Research Article, J Spine Neurosurg Vol: 7 Issue: 2
Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases
Pao-Chun Lin1, Feng-Ming Hsu2, Yi-Hsing Chen1 and Furen Xiao1*
1Department of Neurosurgery, National Taiwan University Hospital, Taiwan
2Department of Radiation Oncology, National Taiwan University Hospital, Taiwan
*Corresponding Author : Furen Xiao
Department of Neurosurgery, National Taiwan University Hospital, No.7, Chung Shan S. Rd., Taipei, Taiwan (R.O.C.)
Tel: +886-2-23123456
E-mail: xiao@ntuh.gov.tw
Received: May 08, 2018 Accepted: May 16, 2018 Published: May 23, 2018
Citation: Lin P, Hsu F, Chen Y, Xiao F (2018) Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases. J Spine Neurosurg 7:2. doi: 10.4172/2325-9701.1000298
Abstract
Study Design: Retrospective study Purpose: The aim of this study was to assess the feasibility and safety of stereotactic body radiation therapy (SBRT) followed by surgery for spine metastases. Overview of Literature: SBRT has emerged as an exciting field in the management of patients with spine metastases, including those who first undergo surgery. Similarly, neoadjuvant SBRT, which means planned preoperative SBRT, should also be promising. However, there was no literature supporting this approach. Methods: Ten consecutive patients who received surgical management for spine metastases within 30 days after SBRT were reviewed. For patients with limited spine metastases, SBRT was performed first if there is no severe myelopathy. Surgery for decompression and fixation was then performed. Perioperative events were recorded and analyzed. If available, they were followed up for at least 12 months. Results: The SBRT was delivered in single fraction of 14 to 18 Gy (median: 16 Gy). Surgical decompression with fixation was performed 0 to 24 days (median: 5.5 days) after SBRT. The blood loss ranged from 100 to 1500 mL (median: 775 mL). The patients were discharged or transferred 6 to 36 days (median: 7 days) after surgery. One patient developed transient Brown-Sequard syndrome postoperatively. There was no wound complication. Five patients passed away due to progressive disease 2.3 to 13 months after surgery. There was no local recurrence and no instrument failure. Conclusions: Our experience showed that neoadjuvant SBRT followed by surgery is safe and promising for spinal metastases. The long term benefit over postoperative radiotherapy should be determined by further investigation.
Keywords: Spine metastasis; Stereotactic body radiation therapy; Surgery
Introduction
Spinal metastasis is a very frequent manifestation of systemic neoplasm, with up to 70% of cancer patients harboring secondary spinal disease [1]. The initial presentation of spine metastasis is usually pain. Other common symptoms included motor weakness and bladder dysfunction caused by epidural compression. As the life expectancy is increasing in malignancy patients, suitable and successful management to the spine metastasis is important. Despite the important role of conventional radiotherapy in treating patients with metastatic spine disease, there is increasing evidence supporting the use of spine radiosurgery or stereotactic body radiation therapy (SBRT) [2-5], considering the excellent pain and local control.
If surgical management is required due to cord compression or instability, radiation therapy is usually given postoperatively for better local control. Due to the short course, SBRT can be also delivered first if the surgery is not emergent. Neoadjuvant spinal radiosurgery, or “planned preoperative” SBRT, may provide several benefits over postoperative radiation therapy. These possible benefits include more accurate tumor volume contouring, more predictable treatment schedule, and more predictable dose delivery [6].
Despite these benefits, there is little, if any, literature on neoadjuvant SBRT for spine metastases. Hereby we report our preliminary experience, focusing the feasibility and safety of such approach.
Materials and Methods
From November 2014 to September 2016, there were total 10 patients with limited spine metastases undergoing SBRT and then surgery within 30 days (Table 1). Three are females and seven are males. Their age ranged from 49 to 74 years (median: 62 years). The primary tumor is renal cell carcinoma in one patient, hepatocellular carcinoma in another, while the others are lung cancer. The indicated levels are at thoracic for 6 patients, cervical for 2 and lumbar for 2.
| No. | Age (years) | Gender | Primary pathology | Level | Symptom of presentation | Margin dose (Gy) | Interval between SBRT and surgery (days) | Follow-up time (months) | Status at last follow-up | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | F | Lung adenocarcinoma | T10 | Back pain, urinary difficulty | 16 | 16 | 26 | Alive | |
| 2 | 49 | M | Lung squamous cell carcinoma | T4 | Back pain | 16 | 5 | 13 | Dead | Transient Brown-Sequard syndrome |
| 3 | 53 | M | Renal cell carcinoma | C1 | Neck pain | 18 | 24 | 2.3 | Dead | Hospital-acquired pneumonia |
| 4 | 50 | M | Lung adenocarcinoma | T5 | Back pain | 16 | 1 | 7.0 | Alive | |
| 5 | 74 | M | Lung adenocarcinoma | L5 | Back pain | 16 | 14 | 15 | Alive | |
| 6 | 58 | M | Lung small cell carcinoma | T3-4 | Back pain, urinary difficulty | 14 | 0 | 3.2 | Dead | |
| 7 | 62 | F | Lung adenocarcinoma | C2 | Neck pain | 16 | 1 | 12 | Alive | |
| 8 | 67 | M | Lung adenocarcinoma | L3 | Back pain | 16 | 4 | 12 | Alive | |
| 9 | 69 | F | Lung adenocarcinoma | T1 | Back pain | 16 | 7 | 7.9 | Dead | |
| 10 | 42 | M | Hepatocellular carcinoma | T1 | Neck pain | 16 | 3 | 5.0 | Dead |
Table 1: Demographic and clinical information for all patients.
For these patients with symptomatic spinal metastases, MRI was performed to evaluate the disease extent and epidural compression. Neurosurgical consultation was then acquired to establish the potential benefit of surgery and exclude the need for emergency operation. The indications for surgery after SBRT include instability, potential instability, and circumferential cord compression on image with stable neurological symptoms.
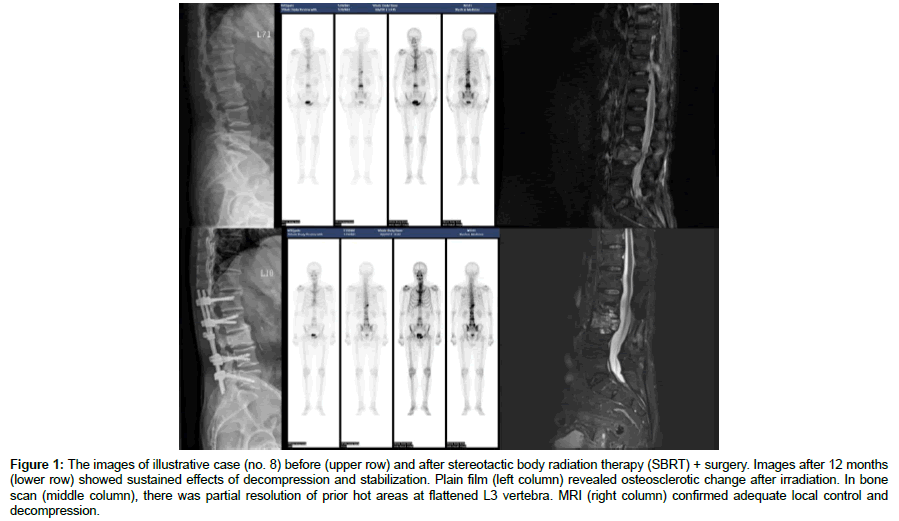
For SBRT, we followed the guidelines of International Spine Radiosurgery Consortium [7] for target volume definition. Dose constraints for critical organs were determined by the suggestion of AAPM Task Group 101 [8]. Single fraction SBRT was delivered by TrueBeam (Varian Medical Systems, Palo Alto, CA) in nine patients and by CyberKnife G4 (Accuray, Inc, Sunnyvale, CA) in one patient. The dose prescribed ranged from 14 to 18 Gy (median: 16 Gy). Surgical management was then arranged. After admission to neurosurgery ward, thecal sac decompression with fixation was performed in all patients. Two patients with high cervical metastases underwent occipital-cervical fixation. Two patients with cervicothoracic junction disease underwent cervical lateral mass screw and thoracic transpedicular screw fixation. The others were treated with transpedicular screw fixation (Figure 1). After the patients stabilized, they were either discharged or transferred to another ward for further treatment.
Figure 1: The images of illustrative case (no. 8) before (upper row) and after stereotactic body radiation therapy (SBRT) + surgery. Images after 12 months (lower row) showed sustained effects of decompression and stabilization. Plain film (left column) revealed osteosclerotic change after irradiation. In bone scan (middle column), there was partial resolution of prior hot areas at flattened L3 vertebra. MRI (right column) confirmed adequate local control and decompression.
Results
The SBRT and surgical management were performed as scheduled except for one patient (case no. 6), whose muscle power of both legs deteriorated from grade 5 to 3 just before SBRT. For this particular patient, we performed emergency operation for cord decompression and fixation right after SBRT. His muscle power returned to the baseline after operation.
The surgery was performed 0 to 24 days (median: 5.5 days) after SBRT. The blood loss ranged from 100 to 1500 mL (median: 775 mL). There was no wound dehiscence or wound infection. The patients were discharged from surgical ward 6 to 36 days (median: 7 days) after surgery. They were discharged from hospital 6 to 36 days (median: 15 days) after surgery, because five of them were transferred to other department (oncology or rehabilitation). The longest hospitalization happened in a patient with renal cell carcinoma and metastases to lung, brain and C1. After surgical decompression and occipitocervical fixation, hospital-acquired pneumonia complicated the postoperative course. Although discharged under stable condition, he passed away after another month due to systemic disease progression.
One patient (case no. 2) experienced neurological deterioration after surgery. He developed transient Brown-Sequard syndrome with left leg weakness postoperatively, which completely recovered in 4 months. He died 13 months after surgery due to progression of systemic disease. The other nine patients had stable neurological status. Local pain was relieved in eight patients.
The follow-up ranged from 2.3 to 26 months after surgery (median: 10.9 months). During this period, five patients died of systemic progressive disease 2.3 to 13 months after surgery (median: 5 months). One patient was lost to follow-up after 7 months. The remaining 4 survivors were followed for more than 12 months after surgery. For all patients, there was no local recurrence. There was no instrument failure and no delayed neurological deterioration attributed to indicated level.
Discussion
Spine radiosurgery, also known as SBRT, is an emerging method in treatment of metastatic spinal tumors. In comparison to conventional radiotherapy, such aggressive local treatment may improve both local control and pain control, which in turn improve patients’ quality of life [3]. If surgery is required to relieve epidural spinal cord compression or to restore mechanical stability, it is usually followed by conventional radiotherapy or SBRT for better local control [9]. However, because of the short treatment course, SBRT can also be performed before surgical management, if there is no surgical emergency.
In comparison to postoperative radiotherapy, there are several benefits of preoperative SBRT. Because of postoperative fluid accumulation, edema and fibrosis, target definition on images after surgery is usually more difficult. In addition, image artifacts caused by metallic implants make it harder to contour the target and organ at risk. It is also possible that these implants reflect or scatter radiation, causing inaccuracy in dosimetry. These concerns can be cleared if SBRT is performed before surgery.
Because of the postoperative discomfort and wound condition, it is common that SBRT or conventional radiotherapy begins weeks after surgery. The interval would be even longer if there is any surgical morbidity, including wound infection or suboptimal healing. Neoadjuvant SBRT, followed by surgery, makes the total treatment course shorter and more predictable. This approach should thus enhance patient convenience and reduce disruption of systemic therapy.
It is also a concern that surgical decompression of epidural metastases might carry risk of tumor cell contamination to the surrounding structures or to distant organs [10]. If SBRT is given to the tumor before surgery, the possibility of tumor dissemination should be lower in theory.
Although evidence showed excellent pain control and local control of SBRT, recently we have learned that vertebral compression fracture (VCF) is a relatively common adverse side effect following SBRT. The risk of VCF following SBRT ranged from 11% to 39% [11-13]. The risk factors included lytic tumor, older age, baseline VCF, misalignment, and high radiation dose [13]. The spinal instability neoplastic score may be used to evaluate the candidate of SBRT. If potentially unstable spine was identified, instrumented surgical procedure following SBRT can restore stability and should prevent VCF [14]. Earlier surgery should minimize the risk of post-irradiation fibrosis, which might increase the technical operative risk.
There are also some possible drawbacks of neoadjuvant SBRT. First, SBRT might take two days or three for setup, planning and delivery, so it will delay the surgery for that long. This approach is not appropriate for patients with severe symptoms of cord compression as immediate surgical decompression is required. It is also possible that preoperative SBRT interfere the healing capacity of surround tissue, causing delayed healing of surrounding tissue, which might be a concern if dura is opened during surgery.
To our knowledge, this is the first series of neoadjuvant SBRT followed by surgery for patients with spine metastases. We believe neoadjuvant SBRT is feasible and safe. There was no wound complication, probably because of relatively low radiation dose on skin. This approach is probably also effective since we saw good local control and stability in these patients. However, the impact of this approach to long-term local tumor control, pain control, and neurological outcome requires further investigation.
The limitation of our study includes small series and relatively short follow-up time due to its pilot nature. We also acknowledged that there was considerable variety of cancer types, spinal regions and surgical timing after SBRT in our series. Nevertheless, our study provides preliminary evidence that neoadjuvant SBRT followed by surgery may be a safe and efficacious treatment option for limited spinal metastases.
Conclusion
We showed that neoadjuvant SBRT followed by surgery is safe and promising for spinal metastases. The long term benefit over postoperative radiotherapy should be determined by further investigation.
Acknowledgments
The research reported in this publication was supported by Taiwan Ministry of Science Technology grant 104-2314-B-002-064.
References
- Jacobs WB, Perrin RG (2001) Evaluation and treatment of spinal metastases: an overview. Neurosurg focus 11: 1-11.
- Chang UK, Cho WI, Lee DH, Kim MS, Cho CK, et al. (2012) Stereotactic radiosurgery for primary and metastatic sarcomas involving the spine. J Neurooncol 107: 551-557.
- Gerszten PC (2014) Spine metastases: from radiotherapy, surgery, to radiosurgery. Neurosurgery 61: 16-25.
- Taunk NK, Spratt DE, Bilsky M, Yamada Y (2015) Spine Radiosurgery in the Management of Renal Cell Carcinoma Metastases. J Natl Compr Canc Netw 13: 801-809.
- Thibault I, Chang EL, Sheehan J, Ahluwalia MS, Guckenberger M, et al. (2015) Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol 16: e595-e603.
- Yao KC, Blacksburg S (2014) Neoadjuvant Spinal Radiosurgery. In: Sheehan JP, Gerszten PC. Controversies in Stereotactic Radiosurgery Stuttgart: Georg Thieme Verlag.
- Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, et al. (2012) International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 83: e597-e605.
- Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, et al. (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med phy 37: 4078-4101.
- Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, et al. (2013) The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 18: 744-751.
- Murakami H, Kawahara N, Tomita K (2015) Primary and Metastatic Tumors of the Spine: Total En Bloc Spondylectomy. Operative Techniques in Orthopaedic Surgical Oncology.
- Cunha MV, Al-Omair A, Atenafu EG, Masucci GL, Letourneau D, et al. (2012) Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys 84: e343-e349.
- Sahgal A, Atenafu EG, Chao S, Al-Omair A, Boehling N, et al. (2013) Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 31: 3426-3431.
- Thibault I, Gravel SB, Whyne C, Mercier D, Sahgal A (2016) Vertebral compression fracture post–spine SBRT. Image-Guided Hypofractionated Stereotactic Radiosurgery: A Practical Approach to Guide Treatment of Brain and Spine Tumors.
- Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, et al. (2010) A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 35: E1221-E1229.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi