Research Article, J Clin Exp Oncol Vol: 9 Issue: 1
Pancreatic Cancer AssociatedCachexia: Role of the Modified Glasgow Prognostic Score in Outcome Prediction
Debora Cardoso1*, Leonor Vasconcelos Matos1, Leonor Fernandes1, Tiago Dias Domingues2, Ricardo João2, Renata Medeiros-Mirra3, Helena Miranda1and Ana Martins11Department of Medical Oncology, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal
2Department of Statistics and Its Applications, Universidade de Lisboa, Lisbon, Portugal
3Cardiff School of Biosciences, Cardiff University, Cardiff, United Kingdom
*Corresponding Author: Débora Cardoso MD Department of Medical Oncology, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal, Tel: 00351 914570416; Fax: 00351 210431714; E-mail: deboravcardoso@hotmail.com
Received: January 13, 2020 Accepted: January 23, 2020 Published: January 30, 2020
Citation:Cardoso D, Matos LV, Fernandes L, Domingues TD, João R, et al. (2020) Pancreatic Cancer Associated-Cachexia: Role of the Modified Glasgow Prognostic Score in Outcome Prediction. J Clin Exp Oncol 9:1.
Abstract
Cancer-associated-cachexia (CAC) is a ubiquitous characteristic of pancreatic cancer (PC) and 1/3 of patients die from its complications. Systemic inflammation is key in CAC and the modified Glasgow Prognostic Score (mGPS) is a reliable inflammation-based prognostic tool. We aimed to evaluate the prognostic value of consensus-based cachexia classification and mGPS, their agreement and to analyze relevant clinical predictors of cachexia. This unicentric, retrospective, cohort study included patients with advanced PC treated over a 5-year period. Cachexia was classified according to weight loss, body mass index and mGPS. Fisher’s test was used to test correlation between classifications and logistic regression models were performed to test their association with other variables. Survival was analyzed with cox regression and Kaplan-Meier curves. 88 eligible patients (mean age 72, 56% female) were reviewed. At baseline, cachectic patients (CP) (77%), when compared with pre-CP, had worse performance status (p=0.016), more NLR>3,5 (p<0.01) and hypoalbuminemia (p 0.01). Of 77% (n=68) categorized as cachectic, only 16% (n=8) had a positive mGPS. No association was found between classifications (p=0.187). In multivariate analysis, NLR>3.5 was a significant predictor of both cachexia (p<0.001) and positive mGPS (p<0.01). Median overall survival (OS) for pre-CP was 19.1 months vs. 4.9 months in the CP (HR 1.94 95% CI 1.10-3.43 p=0.02). A positive mGPS at baseline was an independent predictor of worst OS (HR 2.73, 95% CI 1.126.66, p=0.027). CAC leads to worst survival and a better understanding of this syndrome in PC may improve outcomes for these patients. Our study suggests a baseline predominant fat-only loss phenotype, that patients with positive mGPS are at higher risk of worst outcomes and that NLR is a potential predictor of CAC. A prompt identification of prognostic markers may lead to a better standardized management of CAC
Keywords: Cachexia; Pancreatic cancer; Modified glasgow prognostic score; Weight loss; Neutrophil to lymphocyte ratio
Keywords
Cachexia; Pancreatic cancer; Modified glasgow prognostic score; Weight loss; Neutrophil to lymphocyte ratio
Abbreviations
Alb: Albumin; CAC: Cancer Associated Cachexia; CRP: C-Reactive Protein; BMI: Body Mass Index; mGPS: Modified Glasgow Prognostic Score; NLR: Neutrophil lymphocyte Ratio; OS: Overall Survival; PA: Pancreatic Adenocarcinoma; PLR: Platelet to Lymphocyte Ratio; WL: Weight Loss
Introduction
Cachexia is defined as a multifactorial syndrome characterized by unintended loss of skeletal muscle mass that can be partially, but not fully reversed by adequate nutritional support. It presents with a high prevalence in cancer patients, mostly in advanced stages of disease [1]. Cachexia is nearly omnipresent in patients with pancreatic cancer, with an 80% incidence throughout the course of the disease [2-4].
Pancreatic adenocarcinoma (PA) is the 7th most common cancer in Europe and the 4th cause of death by cancer. 5-year overall survival is less than 12 months and in about 30% of patients the cause of death is attributed to cachexia-related complications [5,6]. The slender association between PA and cachexia might be due to the frequent diagnosis in advanced stages and to the direct effects of this malignancy in ingestion, digestion and absorption of nutrients [7].
The pathophysiology of cachexia is complex, characterized by an energetic and protein imbalance, reduced nutrient ingestion and an hypercatabolic state [8]. A systemic inflammatory response and biochemical alterations (as anemia, rise in c-reactive protein (CRP) and hypoalbuminemia) are also key features of this syndrome. It is frequent to identify in cachectic patients, symptoms as anorexia, reduced muscle strength and fatigue [9].
According to a consensus definition from an expert group, the definition of cancer-associated cachexia (CAC) relies in the presence of at least one of three of the following criteria: weight loss (WL) superior to 5% or>2% WL in patients with body mass index (BMI)<20 mg/m2 or presence of sarcopenia and WL>2% from previous stable weight [10]. The consensus classification suggested 3 stages of cachexia with clinical relevance: pre-cachexia, defined by weight loss of less than 5%, cachexia, defined according to above mentioned criteria and refractory cachexia, which relies on clinical criteria, with variable degree of cachexia. While refractory cachexia implies a very advanced or a rapidly progressive disease, where active intervention to reverse WL may not be effective, the other two stages offer the prospect of active interventions.
Despite its high prevalence, the establishment of a definition that truly reflects the complex pathophysiologic impairments present in cachexia was not achieved yet. Furthermore, specific criteria to define and stage cachexia remain challenging.
Most tools to evaluate and stage cachexia are centered in evaluating its consequences (weight loss, muscle wasting) and not its causes. Despite its importance, WL does not reflect the complexity of the pathophysiological impairments present in cachexia. The systemic inflammatory response is a crucial trigger of the energetic imbalance and muscle wasting that distinguish cachexia. Furthermore, the production of inflammatory cytokines by the tumor plays an important role in the acute-phase response, with a rise in CRP and a fall in albumin (alb) serum levels [11]. The systemic inflammatory response has been proven to have a prognostic value. In this setting, different scores and ratios have been validated as prognostic predictors (the modified Glasgow Prognostic Score (mGPS), as well as neutrophil-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)) [12,13]. The most accepted systemic inflammation-based score is mGPS, a combined index that evaluates malnourishment and inflammation using CRP and alb to define three levels: 0 (CRP≤10 mg/ dL and alb ≥ 3,5 g/dL), 1 (CRP>10 mg/dL) and 2 (CRP>10 mg/dL and alb<3,5 gd/L). This score has a prognostic value, validated for cancer patients [14,15]. The mGPS Score helps stratify prognosis groups and may be a worthy addition to multifactorial evaluations, but as a standalone in individual cases it is unclear at this point how accurate it is likely to be. These two approaches to cachexia, a weight based and an inflammation based, provide different insights on a truly complex and multifactorial syndrome. A simple and practical score as mGPS, that can potentially correlate with other variables present in cachexia (WL, functional decline, worst performance status, uncontrolled symptoms) as well as with survival and that can help in categorizing cachexia in different stages and in identifying subset groups at higher risk, can be a useful tool in investigating and treating cachexia.
This study aimed to compare the agreement and prognostic value of current cachexia classification (based on weight loss) with the systemic inflammation-based mGPS and to evaluate relevant clinical predictors of cachexia development.
Methods
Study design, population and outcomes
This was an observational, descriptive, retrospective cohort study performed in a single institution. All patients presenting to the Oncology Department of a tertiary hospital, with locally advanced or metastatic PA, between the dates of 1 January 2013 and 31 January 2018 were included. Participants were eligible for enrollment if they were aged 18 years or older and had histologically confirmed advanced pancreatic adenocarcinoma, defined by the presence of distant metastasis, locally advanced disease, either unresectable or borderline resectable, that remained unresectable after cytoreductive therapy. Patients were excluded if there was lack of recorded medical data, lack of available survival data or laboratory parameters in clinical files: weight, WL, BMI, albumin and CRP serum levels at diagnosis. The primary outcome was overall survival (OS), defined as the time of first appointment to death from any cause.
From medical files, demographic and clinical data extracted included also age, gender, cancer location in the pancreas, Eastern Cooperative Oncology Group (ECOG)/performance status (PS) at diagnosis, systemic anticancer treatments, loss of appetite, reported by patients, and also readily available serum markers of inflammation, not only CRP and albumin, but also NLR and PLR, calculated from routine full blood neutrophil, lymphocyte and platelet count results, as a simple ratio between absolute counts.
Cachexia assessment
Patients were categorized as having cachexia according to two definitions. The first one was based on consensus definition [16] and incorporated WL and BMI. Consensus definition categorizes patients in 3 stages, being that the most advanced one, refractory cachexia, is defined by clinical criteria. In order to better assess WL among cachectic patients and detect any relevant changes in these population, we’ve subdivided WL into 2 categories. Patients with unintended weight loss≥5% during last 6 months were classified as precachectic, as in the consensus, patients with>5% to 10% weight loss or low BMI<20 kg/m2 and ongoing weight loss>2%-10% as cachectic and patients with>10% weight loss as severe cachectic. The second classification was the mGPS score: 0 defined by CRP≤10 mg/ dL and alb≥3,5 g/dL), 1 by CRP>10 mg/dL and 2 by CRP>10 mg/dL and alb<3,5 g/dL.
Data on Body Mass Index and weight loss
Data on pre-illness weight and time of weight loss were selfreported by patients. Baseline weight and height were collected by clinicians in the first oncology appointment. To calculate the percentage of weight loss, we used the following equation: (pre-illness weight – current weight) x 100/pre-illness weight. BMI was calculated from weight and height (kg/m2).
Statistical analysis
Data analysis of patient baseline demographic and clinical characteristics was performed using absolute and relative frequencies for categorical variables and central tendency, dispersion and range for continuous variables, according to mGPS and cachexia stages. Univariate association of characteristics with student’s t-test and Kruskal-Wallis test was performed, according to data distribution. A comparison between the cachexia classification and mGPS score was done using a 2 x 2 contingency table and Fisher’s exact test. A univariate single-factor logistic regression analyses was performed to assess effect of markers of inflammation, using maximum-likelihood estimation. According to standard cut-offs, NLR >3.5 and PLR>150 were tested. We also conducted multivariate logistic regression analysis with forward delection and likelihood ratio test (LRT). For each factor, we calculated the adjusted odds ratio and 95% CI using maximum likelihood estimation. Overall survival plots were built using Kaplan–Meier methods, according to mGPS and cachexia stages. Univariate differences between survival rates were tested for significance using the log-rank test. Cox proportional hazards models (estimated HRs and 95% CIs) was used to study the association between potential prognostic factors and outcomes. All analysis were performed using SPSS version 26 software. All results with a p value less than 0.05 were considered statistically significant.
Results
We’ve included 88 patients, with a mean age of 72 years, 56% (n=49) females. Most patients in pre-cachectic group received chemotherapy (75%), differently from patients in cachectic and refractory cachectic groups, where less than half (48%) underwent this treatment regimen. Most precachectic patients had good performance status, with 85% patients with ECOGPS 0-1, contrarily to patients with some degree of cachexia, with close to a third classified as ECOG/PS 3. Precachectic patients had significantly lower NLR (p<0.01), compared to patients with some degree of cachexia. An NLR>3.5 was present in 10% (n=2) precachectic patients and in 58% (n=25) patients with severe cachexia. Also, PLR was higher for cachectic patients when compared to prechachectic (p=0.06). Serum values, of hemoglobin (p=0.035) and albumin (p<0.01) were also notably lower in patients with cachexia. All baseline demographics and disease characteristics are presented in Table 1 for the classification according with consensusbased definition for cachexia and (Table 2) presents classification according to mGPS.
| Characteristics | Precachexia (n=20) | Cachexia (n=25) | Severe Cachexia (n=43) | p |
|---|---|---|---|---|
| Gender | 0.44 | |||
| Male | 11 (55.0) | 9 (36.0) | 19 (44.2) | |
| Female | 9 (45.0) | 16 (64.0) | 24 (55.8) | |
| Age (Years) | 69.1 (20) | 76.1 (17) | 76.3 (15) | 0.42 |
| Previous Weight (kg) | 66.5 (14) | 66.0 (11) | 72.0 (18) | 0.07 |
| Weight at diagnosis (kg) | 65.5 (15) | 60.0 (12) | 60.0 (18) | 0.12 |
| Height (cm) | 160.5 (11) | 161.0 (9) | 160.0 (11) | 0.88 |
| % Weight Loss in past 6 months | 0.0 (0) | 7.7 (2.1) | 15.7 (6.8) | <0.01 |
| Body Mass Index at diagnosis | 24.3 (6.2) | 23.4 (4.6) | 22.1 (5.2) | 0.03 |
| Body Mass Index (BMI) | - | |||
| Obese (BMI-30) | 4 (20) | 0 (0) | 2 (4.6) | |
| Overweight (25<BMI<30) | 5 (25) | 7 (28) | 9 (21) | |
| Normal (20<BMI<25) | 10 (50) | 15 (60) | 24 (55.8) | |
| Risk of malnutrition (BMI<20) | 1 (5) | 3 (12) | 8 (18.6) | |
| Loss of appetite | 1 (4.0) | 20 (80.0) | 34 (79.1) | <0.01 |
| Chemotherapy | 15 (75.0) | 12 (48.0) | 21 (48.8) | 0.11 |
| ECOG Performance Status (PS) | 0.016 | |||
| ECOG/PS 0 | 9 (45) | 5 (20) | 9 (21) | |
| ECOG/PS 1 | 8 (40) | 10 (40) | 14 (33) | |
| ECOG/PS 2 | 2 (10) | 3 (12) | 10 (23) | |
| ECOG/PS 3 | 1 (5) | 7 (28) | 9 (21) | |
| ECOG/PS 4 | 0 (0) | 0 (0) | 1 (2) | |
| Neutrophil to Lymphocyte Ratio (NLR) | 2 (1.67) | 6 (5.75) | 4 (4.0) | <0.01 |
| NLR>3.5 | 2 (10) | 13 (52) | 25 (58) | |
| Platelet to lymphocyte ratio (PLR) | 117 (60.6) | 140 (100.3) | 163.7 (112.2) | 0.06 |
| PLR>150 | 6 (30) | 11 (44) | 21 (48.8) | |
| Hemoglobin (g/dL) | 12.5 (1.7) | 11.0 (2.1) | 11.2 (2.9) | 0.01 |
| C Reactive Protein (CRP) (mg/dL) | 1.8 (4.0) | 3.6 (14.9) | 2.8 (3.9) | 0.035 |
| CRP>10 | 1 (5) | 8 (32) | 0 (0) | |
| Albumin (g/dL) | 3.8 (1.0) | 2.6. (1.0) | 3.2 (0.9) | <0.01 |
| Albumin<3,5 | 8 (40) | 17 (68) | 19 (44) | |
Table 1: Demographic and disease characteristics baseline, according to consensus-based cachexia classification
Data are median (IQR) or n (%). IQR: inter quartile range.
| mGPS (diagnosis) | 0 (n=66) |
1 (n=1) |
2 (n=7) |
p |
|---|---|---|---|---|
| Sex, n | 1.0 | |||
| Male | 33 (50.0) | 0 (0.0) | 3 (42.9) | |
| Female | 33 (50.0) | 1 (100.0) | 4 (57.1) | |
| Age (Years) | 72.6 (15) | - | 81.9 (18) | - |
| Baseline Weight Loss | 11.0 (12.6) | - | 7.7 (1.6) | - |
Table 2: Demographic characteristics baseline, according to mGPS classification. Data are median (IQR) or n (%).
Association between Cancer Associated Cachexia and Modified Glasgow Prognostic Score classifications
Based on classification of CAC, 23% (n=20) patients were categorized as pre-cachectic and 77% (n=68) as cachectic or severe cachectic. Using baseline Albumin and CRP values, mGPS index was calculated, resulting in the identification of 84% (n=66) with mGPS of 0 and 16% (n=8) with mGPS of 1 or 2, so the majority of patients classified as cachectic had no change in mGPS at baseline. There was no association between the classification of patients as cachectic or non-cachectic according to consensus definition and mGPS being positive or zero (p=0.187). Specifically, 48 individuals that had been diagnosed with cachexia presented a score of 0, representing a 65% mismatch in outcome. Despite no association, all individuals with a positive mGPS were cachectic and 18 individuals who had not been diagnosed with cachexia presented scores of 0, meaning that 35% of all individuals had matching outcomes.
Predictors of cachexia development
Multivariate analyses with stepwise delection, using sex, age, NLR>3.5 and PLR>3.5, showed that an NLR>3.5 was associated with cachexia development (Table 3), after stepwise procedure (LRT 16.729, p<0.001). Univariate analysis was also performed and showed concordant results. When applying the same analysis with dependent variable being mGPS results, also only NLR>3.5 was statistically significant for punctuating in the mGPS score (LRT 11.832, p<0.01).
| LRT | p-value | |
|---|---|---|
| Sex | 2.14 | 0.142 |
| Age | 2.36 | 0.124 |
| NLR>3.5 | 10.96 | <0.001 |
| PLR>150 | 2.92 | 0.088 |
Table 3: Results of the multivariate logistic regression model for cachexia, based on Sex, Age, NLR and PLR. Presented are the Likelihood ratio test results for each variable and respective p-value. LRT: Likelihood Ratio Test; NLR: Neutrophil to Lymphocyte Ratio; PLR: Platelet to Lymphocyte Ratio.
Survival results
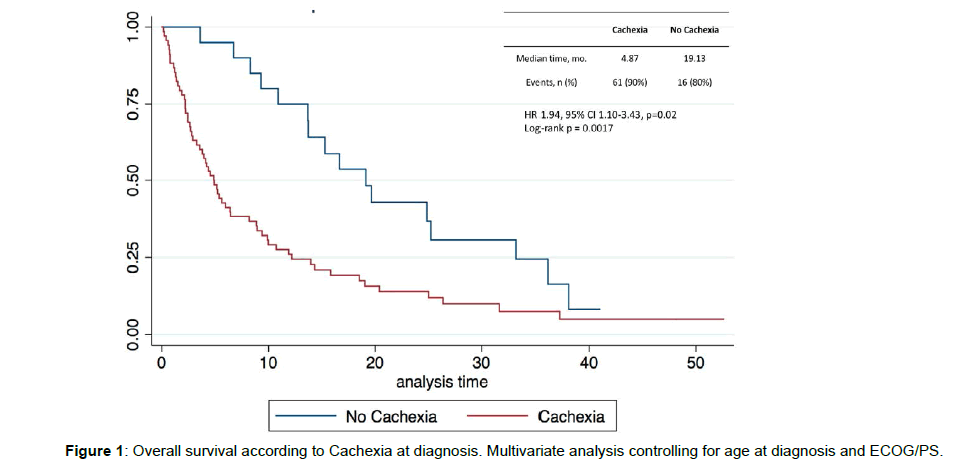
After a median follow-up of 6.9 (IQR 2.7–16.5) months, 77 patients (87.5%) died: 61 (69.3%) vs. 16 (18.2%) in cachectic and noncachectic groups, respectively. Patients without cachexia tended to live longer, with a median survival of 19.1 months, compared to a median survival of 4.9 months of those patients without cachexia. In line with this difference, a statistical tendency was noted disfavoring patients with cachexia, both in the univariate (p<0.01) and multivariate analysis (controlling for age at diagnosis and ECOG/PS; HR 1.94, 95% CI 1.10-3.43, p=0.02) (Figure 1).
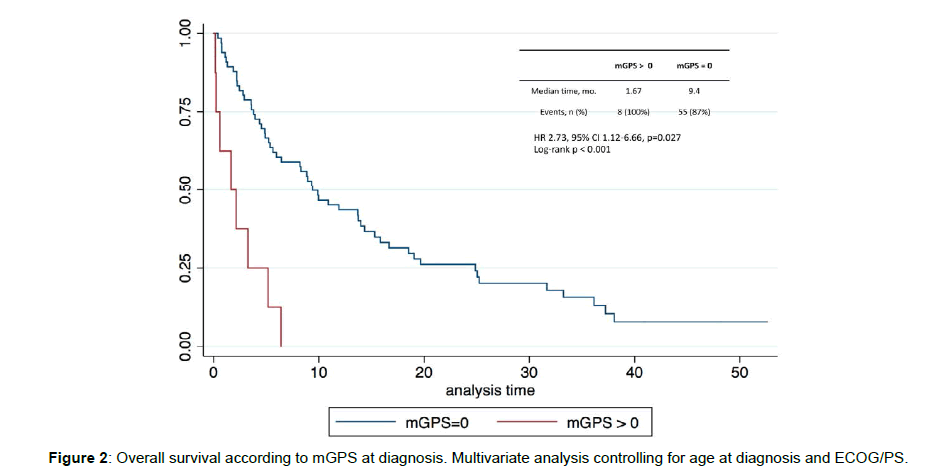
When analyzing patients according to mGPS, all 8 (100%) patients with a positive mGPS died, vs. 55 (87%) patients dying with an mGPS of 0. A positive mGPS was markedly correlated with worse survival, both in univariate (p<0.01) and multivariate analysis (adjusting for age at diagnosis and ECOG/PS; HR 2.73, 95% CI 1.12-6.66, p=0.027) (Figure 2).
Discussion
PA is an illness with high morbidity and mortality and a great majority of patients with PA will present with cachexia at diagnosis. CAC is still largely misunderstood due to its complex physiopathology and, unfortunately, no treatment has so far proven to be successful. Despite extensive efforts, there remains an urgent need to identify reliable and routinely available prognostic factors.
In this retrospective study we aimed to assess the correlation between consensus-based definition of CAC with the validated inflammatory score mGPS and to study relevant clinical predictors of changes with each tool Since cachexia stages are defined according to % of WL alone or together with BMI and mGPS is a score based on inflammatory parameters (albumin and CRP), patient’s baseline classification according to CAC definition and mGPS score suggest that, at diagnosis, fat loss prevails over muscle loss. These results are in line with the theory postulated by the international consensus, of a disease continuum in which early stages are characterized mainly by anorexia and impaired glucose tolerance, which precede substantial involuntary WL [17].
The mGPS score has shown to be a useful aid to stratify risk groups and better characterize the inflammatory component of CAC. We therefore interrogated whether, at diagnosis of PA, mGPS score could be correlated with presence of absence of CAC, according to standard definition. In our cohort, we showed no association between classifications but a relevant positive predictive tendency, since all patients with positive mGPS were cachectic.
Regarding CAC predictors, in our study we’ve incorporated other relevant markers of inflammation, the NLR and PLR, which combine neutrophil, lymphocyte and platelet levels in peripheral venous blood. These quantifications have been found to have predictive value in early diagnosis and malignancies as pancreatic cancer and can reflect prognosis in some extend, while being cost-effective [18]. In our cohort, using NLR clinically relevant cuf-off of 3.5, an NLR>3.5 showed to be a significant predictor of CAC at diagnosis and positive mGPS at diagnosis. These results further validate the predictive role of NLR in identifying at-risk patients of CAC at PA diagnosis: on average, a patient will over 13.5 times more likely to be diagnosed as cachectic if the NLR is>3.5.
Previous studies have already showed the negative impact of cachexia in survival [19, 20] and our results corroborate these findings. Patients with cachexia lived, in median, less 14.2 months than patients with no cachexia. When survival was calculated according to mGPS score, and adjusted for covariates in multivariate analysis, it showed a significantly worse prognosis for patients with a positive mGPS, compared with patients with mGPS of 0.
Conclusion
The results of this study help to shed light in this underappreciated but deathly syndrome that affects more than 80% of PA patients. While mGPS should not be used as a stand-alone tool, it can relevantly identify a patient’s subgroup at higher risk for worst outcomes, Likewise, NLR should be assessed at diagnosis of PA and a value>3.5 act as a trigger for a multimodal approach to CAC. A complete evaluation of multiple cachexia domains is important not only to better understand the complete landscape of CAC, but also to potentially identify treatment strategies to reverse it. Multimodal treatments, targeting the different domains of cachexia have shown promising. Better knowledge on cachexia and the complex web that links this syndrome to PA may prompt the design of targeted therapies that are able to ameliorate wasting in cachectic patients, with reflections in an improvement of quality of life and survival.
References
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489-95.
- Bachmann J, B'chler MW, Friess H, Martignoni ME (2013) Cachexia in patients with chronic pancreatitis and pancreatic cancer: impact on survival and outcome.Nutr Cancer65: 827-33.
- Stewart G, Skipworth R, Fearon KCH (2006) Cancer Cachexia and fatigue. Clin Med 6: 140-3.
- Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N et al. (2014) Pancreatic Cancer Cachexia: a review of mechanisms and therapeutics. Frontiers in Physiology 5: 1-14.
- World Health Organization. Globocan (2018) International Agency for Research on Cancer. Population Fact Sheets: Portugal. 270: 2018-9.
- Bachmann J, Ketterer K, Marsch C, Fechtner K, Krakowski-Roosen H, et al. (2009) Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer 9: 255.
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH, et al. (2018) Cancer-associated cachexia. Nat Rev Dis Primers 4, 17105.
- Ronga I, Gallucci F, Riccardi F, Uomo G (2014) Anorexia-cachexia syndrome in pancreatic cancer: Recent advances and new pharmacological approach. Adv Med Sci 591 :1-6
- Lindenmann J, Fink.Neuboeck N, Koesslbacher M, Pichler M, Stojakovic T, et al. (2014) The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment: CRP and albumin in esophageal cancer. J Surg Oncol 110: 645-50.
- Barry JL, Kaasa S, McMillan DC, Fallon MT, Hjermstad MJ et al. (2013) Prognostic Factors in Patients with Advanced Cancer: A Comparison of Clinicopathological Factors and the Development of an Inflammation-Based Prognostic System. Clin Cancer Res 19: 5456-64.
- Loumaye A, Thissen JP (2017) Biomarkers of cancer cachexia. Clin Bioch 50: 1281-8.
- Protor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD et al. (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 47: 2633-41.
- Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC.et al. (2017) The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 116: 134-46.
- Imaoka H, Mizuno N, Hara K, Hijioka S, Tajika M, et al. (2016) Evaluation of Modified Glasgow Prognostic Score for Pancreatic Cancer: A Retrospective Cohort Study.Pancreas 45: 211-7.
- Proctor MJ. Morrison DS, Talwar D, Balmer SM, O'Reilly DS et al. (2011) An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumor site: a Glasgow Inflammation Outcome Study. Br J Cancer 104: 726-34.
- Gao Y, Wang WJ, Zhi Q, Shen M, Jiang M, et al. (2017) Neutrophil/lymphocyte ratio is a more sensitive systemic inflammatory response biomarker than platelet/lymphocyte ratio in the prognosis evaluation of unresectable pancreatic cancer. Oncotarget.8: 88835-44.
- Fearon KC, Voss AC, Hustead DS. (2006) Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr.83: 1345-50.
- Fearon KC, Voss AC, Hustead DS. (2006) Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr.83: 1345-50.
- Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler M, Friess H, et al.(2008) Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 12: 1193-201.
- Solheim TS, Laird BJA, Balstad TR, Bye A, Stene G, Baracos V, et al. (2018) Cancer cachexia: Rationale for the MENAC (Multimodal - Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 1-8.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi