Research Article, J Clin Exp Oncol Vol: 6 Issue: 3
Orphan Nuclear Receptor TR3/Nur77 is a Specific Therapeutic Target for Hepatic Cancers
Yingling Zeng1*, Xiaoguang Ye2, Degui Liao3, Shizhang Huang3, Huinan Mao1, Dezheng Zhao4,5 and Huiyan Zeng4,6*
1Departments of Preventative Medicine, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, P. R. China
2Departments of Infectious Diseases, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, P. R. China
3Departments of Pathology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, P. R. China
4Center for Vascular Biology Research, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
5Divisions of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
6Department of Medicine, Hematology and Oncology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
*Corresponding Authors : Huiyan Zeng
Center for Vascular Biology Research and Division of Molecular and Vascular Medicine, Department of Medicine
Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, 02215, USA
Tel: 617-667-2329
Fax: 617-667-3591
E-mail: hzeng@bidmc.harvard.edu
Yingling Zeng
Department of Preventative Medicine, The Second Affiliated Hospital of Guangzhou Medical University
250 Changgang Dong Road Guangzhou, Guangdong, P. R. China 510260
Tel: 86-20-34153412
E-mail: zyl1308@163.com
Received: April 14, 2017 Accepted: May 02, 2017 Published: May 09, 2017
Citation: Zeng Y, Ye X, Liao D, Huang S, Mao H, et al. (2017) Orphan Nuclear Receptor TR3/Nur77 is a Specific Therapeutic Target for Hepatic Cancers. J Clin Exp Oncol 6:3. doi: 10.4172/2324-9110.1000184
Abstract
Objective: Although great success has been achieved in cancer treatment, current cancer therapies, including anti-tumorigenesis and anti-angiogenesis, still face the problems of insufficient efficacy, resistance and intrinsic refractoriness, in addition to their toxic side effects. There is a demand to identify additional targets that can be blocked to turn off the downstream effects of most, if not all, pathways. Our previous studies suggest that orphan nuclear receptor TR3 (human) / Nur77 (mouse) is such a target. However, the correlation of TR3 expression and clinical tumor progression has not been studied.
Methods: The expression of TR3 was analysed in human primary hepatic cancer specimens from patients that have complete medical records with Immunohistochemical staining. The statistical analysis was used to assess the significance of TR3 expression in tumor tissues, paratumor tissues and normal tissues, and to investigate the correlation of TR3 expression and clincopathologic characteristics.
Results: TR3 is highly expressed in human hepatic cancer tissues, but not in normal liver tissues. The positive expression yields of TR3 are 67.67% (14/21), 19.05% (4/21) and 0% (0/10) in cancer tissues, para cancer tissues, and normal liver tissue, respectively, which are statistic significant (χ2=17.07, p<0.005). The expression of TR3 is significantly higher in cancer tissues than in para cancer tissues (χ2=9.722, p<0.005) and in normal tissues (p< 0.0005). The levels of TR3 expression in human hepatic cancer tissues correlates well with tumors that are at low/middle degree of tumor differentiation and have portal vein thrombosis, metastasis and recurrence, but not with age, gender, tumor number and Alpha-fetal protein (AFP) volume.
Conclusion: The results indicate that TR3 is a specific therapeutic target for hepatic cancers.
Keywords: TR3; Nur77; Tumor; Hepatic cancer; Expression; Progression; Clinical; Correlation; Metastasis; Therapeutic target
Abbreviations
VEGF-A: Vascular Endothelial Growth Factor-A; Bfgf: Basic Fibroblast Growth Factor; PLGF: Placental Growth Factor; PDGF: Platelet-Derived Growth Factor; AFP: Alpha-Fetoprotein; EGF: Epidermal Growth Factor; HGF: Hepatocyte Growth Factor; FGF: Fibroblast Growth Factor
Introduction
We were the first to identify that TR3/Nur77, a member of nuclear receptor IV subfamily of transcription factors [1], is a critical mediator of angiogenesis [2-4]. TR3/Nur77 is highly and transiently up-regulated by angiogenic factors vascular endothelial growth factor-A (VEGF-A), histamine and serotonin, but not by basic fibroblast growth factor (bFGF), placental growth factor (PlGF) and platelet-derived growth factor (PDGF) in cultured endothelial cells (EC) and during angiogenesis in vivo [2-4]. Endothelial cell proliferation, migration and tube formation induced by VEGF-A, histamine and serotonin in vitro, tumor growth, angiogenesis and micro vessel permeability induced by VEGF-A, histamine or serotonin, are inhibited by TR3 antisense DNA or shRNA in vitro and in Nur77 knockout mice, respectively [2-4]. Overexpression of TR3/ Nur77 is sufficient to induce endothelial cell proliferation, migration and tube formation in vitro, angiogenesis, microvessel permeability and normal skin wound healing in our transgenic mice, in which, Nur77 full-length cDNA is inducibly and specifically expressed in mouse endothelium (EC-Nur77-S transgenic mice), respectively [2-4]. However, both the Nur77 null mice and EC-Nur77-S transgenic mice are healthy [5,6]. Therefore, TR3/Nur77 is an excellent target for pro-angiogenesis and anti-angiogenesis therapies.
It was also reported that TR3/Nur77 plays important roles in carcinogenesis, apoptosis [5], brown fat thermogenesis [7,8] inflammation, metabolism diseases, stress and addiction [9-12]. However, the clinical correlation of TR3 expression in human cancers is understudied. Hepatocellular carcinoma (HCC) that accounts for 80% of all liver cancers worldwide [13] is the sixth most common neoplasm and the third most frequent cause of cancer death [14]. In the clinic, most HCC are diagnosed in advanced stage without effective treatment [13]. Here, we examined the expression of TR3 in human hepatic cancer tissues and analysed its clinical correlation. We found that TR3 is highly expressed in human hepatic cancer tissues, but not in human normal liver tissues. The positive expression yields of TR3 are 67.67% (14/21), 19.05% (4/21) and 0% (0/10) in cancer tissues, paracancer tissues, and normal liver tissue, respectively, which are statistic significant (χ2=17.07, p<0.005). Further, the expression of TR3 is significantly higher in cancer tissues than in paracancer tissues (χ2=9.722, p<0.005) and in normal tissues (p<0.0005). The levels of TR3 expression in human hepatic cancer tissues correlates well with tumors that are at low/middle degree of tumor differentiation and have portal vein thrombosis, metastasis and recurrence, but not with age, gender, tumor number and Alpha-fetal protein (AFP) volume. The results suggest that TR3 could be a novel target for human cancer therapy.
Material and Methods
Patient material and tissue
The paraffin-embedded postoperative tissues specimens classified by pathological analysis were obtained from the archives of Department of Pathology, the Second Affiliated Hospital of Guangzhou Medical University, P. R. China, between January 2010 to December 2013. Twenty-one human primary liver cancer specimens from patients that have complete medical records were retrieved. Approval of the current project was obtained from the local ethics committee.
The main characteristics of 21 patients were summarized in Table 1. Ages of all patients in this study are between 32 and 70 years (median, 58 years), with 13 cases of males and 8 cases of females. There are 8 cases and 13 cases with the tumor diameter less/equal or greater than 5 cm, respectively. Histological analysis indicated that 13 cases and 8 cases belong to low/middle and highly differentiated tumors, respectively. Among the 21 patients, 14 and 13 patients complicated with portal vein thrombosis and tumor metastasis, respectively.
| Variables | No. of patients | Positive expression rate | p value |
|---|---|---|---|
| Age (years) | |||
| = 60 | 15 | 10 (66.67%) | 0.686 |
| < 60 | 6 | 4 (66.67%) | |
| Gender | |||
| Male | 13 | 9 (69.23%) | 0.557 |
| Female | 8 | 5 (62.5%) | |
| Tumor diameter (cm) | |||
| = 5 | 9 | 4 (44.44%) | *0.080 |
| > 5 | 12 | 10 (83.33%) | |
| Degree of tumor differentiation | |||
| Low and middle | 13 | 11 (84.61%) | **0.040 |
| High | 8 | 3 (37.5%) | |
| Portal vein thrombosis | |||
| With | 14 | 12 (85.71%) | **0.017 |
| Without | 7 | 2(28.57%) | |
| Metastasis | |||
| Yes | 13 | 11 (84.62%) | **0.040 |
| No | 8 | 3(37.5%) | |
| Recurrence | |||
| Yes | 11 | 10 (90.91%) | **0.020 |
| No | 10 | 4 (40%) | |
| Tumor number | |||
| Single | 14 | 10 (71.43%) | 0.428 |
| Multiple | 7 | 4 (57.14%) | |
| AFP volume | |||
| > 400 µg/L | 12 | 9 (75%) | 0.318 |
| < 400 µg/L | 9 | 5 (55.56%) |
Table 1: Correlation of TR3 expression and clincopathologic characteristics.
Compliance with ethical standards
The research involved human samples. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Guangzhou Medical University.
Tissue processing and Immunohistochemical staining
All tissues were fixed with 10% formalin, embedded with paraffin. Immunochemical staining on 4 μm sections was performed with an antibody against TR3/Nur77 (Univ-bio, Shanghai, CHINA), using immunological staining reagents following the protocol provided by the manufacture (Univ-bio, Shanghai, CHINA). PBS buffer was used as negative control.
Data analysis
Stained sections were analyzed by two independent pathologists. At least 10 fields with 400x amplification from each stained section were randomly chosen to be photographed with Olympus BX41 microscope (Olympus Scientific Solutions Americas Inc. Waltham, MA). Brown staining is referred as positive. Using half-quantitative measurement, the staining intensity was recorded as two levels, 1 as no staining and 2 as positive staining. The positive percentage of cancer cells was counted as 3 levels, level 1 as ≤ 25%, level 2 as 26% ~75%and level 3 as ≥ 76%. The final score for each section was the multiplication of these two scores (1~6). The sections were referred to be positive and negative with final score ≥ 3 and <3, respectively.
Statistics
Chi-square test and Fisher exact test were used for statistical analysis. A level of p<0.05 is considered significant.
Results
Expression of TR3/Nur77 in human hepatic cancer tissues, paracancer tissues and normal tissues
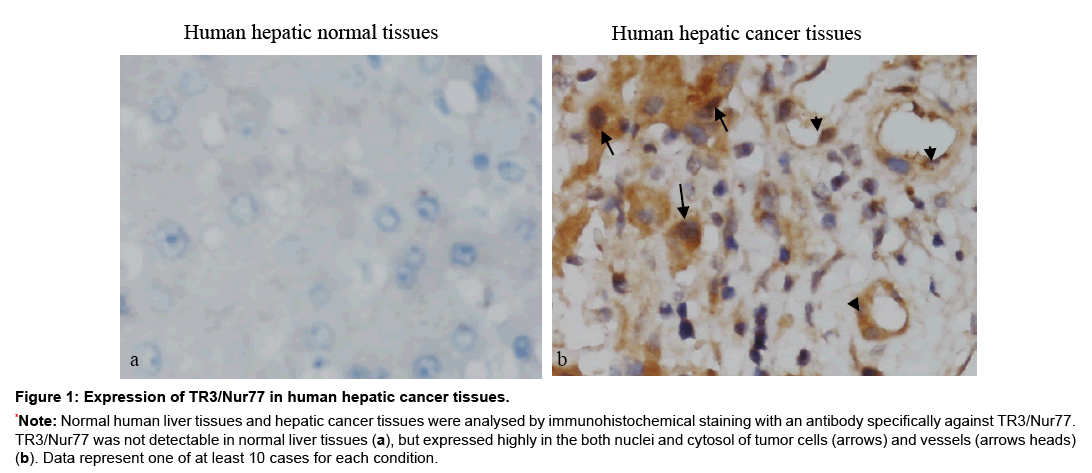
In order to study whether TR3/Nur77 is a potential target for human tumors, we studied the correlation of TR3 expression with hepatic cancers. Human normal liver tissues and hepatic cancer tissues were immunostained with an antibody against TR3/Nur77. The stained sections were analysed by two independent pathologists. At least 10 fields with 400x amplification were randomly chosen from each stained section. Using half-quantitative measurement, the staining intensity was recorded as two levels, 1 as no staining and 2 as positive staining. The positive percentage of cancer cells was counted 3 levels, level 1 as ≤ 25%, level 2 as 26%~75% and level 3 as ≥76%. The final score for each specimen was the multiplication of these two scores. They were referred to as high and ≥ 3 low and with <3, final score respectively. We found that the positive expression yields of TR3 are 67.67% (14/21), 19.05% (4/21) and 0% (0/10) in cancer tissues, paracancer tissues, and normal liver tissue, respectively, which are statistic significant (χ2=17.07, p<0.005). The expression of TR3 is significantly higher in cancer tissues than in paracancer tissues (χ2=9.722, p<0.005) and in normal tissues (p<0.0005) (Table 2). Further, TR3 is expressed in both cancer cells and vasculature of human hepatic cancer tissues, but not in normal liver tissues (Figure 1).
| Tissues | No. of patients | TR3 expression level | Χ2 | p | |
|---|---|---|---|---|---|
| High (%) | Low or no (%) | ||||
| Cancer tissues | 21 | 14/21 -67% | 7/21 (33) | 17.07 | <0.005 |
| Paracancer tissues | 21 | 4/21 (19%) | 17/21 (81%) | ||
| Normal tissues | 10 | 0/10 (0%) | 10/10 (100%) | ||
Table 2: Expression of TR3 in human hepatic cancer tissues, paracancer tissues and normal tissues.
Figure 1: Expression of TR3/Nur77 in human hepatic cancer tissues.
*Note: Normal human liver tissues and hepatic cancer tissues were analysed by immunohistochemical staining with an antibody specifically against TR3/Nur77. TR3/Nur77 was not detectable in normal liver tissues (a), but expressed highly in the both nuclei and cytosol of tumor cells (arrows) and vessels (arrows heads) (b). Data represent one of at least 10 cases for each condition.
Correlation of TR3 expression and clincopathologic characteristics
The positive expression rate of TR3 is (a) 10/15 (66.7%) cases and 4/6 (66.67%) cases for age ≥ 60 and <60, respectively; and (b) 9/13 (69.23%) cases and 5/8 (62.5%) cases for males and females, respectively (Table 1). There is no significant difference of TR3 positive expression in terms of age and gender (p>0.5). Some, but not significant, difference of TR3 expression was found in 4/9 (44.44%) cases and 10/12 (83.33%) cases for tumors with a diameter ≤ 5 cm and >5 cm, respectively (Table 1, 0.05 <*p<0.5). Expression of TR3 is significantly higher in tumors with low/middle degree of differentiation, 11/13 (84.61%) cases, than in tumors with high degree of differentiation, 3/8 (37.5%) cases. Tumors with portal vein thrombosis showed significantly higher levels of TR3 expression, 12/14 (85.71%) cases, than tumors without portal vein thrombosis, 2/7 (28.57%) cases. TR3 is expressed in 11/13 (84.61%) cases tumors with metastasis, which is significantly greater than those tumors without metastasis, 3/8 (37.5%) cases. TR3 expression was found significantly higher in tumors with recurrence, 10/11 (90.91%) cases, than in tumors without recurrence, 4/10 (40%) cases (Table 1, all **p<0.05). Single tumors, 10/14 (71.43%) cases, did not show any difference in TR3 expression from multiple tumors, 4/10 (40%) cases. There is no difference of TR3 expression in tumors with AFP >400 μg/L, 9/12 (75%) cases, or <400 μg/L, 5/9 (55.56%) cases (Table 1, p>0.5). These data clearly indicate that expression of TR3 in human hepatic cancer tissues correlates very well with tumors that are at low/middle degree of tumor differentiation, have portal vein thrombosis, metastasis and recurrence, somehow with tumor diameter, but does not correlate with age, gender, tumor number and AFP volume.
Conclusions
The data presented here indicate that TR3 is highly expressed in human hepatic cancer tissues, but not in human normal liver tissues. The positive expression yield of TR3 is 67.67% and 19.05% in cancer tissues and paracancer tissues, respectively. Expression of TR3 in human hepatic cancer tissues correlates very well with tumors that are at low/middle degree of tumor differentiation and have portal vein thrombosis, metastasis and recurrence, but does not have any difference in regarding of age, gender, tumor number and Alphafetoprotein volume.
Angiogenesis is critical for tumor growth [15-19]. Anti-VEGF neutralizing antibodies and VEGFR kinase/multiple kinase inhibitors have been successfully developed and widely used in the clinic [20]. However, in addition to their toxic side effects [21] VEGF-targeted therapies in cancer face the problems of insufficient efficacy [22-31], resistance, and intrinsic refractoriness [29,32,33]. Current anti-angiogenesis therapies mainly focus on targeting a single molecule or a single pathway, although there are some combination therapies that target a couple or a few pathways. The map of signaling pathways resembles a spider web. Hence, targeting one or a few major pathways results in other pathways becoming dominant. It is desirable to identify additional angiogenesis targets, blocking of which will turn off the downstream effects of most, if not all, pathways. Our studies found that orphan nuclear receptor TR3 / Nur77 is a master transcription factor that is down-stream of almost, if not all, signaling pathways, including VEGF-A, histamine and serotonin that control the various transcription profiles in pathological angiogenesis [2-4]. Microvessel permeability induced by VEGF-A, histamine or serotonin, and tumor growth are almost completely inhibited in Nur77 knockout mice [2-4]. However, Nur77 null mice are viable, fertile, develop an apparently normal adult vasculature [5], and have no defects in normal skin wound healing [34], suggesting that TR3 is required for pathological angiogenesis, but is not essential for developmental and physiological angiogenesis. The data that TR3 is not expressed in human normal liver tissues, but highly induced in human hepatic cancer tissues, are consistent with previous report that Nur77-/- mice 1) are viable and fertile, can develop an apparently normal adult vasculature [2,5] have no defects in normal skin wound healing whereas tumor growth is inhibited in Nur77-/- mice. These finding strongly support our hypothesis that TR3 is required for pathological angiogenesis.
TR3/Nur77 is induced by epidermal growth factor (EGF) and by serum in cancer cells, and knockdown of TR3/Nur77 inhibits the proliferation of tumor cells [34]. TR3/Nur77 plays important roles in cancer cell biology [9-12]. Our results that TR3 is expressed in both endothelial cells and tumor cells correlate very well with the function of TR3 in tumor cells. Targeting TR3 will inhibit not only tumor angiogenesis, but also tumorigenesis induced by EGF and other serum factors.
Other problems that VEGF-targeted therapy faces are resistance and intrinsic refractoriness that may be regulated by three mechanisms, 1) other angiogenic factors, such as hepatocyte growth factor (HGF) and fibroblast growth factor (FGF), regulate angiogenesis [35]; 2) tumor metastasis is increased [36]; 3) tumors increase resistance to anti-VEGF/VEGFR therapy [37]. The results obtained from current studies indicate that expression of TR3 is significantly increased in tumor tissues with metastasis and recurrence.
It was reported that TR3/Nur77 mediates VEGF-A-induced thrombomodulin expression to protect mice from arterial thrombus formation [38]. However, we found that TR3 expression is significantly increased in tumor tissues with portal vein thrombosis. The function of TR3 in mouse hepatic tumor model with or without thrombosis needs to be further studied.
In summary, we found that expression of TR3 correlates very well with the stages of hepatic cancers. To our knowledge, this is the first report about the clinical correlation of TR3 expression and human cancers. The results strongly support our previous hypothesis that TR3 is an excellent specific target for human cancers.
Consent to Publish
Informed consent was obtained from all individual participants included in the study.
Authors’ Contributions
YZ and HZ have the overall responsibility to the manuscript. They contributed to design all experiments, analyze and interpret data, write and approve the final version of manuscript to be published. XY participated in the design of experiments. DL acquired all data. SH performed analysis and interpretation of data. HM took part in analysis of Data. DZ took part in analysis and interpretation of data, and final approval of the version to be published.
Acknowledgement
This work was supported by National Institutes of Health [grant numbers R01CA133235, R03 CA194795 and R03CA191463 to HZ] and by Guangdong Science and Technology Department [grant numbers 2012B031800491 to YZ].
References
- Flaig R, Greschik H, Peluso-Iltis C, Moras D (2005) Structural basis for the cell-specific activities of the NGFI-B and the Nurr1 ligand-binding domain. J Biol Chem 280: 19250-19258.
- Qin L, Zhao D, Xu J, Ren X, Terwilliger EF, et al. (2013) The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood 121: 2154-2164.
- Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, et al. (2006) Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med 203: 719-729.
- Zhao D, Qin L, Bourbon PM, James L, Dvorak HF, et al. (2011) Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions. Proc Natl Acad Sci U S A 108: 12066-12071.
- Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, et al. (1995) Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science 269: 532-535.
- Niu G, Ye T, Qin L, Bourbon PM, Chang C, et al. (2014) Orphan nuclear receptor TR3/Nur77 impr oves wound healing by upregulating the expression of integrin beta4. Faseb J 29:131-140.
- Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, et al. (2005) Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem 280: 12573-12584.
- Kanzleiter T, Schneider T, Walter I, Bolze F, Eickhorst C, et al. (2005) Evidence for Nr4a1 as a cold-induced effector of brown fat thermogenesis. Physiol Genomics 24: 37-44.
- Campos-Melo D, Galleguillos D, Sánchez N, Gysling K, Andrés ME1 (2013) Nur transcription factors in stress and addiction. Front Mol Neurosci 6: 44.
- McMorrow JP, Murphy EP (2011) Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans 39: 688-693.
- Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, et al. (2012) Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin Cancer Res 18: 3223-3228.
- Pearen MA, Muscat GE (2010) Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24: 1891-1903.
- Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379: 1245-1255.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
- Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029-1039.
- Ferrara N (1999) Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol 237: 1-30.
- Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9: 669-676.
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306-1309
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, et al. (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983-985.
- Shibuya M (2014) VEGF-VEGFR Signals in Health and Disease. Biomol Ther (Seoul) 22: 1-9.
- Hayman SR, Leung N, Grande JP, Garovic VD (2012) VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 14: 285-294.
- Bautch VL (2010) Cancer: Tumour stem cells switch sides. Nature 468: 770-771.
- Ebos JM, Kerbel RS (2011) Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8: 210-221.
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS (2007) Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A 104: 17069-17074.
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, et al. (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16: 116-122.
- Li JL, Sainson RC, Oon CE, Turley H, Leek R, et al. (2011) DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res 71: 6073-6083.
- Loges S, Schmidt T, Carmeliet P (2010) Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 1: 12-25.
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, et al. (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468: 824-828.
- Shojaei F (2012) Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 320: 130-137.
- Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, et al. (2009) G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A 106: 6742-6747.
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, et al. (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468: 829-833.
- Helfrich I, Scheffrahn I, Bartling S, Weis J, von Felbert V, et al. (2010) Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J Exp Med 207: 491-503
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, et al. (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 25: 911-920.
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, et al. (2003) Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol Cell Biol 23: 8651-8667.
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, et al. (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15: 220-231.
- Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, et al. (2013) Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS One 8: e56505
- Hida K, Ohga N, Akiyama K, Maishi N, Hida Y (2013) Heterogeneity of tumor endothelial cells. Cancer Sci 104: 1391-1395.
- Yang P, Wei X, Zhang J, Yi B, Zhang GX, et al. (2016) Antithrombotic Effects of Nur77 and Nor1 Are Mediated Through Upregulating Thrombomodulin Expression in Endothelial Cells. Arterioscler Thromb Vasc Biol 36: 361-369.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi