Case Report, J Vet Sci Med Diagn Vol: 11 Issue: 3
Old Disease in a Young Mouse: Case Report for Lymphocytic Leukemia in an 11-Week-Old Female SCID/Beige Mouse
Nguyen K1*, Mills PO2, Blair R3, Collins-Burow BM1, Burow ME1
1Department of Medicine, Tulane University School of Medicine, New Orleans, LA, United States
2Department of Comparative Medicine, Tulane University School of Medicine, New Orleans, LA, United States
3Department of Pathology and Laboratory Medicine, Tulane University National Primate Research Center, Covington, LA, United States
*Corresponding author: Nguyen K, Department of Medicine, Tulane University School of Medicine, New Orleans, LA, United States, Tel: 5049886704; E-mail: KNguyen11@tulane.edu
Received date: 24 January, 2022, Manuscript No. JVSMD-22-52350;
Editor assigned date: 26 January, 2022, Pre QC No. JVSMD-22-52350 (PQ);
Reviewed date: 07 February, 2022, QC No. JVSMD-22-52350;
Revised date: 17 February, 2022, Manuscript No. JVSMD-22-52350 (R);
Published date: 23 March, 2022, DOI:10.4172/2325-9590.306
Abstract
The SCID (Prkdcscid) Beige (Lystbg) (Fox Chase SCID Beige) strain is an inbred and severely immune deficient mouse model that is commonly used for tumor biology and xeno graft research. The SCID mutation results in defective antibody responses due to improper maturation and function of B cells and T cells. Furthermore, the SCID mutation impairs DNA double-strand break repairing mechanisms. The beige mutation further diminishes the animal’s immune system by causing defective Natural Killer (NK) cells. Altogether, these mutations induce susceptibility to diseases such as cancers. Although there are reports of spontaneous hematopoietic cancers developing in immune deficient mice, this phenomenon is typically seen in advanced age mice. In this report, we discuss a unique case of a spontaneous lymphoma developing in a female SCID/Beige mouse at 11 weeks old. This rare occurrence emphasizes the importance of distinguishing spontaneously arising diseases from xeno grafted tissues when interpreting studies.
Keywords: SCID mutation; Animal's immune system; Tumor biology
Report
Clinical history
An 11-week-old, sexually intact female SCID Beige mouse was examined, at Tulane University School of Medicine, because of a 2-week history of progressive abdominal distension and swelling. The mouse was socially housed with a cohort of 4 other genetically identical mice of the same age and sex.
Gross necropsy
The mouse was humanely euthanized following the AVMA euthanasia guidelines and approved IACUC protocols. Dissection revealed poor body condition evident by lack of musculature, minimum fat stores, and mild autolysis. Visceral examination showed a severely enlarged liver (Figure 1A).
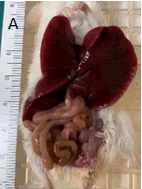
Figure 1A: View of reflected liver lobes and exposed GI tract. The liver is enlarged with ruler (cm side) for scale.
And spleen (Figure 1B) with rounded edges; ingesta was found throughout the entire gastrointestinal tract. Remaining thoracic and abdominal cavity tissues were grossly unremarkable.
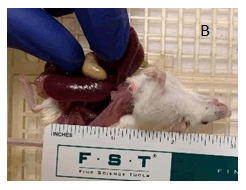
Figure 1B: View of exposed spleen with ruler for scale. Enlarged spleen is approximately 2.5 cm in length
Microscopic Findings
Tissues examined: Liver, lung, kidney, heart, spleen, small intestine, pancreas, and mesenteric lymph node.
Liver–invading and effacing the normal hepatic parenchyma is a densely cellular, unencapsulated, poorly circumscribed neoplasm. The neoplasm is composed of sheets and aggregates of round cells that surround vessels, expand sinusoids, and compress hepatic cords (Figure 2A).
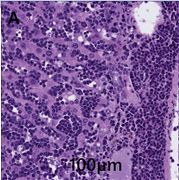
Figure 2A: Histological view of H&E stained tissues. Liver contains neoplastic cells and has a mitotic index of >40 in 10 high powered fields.
Neoplastic cells have round to oval nuclei with finely to coarsely stippled chromatin and 1-2 prominent nucleoli. Neoplastic cells exhibit moderate anisocytosis and anisokaryosis. The mitotic index is >40 mitoses in 10 high powered fields. Neoplastic cells frequently exhibit single cell necrosis. Hepatocytes are frequently atrophic.
Spleen–diffusely effacing and replacing the spleen is a densely cellular, unencapsulated neoplasm similar to the one described above in the liver. Neoplastic cells form a continuous sheet that replaces the entire splenic parenchyma. Megakaryocytes and rare smooth muscle trabeculae are the only remaining structures to aid in organ identification (Figure 2B).
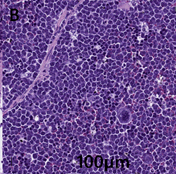
Figure 2B: Spleen displays dense cellular neoplasms that diffusely effaces and replaces the normal spleen parenchyma.
Neoplastic cells exhibit moderate to marked anisocytosis and anisokaryosis. The mitotic rate is 5-6 mitoses per 40 x field.
Mesenteric lymph node–invading and effacing the lymph node is a densely cellular, unencapsulated neoplasm similar to the one described in the liver (Figure 2C).
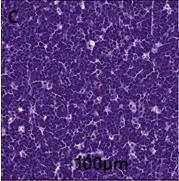
Figure 2C: Mesenteric lymph node contains unencapsulated neoplastic cells and has a mitotic rate of 8-9 mitosis per 40 x field.
Neoplastic cells exhibit moderate to marked anisocytosis and anisokaryosis. The mitotic rate is 8-9 mitoses per 40 x field. There is a focal area of necrosis along the margin of the lymph node.
Lung–alveolar septa and pulmonary vessels are diffusely infiltrated by neoplastic round cells similar to those described above (Figure 2D).
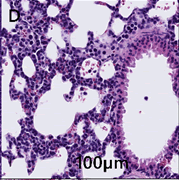
Figure 2D: Diffuse infiltration of neoplastic cells into the alveolar septa and pulmonary vessels of the lung can be seen. Furthermore, there is alveoli hemorrhage and edema.
Invasion into the interstitial and perivascular spaces occurs less frequently. Alveoli multifocally contain hemorrhage and edema.
Kidney–multifocally invading and expanding the renal interstitium surrounding vessels are neoplastic cells similar to those described above in other organs (Figure 2E).
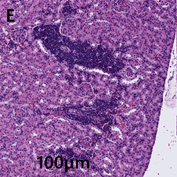
Figure 2E: Kidneys display multifocally invading neoplastic cells that have expanded into the renal interstitial spaces. Mitotic figures are occasionally observed.
Neoplastic cells exhibit moderate anisocytosis and anisokaryosis. Mitotic figures are occasionally observed.
Heart–The epicardium is multifocally expanded by neoplastic cells similar to those described above (Figure 2F).
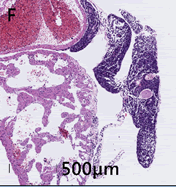
Figure 2F: Heart displays multifocal epicardial expansion by neoplastic cells. Neoplastic cells are also detected within the vessels and chambers of the heart.
Similar neoplastic cells are seen within vessels and the chambers of the heart. Small intestine–no significant findings (NSF).Pancreas–NSF.
Discussion
Immunodeficient mice are popular models for translational research because they are receptive to xenografts of immortalized cell lines, patient-derived-xenograft (PDX) tumors, and primary patient samples. The Fox Chase SCID Beige mouse is an inbred, congenic strain that possess both the Prkdscid (SCID) and Lystbg (Beige) autosomal recessive mutations. [1] referred to as SCID (severe combined immunodeficiency), eliminates adaptive immunity by preventing V(D)J recombination in developing T and B lymphocytes. Furthermore, this mutation also leads to defects in nonhomologous DNA end joining (NHEJ), a major mechanism for DNA damage [2,3] the human homologous equivalent of this mutation is Chediak-Higashi syndrome [4,5] Lymphoma incidence is positively correlated with mouse age with a study showing 21 weeks as the earliest age of onset [6-8].
Conclusion
This case report describes the earliest known case of spontaneous lymphoma development in a female SCID Beige mouse at 11 weeks. Although this is an unusual presentation, we wanted to make researchers aware that it is a possibility and to remind about the importance of vigilance in monitoring research animals.
References
- https://en.wikipedia.org/wiki/NSG_mouse
- Schwarz K, Ma Y, Pannicke U, Lieber MR (2003) Human severe combined immune deficiency and DNA repair. Bioessays 25 : 1061-1071.
[Cross Ref] [Google scholar] [Pubmed]
- Brandt EJ, Swank RT (1976) The Chediak-Higashi (beige) mutation in two mouse strains. Allelism and similarity in lysosomal dysfunction. Am J Pathol 82: 573–588.
[Google Scholar] [Pubmed]
- Sundberg JP (1992) The Beige (Bgj) Mutation.
- Huang P, Westmoreland SV, Jain RK, Fukumura D (2011) Spontaneous nonthymic tumors in SCID mice. Comp Med 61: 227-234.
[Google Scholar] [Pubmed]
- Sundberg JP, Adkison DL, Bedigian HG (1991) Skeletal Muscle Rhabdomyosarcomas in Inbred Laboratory Mice. Vet Pathol 28: 200-206.
[Cross Ref] [Google scholar] [Pubmed]
- Serreze DV, Leiter EH, Hanson MS, Christianson SW, Shultz LD, et al. (1995) Emv30nuu NOD-scid Mice An Improved Host for Adoptive Transfer of Autoimmune Diabetes and Growth of Human Lymphohematopoietic Cells. Diabetes 44: 1392-1398.
[Cross Ref] [Google scholar] [Pubmed]
- Chiu PPL, Ivakine E, Mortin-Toth S, Danska JS (2002) Susceptibility to Lymphoid Neoplasia in Immunodeficient Strains of Nonobese Diabetic Mice. Cancer Res 62: 5828-5834.
[Google scholar] [Pubmed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi 
