literature of incidental durotomies resulting in nerve injuries. We discuss some of the factors involved as well as the anatomical and pathophysiological correlations to such nerve injuries. Our case is unique in that it is the first reported case of multiple nerve herniation through the durotomy defect leading to bilateral nerve injuries.
Conflict of Interest
I have no conflicts of interest or ethical issues to report. There was no financial support for this case report. No parts of this paper have been presented or published previously. There was no financial support or industry affiliation for this manuscript. I, Joseph Yazdi, certify that this manuscript is a unique submission and has not been previously nor is now being considered for publication or review, in part or in full, with any other source in any medium.
References
- Eismont FJ, Wiesel SW, Rothman RH (1981) Treatment of dural tears associated with spinal surgery. J Bone Joint Surg 63A: 1132-1136.
- Jones AM, Stambough JL, Balderson RA, Rothman RH, Booth RE (1989) Long-term results of lumbar surgery complicated by incidental durotomy. Spine 14: 443-446.
- Opel F, Schramm J, Schirmer M, Zeitner M (1977) Results and complicated course after surgery for lumbar disc herniation. Adv Neurosurg 4: 6-51.
- Silvers HR, Lewis J, Asch HL (1993) Decompressive lumbar laminectomy for spinal stenosis. J Neurosurgery 78: 695-701.
- Smorgick Y, Baker K, Herkowitz H, Montgomery D, Badve S, et al. (2015) Predisposing factors for dural tear in patients undergoing lumbar spine surgery. J Neurosurg Spine 22: 483-486.
- Williams B, Sansur C, Smith J, Berven S, Broadstone P, et al. (2011) Incidence of unintended durotomy in spine surgery based on 108,478 cases. Neurosurgery 68: 117-124.
- Baker G, Cizik A, Bransford R, Bellabarba C, Konodi M, et al. (2012) Risk factors for unintended durotomy during spine surgery: a multivariate analysis. Spine J 12: 121-126.
- Hubbe U, Franco-Jimenez P, Klinger J, Vasilikos I, Scholz C, et al. (2016) Minimally invasive tubular microdiscectomy for recurrent lumbar disc herniation. J Neurosurg Spine 24: 48-53.
- Tafazal S, Sell P (2005) Incidental durotomy in lumbar spine surgery: incidence and management. Eur Spine J 14: 287-290.
- Chang MY, Chan JY, Huang CT, Liu YK, Huang JS (2012) Cauda equina incarceration secondary to dural tears after lumbar microsurgical discectomy. Formosan J Surg 45: 37-40.
- Choi JH, Kim JS, Jang JS, Lee DY (2013) Transdural nerve rootlet entrapment in the intervertebral disc space through minimal dural tear: report of 4 cases. J Korean Neurosurg Soc 53: 52-56.
- Toppich HG, Feldmann H, Sandvoss G, Meyer F (1994) Intervertebral space nerve root entrapment after lumbar disc surgery. Spine 19: 249-250.
- McCabe JS, Low FN (1969) The subarachnoid triangle: an area of transition in peripheral nerve. Anat Rec 164: 15-34.
- Parke W, Watanabe R (1985) The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine 10: 508-515.
- Olmarker K, Holm S, Rosenqvist A, Rydevik B (1991) Experimental nerve root compression: A model of acute, graded compression of the porcine cauda equina and an analysis of neural and vascular anatomy. Spine 16: 61-69.
- Wall E, Cohen M, Massie J, Rydevik B, Garfin S (1990) Cauda equina anatomy I: Intrathecal nerve root organization. Spine 15: 1244-1247.
- Hauck E, Wittkowski W, Bothe H (2008) Intradural anatomy of the nerve roots S1-S5 at their origin from the conus medullaris. Laboratory investigation. J Neurosurg Spine 9: 207-212.
- Djindjian M, Ribeiro A, Ortega E, Gaston A, Poirier J (1998) The normal vascularization of the intradural filum terminale in man. Surg Radiol Anat 10: 201-209.
- Parke W, Gammell K, Rothman R (1981) Arterial vascularization of the cauda equina. J Bone Joint Surg 63A: 53-62.
- Martirosyan N, Kalani M, Lemole M, Spetzler R, Preul M, et al. (2015) Microsurgical anatomy of the arterial basket of the conus medullaris. J Neurosurg Spine 22: 672-676.
- Nathoo N, Caris E, Wiener J, Mendel E (2011) History of the vertebral venous plexus and the significant contributions of Breschet and Batson. Neurosurgery 69: 1007-1014.
- Dommisse GF, Grobler L (1976) Arteries and veins of the lumbar nerve roots and cauda equina. Clin Orthop Relat Res 115: 22-29.
- McCutchen I, Doppman J, Oldfield E (1996) Microvascular anatomy of dural arteriovenous abnormalities of the spine: a microangiographic study. J Neurosurg 84: 215-220.
- Fyneface-Ogan S (2012) Anatomy and Clinical Importance of the Epidural Space, Epidural Analgesia - Current Views and Approaches. (1st edtn), InTech.
- Brockstein B, Johns L, Gewertz B (1994) Blood supply to the spinal cord: anatomic and physiologic correlations. Ann Vasc Surg 8: 394-399.
- Biceroglu H, Albayram S, Ogullar S, Hasiloglu ZI, Selcuk H, et al. (2012) Direct venous spinal absorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits. J Neurosurg Spine 16: 394-401.
- Tubbs RS, Hansasuta A, Steller W, Kelly DR, Blevins D, et al. (2007) Human spinal arachnoid villi revisited: immunohistological study and review of the literature. J Neurosurg Spine 7: 328-331.
- Sklar FH, Beyer CW, Diehl JT, Clark K (2012) Significance of the so-called absorptive reserve in communicating hydrocephalus: a preliminary report. Neurosurgery 8: 525-530.
- Stambough JL, Simeone FA (1999) Neurological complications in spinal surgery, Rothman – Simeone. The Spine (4th edtn), Philadelphia: W. B. Saunders Company.
- Hadani M, Findler G, Knoler N, Tadmor R, Sahar A, et al. (1986) Entrapped lumbar nerve root in pseudomeningocele after laminectomy: report of three cases. Neurosurgery 19: 405-407.
- Matsumoto T, Okuda S, Haku T, Maeda K, Maeno T, et al. (2015) Neurogenic shock immediately following posterior lumbar interbody fusion: report of two cases. Global Spine J 5: e13-e16.
- Oterdoom D, Groen R, Coppes M (2010) Cauda equine entrapment in a pseudomeningocele after lumbar Schwannoma extirpation. Eur Spine J 19: S158-S161.
- Burks C, Werner B, Yang S, Shimer A (2015) Obesity is associated with an increased rate of incidental durotomy in lumbar spine surgery. Spine 40: 500-504.
- Roth C, Ferbert A, Deinberger W, Kleffmann J, Kastner S, et al. (2014) Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care 21: 186-191.
- Robba C, Bragazzi N, Bertuccio A, Cardim D, Donnelly J, et al. (2016) Effect of prone position and positive end-expiratory pressure on non-invasive estimators of ICP: a pilot study. J Neurosurg Anesthesiol.
- Dharmavaram S, Jellish W, Nockels R, Shea J, Mehmod R, et al. (2006) Effect of prone positioning systems on the hemodynamic and cardiac function during lumbar spine surgery: an electrocardiographic study. Spine 31: 1388-1393.
- Palmon S, Kirsch J, Depper J, Toung T (1998) The effect of prone position on pulmonary mechanics is frame dependent. Anesth Analog 87: 1175-1180.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi