Review Article, Cell Biol Res Ther Vol: 13 Issue: 1
Pathogenic and Preventive Role of MAIT Cells in Chronic Diseases
Narkunaraja Shanmugam*, Arun Sundaramoorthy, Vaishnavi Mahadevan and Nerethika Ravichandiran
Department of Biomedical Science, Bharathidasan University, Tiruchirappalli 620024, Tamil Nadu, India
*Corresponding Author: Narkunaraja Shanmugam
Department of Biomedical Science, Bharathidasan University, Tiruchirappalli 620024, Tamil Nadu, India
E-mail: nshanmugam@bdu.ac.in
Received date: 12 July, 2023, Manuscript No. CBRT-24-105836;
Editor assigned date: 14 July, 2023, PreQC No. CBRT-24-105836 (PQ);
Reviewed date: 27 July, 2023, QC No. CBRT-24-105836;
Revised date: 15 January, 2024, Manuscript No. CBRT-24-105836 (R);
Published date: 22 January, 2024, DOI: 10.4172/2324-9293.1000201
Citation:Shanmugam N, Sundaramoorthy A, Mahadevan V, Ravichandiran N (2024) Pathogenic and Preventive Role of MAIT Cells in Chronic Diseases. Cell Biol 13:1.
Abstract
Background: Chronic disease encompasses a wide range of metabolic abnormalities. These factors largely account for this emerging interest and are notably marked by inflammation that implicates the immune system. MAIT (Mucosal-Associated Invariant T) cell expression is linked to chronic diseases.
Scope of the review: The current study reviews the MAIT cells role in metabolic pathologies in human chronic diseases like diabetes, asthma, and cancer.
Conclusion: MAIT cells are influenced by severe concepts, different expressions with a frequency reduction, and a protective to deleterious from phenotype shift, including chronic viral infections, autoimmune, and inflammatory conditions. The MAIT cells can play a pathogenic role by sustaining inflammation and cytotoxicity and physiological and pathological situations which may lead us to novel therapeutic approaches.
Keywords: Mucosal-Associated Invariant T cells (MAIT); Metabolic disease; Diabetes; Asthma; Cancer
Introduction
The immune system is conventionally classified into adaptive and innate immune systems that together organize and initiate immune responses against invading pathogens and preserve body integrity [1]. T cells from the activated adaptive immune system play a critical role in the establishment and maintenance of immune response. T cells are subdivided into conventional and unconventional cells that operate in fundamentally different ways to mediate and coordinate immune responses [2]. Conventional T cells mediate immune response through T Cell Receptor (TCR) recognition of processed antigenic peptides within the grooves of Major Histocompatibility Complex (MHC) molecules by Antigen Presenting Cells (APC) and are known for their immunology whereas; unconventional T cells differ from the memory. Conventional T cells in the rapidity of their initial recognition and response to a foreign antigen. Thus called innate-like T cells and possess an intermediate characteristic [3].
Mucosal-Associated Invariant T cells (MAIT) cells are αβCD4-CD8-T cells found in the peripheral blood reported by Porcelli et al., in 1993 [4-6]. MAIT cells are innate-like T cells, spanning both the innate and adaptive immune systems. MAIT cells are typically defined by their expression of an invariant TCR α-chain (Vα7.2–Jα33 in humans, and Vα19–Jα33 in mice) associated with a limited TCR β-chain repertoire [1,7] that recognize the single antigen in human and found in periphery and 50% in the liver which comprises 10% of T cells. Vitamin B-based antigens presented by the non-polymorphic MHC class I related-1 molecule (MR1) are recognize by MAIT cells. The MR1-MAIT cells were conserved across ~150 million years of mammalian evolution with 90% of sequence homology between MR1 of mice and humans [8]. Ligands such as 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5- OP-RU) and 5-(2-oxoethylideneamino)-6-D-ribitylaminouracil (5- OERU) produced by various bacteria, mycobacterium, and yeast during riboflavin synthesis were presented by MR1 for the activation MAIT cells. The absence of the riboflavin synthesis pathway in mammals provides host-pathogen discrimination. On activation, MAIT cells rapidly produce cytokines and mediate cytolytic activity against the infected cells [9]. Depending on the nature of the disease condition, these can range from host protective functions to pathogenic functions [10]. This review will focus on recent advances, and the preventive and pathogenic role of mucosal-associated invariant T cells.
Literature Review
Phenotype and localization: MAIT cells are defined by the surface expression of their semi-invariant TCR in combination with the expression of the C-type lectin CD161 and Interleukin-18 Receptor α- subunit (IL-18R α) [11]. Many studies reported that they are classified into three subsets on the basis of the expression of CD4 and CD8 surface markers: CD4+/CD8−(CD4+), CD4−/CD8+ (CD8+), and CD4/ CD8 Double-Negative (DN) subsets [12]. CD8+ and CD8-CD4-(double negative, DN), usually accounting for 80% and 15% of total MAIT cells, respectively. MAIT cells are predominantly CD8+ with a small fraction of DN subset in peripheral blood of healthy adults and DN subsets are relatively rare in the thymus. At steady-state, mature MAIT cells display an effector phenotype (CD45RO_CD27) with chemokine receptor expression CCR7_CCR9intCCR5hiCCR6hiCXCR6hi, thus confirming MAIT cells show preferential homing in tissues [13,14]. In humans MAIT cells express effector memory phenotype, usually CD45RA−CD45RO+CD95HiCD62LLoCD44Hi for rapid responses to pathogens. MAIT cells exhibit a distinct transcriptional profile, featured by the expression of Promyelocytic Leukemia Zinc Finger (PLZF), Retinoic acid-related Orphan Receptor (ROR)γt. They also express classical effectors T cells T-box transcription factors T-bet and Eomesodermin (Eomes) [14,15].
Tissue distribution
MAIT cells are distributed in two forms, circulatory and stagnant. MAIT cells are mainly found in mucosal tissues and lamina propria, at the boundary with environment and circulatory MAIT cells circulate in blood and lymph [16]. These cells bear distinctiveness of the innate immune system and also develop specific adaptive immune system properties like semi-variant T-Cell Receptor (TCR) expression. In humans, MAIT cells represent an abundant T cell subset with a tissue distribution of 1-35% of total T cells.
Abundant MAIT cells are found in the peripheral blood (1-8%) and liver (20-50% of total T cells [17]. MAIT cells are rarely found in lymphoid tissues due to a lack of CCR7 and CD62L expression which is required for lymph node homing [18]. MAIT cells in female genital tract constitute about 1-2% by predominantly secreting IL-17 and IL-22 on activation. MAIT cells in the placenta are about 3.9% of the total CD3+ population of T cells and have a naive phenotype [19]. Oral mucosa MAIT cell population range from 0.7 to 7% with the production of IL-17, in lung (3%), gastric mucosa (0.1-12%), adipose tissue (2%), spleen 1%, ileum 2%, colon 2-7% (1). In the kidney, prostate, and ovaries MAIT cell TCR transcripts had been reported [20].
Development of MAIT cells
T and B cells develop from a common lymphoid progenitor in the bone marrow cells of which, cells committed to T cell lineage leave the bone marrow and enter the thymus.
Intrathymic development
MAIT cells exhibit minimum intrathymic proliferation and naïve phenotype in both thymus and cord blood. MAIT cells in the thymus are selected/activated by MR1-expressing Double Positive (DP) thymocytes (CD8+CD4+ cells). This function is affected by some mutations in the groove (of where or which molecule) where the ligands bind. Hui-Fern et al., reported that the CD8+ MAIT cells in human thymus expressing the CD8αβ heterodimer irrespectively whether at stage 1, stage 2 or stage 3 of their development whereas a large proportion of blood MAIT cells were CD8αα+. Stages: Studies in humans, reported that there are three different stages in the intrathymic MAIT cell formation. Stage 1 MAIT cells could be defined as CD27- CD161- and Promyelocytic eukemia Zinc Finger (PLZF) is not expressed. T-cell receptor TCR of MAIT cells and MR1 of DP thymocytes interaction is required for MAIT cell positive selection. This TCR-MR1 interaction produces downstream (Signaling Lymphocytic Activation Molecule) SLAM-(SLAM-Associated Protein) SAP signaling that activates transcription factor Erg2. The expression of specific transcription factors drives these cells to stage 2 (CD27+CD161-) MAIT cells, and also involves microRNA. PLZF, a transcription factor that is encoded by Zbtb16 is a direct target of Erg2. PLZF expression is essential for stage 2 to stage 3 (CD27lo/+CD161+) maturation and gaining effector function. RORγt and T-bet are coexpressed in stage 3 mature MAIT cells. IL-18R expression in stage 3 coincides with the expression of CD161. MAIT cells exit thymus as naïve phenotype. Self-reactive MAIT thymocytes undergo negative selection and peripheral MAIT cell activation is controlled by mechanisms such as dampened TCR signaling.
Microrna role in the development of MAIT cells
MicroRNAs were short 18-22 nucleotide long non-coding RNAs that influence gene expression at the post-transcriptional level by destabilizing mRNA targets and/or inhibiting translation. Koayet al., concluded that the micro RNAs were essential for the development of MAIT cells beyond stage 1 and conditional deletion of Dorsha in T cell results in a lack of most mature miRNA which leads to a severe reduction in MAIT cells population due to block at stage 1. Winter et al., demonstrated that miR-181a/b-1 was required to drive the development of MAIT cells in the thymus. MiR-181a/b-1 controls the earliest defined stage of MAIT cell development and profound block in proliferation both within the thymus and in the periphery as well. miR-181a/b-1 is required for the differentiation of MAIT cell effectors subsets in the lymph nodes and peripheral tissue.
Extrathymic development
MAIT cells can exit the thymus as stage 2 cells, prior to further maturation to stage 3 cells in the periphery. Stage 3 MAIT cells from the human thymus have a limited capacity to produce cytokines compared to stage 3 MAIT cells from human blood, suggesting that they further undergo maturation in the periphery. It is currently unclear what factors drive extrathymic development of MAIT cells, whether it is direct exposure to microbial Ags or B cell interaction or other environmental signals such as IL-18 and/or other cytokines.
Activation and function
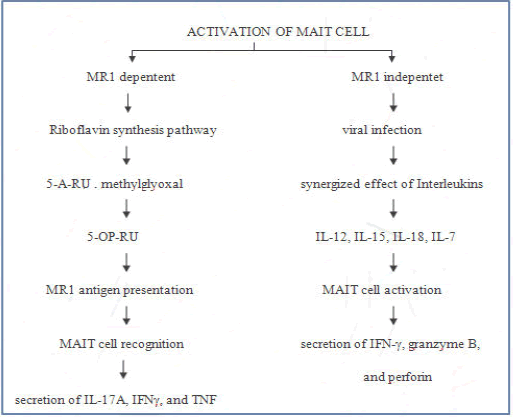
Several research articles have illuminated how MAIT cells are activated in response to their antigen in MR1 dependent manner and MR1 independent manner (Figure 1).
MR1-dependent manner
One of the essential metabolic pathways for many bacteria and yeasts is highly conserved biosynthetic riboflavin synthesis pathway. In riboflavin synthesis, in some microorganisms, RibD, is a gene that encodes for pyrimidine deaminase/reductase that forms 5-amino-6- Dribitylaminouracil (5-A-RU) which is a precursor of 5-OP-RU and 5-OE-RU generated by non–enzymatic condensation with methylglyoxal or glyoxal acts as a ligand for activation of T-cells. MR1 is a non-polymorphic, β2-microglobulin-associated antigen presenting molecule and they present in the endoplasmic recticulum of all cells in ligand receptive conformation without β2 microgobulin. The riboflavin metabolites are transported through ER and binds with MR1 with Schiff base formation and association of β2-microglobulin and move towards the cell surface to present the ligand to MAIT cell for activation. Gram-positive and gram-negative riboflavin metabolites are presented by MR1, differ in their grouped in a highly regulated operon and dispersed in the genome respectively. These riboflavin metabolites are presented by MR1, recognized by MAIT cells invariant T-Cell Receptor (iTCR).
In the presence of ligand, MAIT cells can lyse MR1 expressing cells with the secretion of IL-17A, IFNγ, and TNF. Activated MAIT cells express CD107a, IFN-γ, CD40L, GrB, and PD-1 to varying degrees in an MR1-dependent manner. The bacteria such as Escherichia, Lactobacillus, Staphylococcus, Shigella flexneri, Salmonella, Mycobacteria, and Clostridioides species and fungi equipped with riboflavin synthesis, such as Saccharomyces, Candida, and Aspergillus can activate MAIT cells in an MR1-dependent manner.
MR1 independent manner
Unlike bacteria, viruses are unable to generate riboflavin-derived ligands to activate MAIT cells. Exposure of bacteria or viruses to APCs results in the expression of a wide range of pro-inflammatory cytokines such as IL-12 and IL-18. Combination of at least two cytokines essential for efficient cytokine-dependent activation of MAIT cells, for example, a combination of IL-12 and IL-18 was shown to be a potential inducer of IFN-γ expression by binding to their respective receptors IL-12R and IL-18R, however, either alone does not. In synergy with IL-12 and IL-18, IL-15 specifically activates the unique functions of MAIT cells. IL-15 can activate MAIT cell production of IFN-γ, granzyme B, and perforin, but only in the presence of IL-18 producing monocytes in response to I L-15.
Similarly, IL-15 can act in synergy with either IL-12 or IL-18 to drive IFN-γ production by MAIT cells. Secretion of IFN-γ by MAIT cells is in response to IFN-α/β in combination with IL-12 and IL-18. IL-1β and IL-7 can preferentially promote IL-17A production by MAIT cells. IL-7 (in conjunction with TCR signals) also strongly enhances the cytotoxic effectors function of MAIT cells, including expression of granzyme A, granzyme B, perforin and the degranulation marker CD107a. TLR8 and TLR3 induce MAIT cell activation via IL-18and IL-12, which have an antibacterial and antiviral response. Prolonged Hepatitis C virus infection they observed up regulation of granzyme B. In response to DENV MAIT cells show high expression of CD38 and production of TNF-α. Furthermore, it is reported that MAIT cells show up regulation of CD69 and INF-γ in response to the influenza virus.
MAIT cells have been reported to be involved in several chronic inflammatory. MAIT cells are bearing tissue homing properties and produce inflammatory cytokines, suggested that MAIT cells involved in inflammatory diseases. Deleterious role of MAIT cells in tissue inflammation and destruction are revealed by many reports, few recent reports suggest a protective role of MAIT cells in inflammation (Table 1).
| Disease | Role of MAIT cell | A MAIT cell phenotypes | Effect of MAIT cell activation | Significance |
|---|---|---|---|---|
| Asthma | Glucose homeostasis impairment | CD161high Vα7.2+ T cells. | Ki67+, CD56+, IL-4+ and GzB+ PD1+ MAIT cell Frequency positively correlated with blood HbA1c level. | MAIT cells as a biomarker of disease progression |
| Asthma | Asthma severity | MAIT-17/IL-4R | MAIT cells, which secrete IL-17 and IFN-γ; both of which are considered mediators of steroid-resistant asthma. Decrease in MAIT cell frequency uponrepeated exposure to allergens. | MAIT cell deficiency may lead to increased inflammation in response to allergens |
| Cancer | Tumor proliferation | CD3+, TCRγδ-,CD161+,Vα7.2+ | Tumor infiltrating MAIT cells shows decreased INF- γ and TNF-α production and increased production of IL-17 induced tumor proliferation. | MAITs infiltrate tumor, tumor impairs MAIT functionality |
| Liver disease | Anti-inflammatory macrophage polarization, hepatic stellate cell activation | CD69, CD38, HLA-DR, PD-1 | secreting inflammatory cytokines and produce regulatory cytokines, indicate the disease progression. | attractive biomarkers and therapeutic targets for liver disease |
| COVID-19 | Pulmonary fibrosis, toxic shock syndrome | CD4+, CD 25, CD45RA+, CD62L+,CD45RA, CD62L−, CXCR3- | IL-18, TNF-α, INF-γ were up regulated by MAIT cell may potentially eliminate virus and other pathogens. Also IL-17A may act as profibrinogenic factor causing pulmonary fibrosis. | MAIT cell can boost antiviral immunity and on contrary induce cytokine stroms |
| Obesity | Metabolic dysfunction | CD45+CD19-CD11b-TCRγδ−TCRαβ+TetMR1+ | Ileum MAIT cells produce iIL-17 A and TNF-α and overexpression of IL-18R and CCR6 by Th1 cells causing chronic inflammation. | MAIT cell express pro apoptotic gene and down regulated anti apoptotic BCL–2 gene which favours local inflammation in VAT and Ileum. |
| Cardiovascular diseases | Heart dysfunction | CD26/DPP IV | Decreased MAIT cell frequency is inversely propotional to increased level of IP–10, Pro BNP which results in heart failure. | Diagnostic marker for cardiovascular disease. |
Table 1: Protective role of MAIT cells in inflammation.
Discussion
MAIT cells in diabetes
Diabetes mellitus is a group of metabolic disorders characterized by hyperglycemia resulting from defects in insulin secretion or efficacy. This can be divided into two broad etiopathogenetic categories: Type 1 diabetes mellitus and type 2 diabetes mellitus.
Type 1 diabetes: Type 1 Diabetes (T1D) is an autoimmune disease characterized by the selective destruction of pancreatic islet β-cells that secrete insulin, with the activation of both the innate and adaptive immune systems. Innate immune cells are involved at various stages of the disease, which initiate a local immune response in the pancreas. When most of the β-cells are ruined, the resulting lack of insulin fallout in hyperglycemic conditions and requires enduring insulin-replacement therapy. With the recent onset of T1D, diabetic patients unlike the control subject show a lower frequency of CD8+MAIT cells and CD4- CD8-DN MAIT cells in the blood. This reflects their migration to inflamed tissues and/or their death by apoptosis subsequent to their activation. Phenotypic analysis showed a lower frequency of MAIT cells expressing tissue-recruitment or adhesion molecule (CCR6 and CD56) at the onset of disease in relation to the frequency of such cells later in the disease. In addition to these data, they also observed a higher frequency of MAIT cells expressing activation and/or exhaustion markers CD25 and PD-1, and a lower frequency of MAIT cells expressing the anti-apoptotic molecule BCL-2. With recent-onset T1D, MAIT cells produced fewer IFN-γ, whereas their production of TNF, IL-4, and GzBwas higher than that of MAIT cells from their respective control. At the onset of disease production of GzB by MAIT cells was inversely correlated with the concentration of glycated Hemoglobin (HbA1c) and suggested that the MAIT cells might participate in β-cell death. In human β-cell line EndoC-βH1, it was observed that IL-1β, IFN-γ, and TNF produced; in inflamed β-cell line during the progression of T1D induces up regulation of MR1 expression and MR1-dependent apoptosis of EndoC-βH1. All of these suggested that MAIT cells participate in β-cell destruction.
The development of T1D is also associated with changes in the gut micro-biome can cause alteration in the circulating MAIT cells. Children diagnosed with T1D show an increased proportion of CD8- CD27-(DN) MAIT cells with increased expression of homing receptors CCR5 and β7 integrin but lesser production of IFN-γ by MAIT cells. The frequency of MAIT cells was decreased in children with auto antibodies who progressed to T1D as well was no change in the frequency of MAIT cells in children with auto antibodies does not progress toT1D. Moreover, sex, HLA class II genotype, BMI or level of dysglycaemia does not affect MAIT cell frequencies in T1D patients. There is a decrease in MAIT cell frequencies in other patients with Inflammatory Bowel Disease (IBD) and Systemic Lupus Erythematosus (SLE) and no alteration in MAIT cell frequency in other autoimmune diseases (multiple sclerosis and rheumatoid arthritis) shows that alterations in blood MAIT cells may be less prominent in organ-specific autoimmune diseases.
The frequencies of other circulating unconventional T subtypes, namely γδ T cells and iNKT cells, were not altered either in children with newly diagnosed type 1 diabetes or AAb+ at-risk children (49→5). Extrinsic factors like 25(OH) vitamin D influence the MAIT cell frequency. The lowest levels of 25(OH) vitamin D with a higher frequency of MAIT cells and a higher level of vitamin D binding protein with a higher frequency of MAIT cells. A∼7-fold increase in VDR transcripts among stimulated MAIT cells compared to unstimulated shows it can interfere with the proliferation of MAIT cells with a higher level of vitamin D.
Type 2 diabetes: T2D is the most common type of diabetes characterized by hyperglycemic conditions due to insulin resistance. The major cause is obesity. Obesity is associated with dysbiosis, an altered composition and functional properties of gut microbiota which play a major role in the regulation of body weight and inflammatory status. One major outcome of this is increased intestinal permeability, involving reduced expression of epithelial tight junction proteins such as zonulaoccudens 1 and occudin. Intestinal permeability increases the leakage of bacteria and bacterial products across the intestinal barrier, thus alternating the MAIT cell numbers. The frequency of circulating MAIT cells was negatively associated with disease progression and BMI. This reduction was more significant in obese adults with uncontrolled HbA1c and insulin resistance. Decreased frequency accompanied by activation markers CD69 and CD25 suggests abnormal activation of MAIT cells. EirinCrolanet al., reported that in obese people, proportions of circulating MAIT cells were significantly higher in adipose tissue than in circulation. Both omental and subcutaneous adipose tissue of obese showed a reduction in the proportion of MAIT cells producing INF-γ, and IL-10, with a high frequency of MAIT cells producing IL-17. IL-17 plays a major role in obesity associated insulin resistance which causes diabetes mellitus. Local chronic activation of the MAIT cell population takes place which is characterized by increased proliferation of MAIT cells and decreased expression of Bcl-2, which acts as an apoptosis regulator. On activation MAIT cells display a pro-inflammatory Th17 phenotype, producing increased levels of IL-2, GrB, IL-17, IFN-É£, and TNF-α in T2D patients and only IL-17 in obese patients, compared to healthy people. In the pancreas, MAIT cells correlated with the diseases progress. They could produce granzyme B and IFN-γ, which directly killed β-cells and aggravated the diseases.
MAIT cells in asthma
Asthma is a chronic airway inflammation leading to reversible airflow obstruction (hyper mucus secretion) in association with hyperresponsiveness and tissue remodeling of the lower respiratory tract. Most asthma starts from childhood in relation to sensitization of the respiratory tract on inhalation of allergens. Various cells are involved in the pathophysiology of asthma. Inhaled allergens stimulate T helper type 2 (Th2) cells that subsequently secrete IL-4, IL-5, and IL-13. Recent studies show that Th17 cells also modulate the airways with the production of IL-17A, IL-17F, and IL-22. These cytokines induce airway inflammation and IL-17A enhances smooth muscle contractility. Though, Th17 cells are deemed to be the primary source of IL-17 in asthma. Other cells such as MAIT cells also produce IL-17.
Lezmi G et al., studied whether circulating MAIT cells are also a source of IL-17 in asthma and whether the number in circulation is correlated with the degree of symptoms. They concluded that increased MAIT cells are found and they had a potential role in exacerbators than the non-exacerbators. Bacterial and viral infections trigger exacerbations, which are major drivers of morbidity, mortality, and health care use in chronic obstructive pulmonary disease. Several studies have reported the role of MAIT cells in patients with Chronic Obstructive Pulmonary Disease (COPD) whose number decrease in the airways of patients being administered Inhaled Corticosteroids (ICS) compared with healthy participants. Furthermore, the study elucidated that the Haemophilus influenza (Hi) infection-induced surface up regulation of MR1 on human pulmonary macrophages, resulted in activation and secretion of INF-γ by MAIT cells. Administration ICS significantly decreased the Hi-induced INF-γ production. However, no significant difference in MAIT cell numbers in sputum or BAL was reported. ICS suppressed the local expansion of pulmonary MAIT cells, induces apoptosis due to their high expression of caspase 3, and inhibits the transcription factors involved in the expression of INF-γ. MAIT cells could be asthma protective in many children and could reflect immune programming or imprinting phenomena encouraging Th1 response thus conferring protection from the disease. AyakoIshimori et al., showed that MAIT cells are positively associated with other cells like CD69+-activated NK cells, ILC1s, ILC2s, and ILC3s and orchestrate immune response but not behave as an individual population in asthmatic airways.
MAIT cells in cancer
The first study of MAIT cell role in cancer was reported by Peterfalvi et al., in kidney and brain tumors. They reported that MAIT cells infiltrate tumors and MR1 presented by tumors activates the MAIT cells locally. Shaler et al., found increased tumor infiltration in the hepatic metastases of (Colorectal cancer) CRC. Colorectal Liver Metastasis (CRLM) penetrating MAIT cells exhibit functional impairment such as hampered ability to produce INF-γ, failure to up regulate GzB, and a negligible amount of IL-17 which are dictated by the tumor microenvironment. MAIT cells express NKG2D, which is known to facilitate anticancer immune surveillance. Therefore they provide an attractive therapeutic target in CRC and CRLM. MAIT cells are found in epithelial ducts of human breast tissue and mediate effector response to breast carcinoma cells that are exposed to microbial compounds by secretion of IL-17. MAIT cells in tumors are activated in an MR1-dependent manner and byNKG2D mediated costimulation. It is hypothesized that the NKG2D pathway promotes MAIT cells to respond to breast carcinoma and IL-17 hinders the initial stages of epithelial carcinogenesis. Won et al., compared MAIT cell levels between Mucosal Associated Cancer (MACs) and non-MACs. It showed a significant decline in MAIT cell levels in MACs than in non- MACs. MAIT cells infiltrating MAC cells express high IFN-γ, IL-17, and TNF-α which are antagonists to the cancer cells. MAIT cell numbers in peripheral blood have decreased due to the expression of CCL20 and CXCL16 tumor homing chemokine and are correlated with the prognosis of cancer tissue. Collectively, these findings suggest that activated MAIT cells have Lymphokine Activated Killer (LAK) activity and direct cytotoxicity on cancer cells. Chronic Lymphocytic Leukemia (CLL) is a malignancy of mature B lymphocytes, characterized by clonal malignant B lymphocytes in peripheral blood. As stated before MAIT cells require B cells and commensal bacteria for their extrathymic development. It has been observed deficiencies of MAIT cells in patients with CLL which may lead to bacterial lung infections. The exact role of MAIT cells in CLL is far to be established. It has been indicated that there was a significantly lower frequency of circulating MAIT cells but a higher number of infiltration in tumor tissue. This is due to the persistent expression of CCR6 and CXCR6 that help migrate to tumor tissue. Unaffected Colon tissue MAIT cells produced IFN-γ and fewer IL-17. Whereas in tumors, significantly lower frequencies of IFN-γ producing cells and a two-fold increase in MAIT cells producing IL-17. These functionally activated MAIT cells induce human CRC cell cycle arrest in an MR1-dependent manner in in vitro. All these studies suggest that MAIT cells could represent attractive therapeutic targets; however, their precise role in cancer needs further investigation.
The immunosuppression in the Tumor Micro Environment (TME) is due to the altered levels of metabolites, cytokines, nutrients, and oxygen. Since the cancer cells are highly glycolytic that results in the limited availability of glucose that can impact the effector functions of infiltrating immune cells. MAIT cells depend on glycolysis for their production of IFN-γ, demonstrating reduced production under acute glucose restriction. The MAIT17 cells can promote pro-tumor by the production of INF-17 that leads to the depletion of NK cells, but a recent study shows the in vivo expansion of MAIT cells can promote the NK-mediated anti-tumor effect through 5-OP-RU mediated effect that leads to the activation and proliferation of macrophages and that contributes to the anti-tumor effect through chemokine mediated recruitment of NK cells in anti-tumor activity. The activated CD1d restricted NKT cells also promote anti-tumor immunity by the activation of NK cells in an IFN-γ-dependent manner. The RNA sequence based-experiment showed that the production of IFN-γ by MAIT cells leads to the activation of genes responsible for the activation of IFN-γ producing genes in the NK cells and mediates antitumor activity through IFN-γ. In cancer MAIT cell shows alterations in the frequencies in the periphery to altered cytokine levels this aids to target MAIT cells in cancer therapy by immune checkpoints Programmed cell Death Protein 1 (PD-1) and CTLA-4 can inhibit T cell response by binding with their ligands and another immune checkpoint in therapeutic cancer therapy is T cell immunoglobulin and Mucin domain-containing protein 3 (TIM-3) expressed on dysfunctional and exhausted T cells to limit the production of IFN-γ by conventional T cells.
MAIT cells in liver disease
The liver is an important immune organ where innate and adaptive immune cells are present in the hepatic sinusoid that plays a major role in the elimination of pathogens that entered the liver through the portal veins and are eliminated from circulation. One of the most abundant innate-like cells in the liver is MAIT cells which constitute up to 10 to 50% of body T cells. TCR-stimulated liver-derived MAIT cells play a potential role in liver homeostasis and progressive regeneration of liver cells. Liver MAIT cells secrete large amounts of pro-inflammatory and fibrogenic cytokines, including IFN-γ, TNF-α, and IL-17. Hence the abundance of MAIT cells in the liver and the cytotoxic effect show the prominent role of MAIT cells in liver inflammations. Wanget al., explored that alcoholic hepatitis patients were activated with high levels of activation markers CD69, CD38, and HLA-DR in MAIT cells. MAIT cells can express higher levels of exhaustion marker PD-1, not observing an increase in apoptotic of MAIT cells and MAIT cell abnormalities can be partially reversed in long-term alcohol abstinence. Severe alcoholic hepatitis shows a reduction in the MAIT cell frequency.
Lately, Li Y, et al., indicated that MAIT cells may have a positive effect on NAFLD patients by producing regulatory cytokines including IL-4 and IL-10, resulting in anti-inflammatory macrophage polarization.
In autoimmune liver disease, the MAIT cells tended to decrease with increasing fibrosis stage associated with long-term exposure to the cytokines, and bacterial infections down-regulated the T-bet and EOMEs, resulting in cell exhaustion. Meanwhile, activated MAIT cells could secrete IL-17A, which induced Hepatic Satellite Cell (HSC) proliferation, and the granzyme B expression by MAIT cells correlated with fibrosis. Peripheral MAIT cells significantly reduced and produced less granzyme B and IFN-γ, which correlated with the levels of CD69 expression in viral hepatitis. The high expression of PD-1 on MAIT cells could predict plasma HBV-DNA levels. After anti-viral therapy, the MAIT cells in the liver have lesser activation status, cytotoxic effect, and expressed a high level of degranulation marker CD107a though the ability to respond to E. coli stimulation in chronic HCV infection is decreased. The up regulation of the cancer-promoting FOXM1 gene by Cannabinoid Receptor1 (CB1R) via a Gi/o/CAMP/ CREBP pathway causes the occurrence and development of cancer in the liver. Duanet al., found that tumor-infiltrating MAIT cells show unfavorable outcomes in HCC patients who expressed higher levels of immune checkpoints PD-1 cause immune exhaustion rather than apoptosis. Tumor-derived MAIT cells secreted fewer IFN-γ, IL-17, granzyme B, and perforin, but they secreted more IL-8, which is of great importance in promoting tumor angiogenesis and progression. In patients with decompensated cirrhosis, the peritoneal MAIT cells have moved ascites which can be proven by the expression of the homing chemokine, CXCR3, by the peritoneal MAIT cells and the presence of high levels of CXCL10 in the ascites and may have a protective function in uninfected ascites and a critical antimicrobial function in infected ascites.
MAIT cells in COVID-19
COVID-19 is a respiratory disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). That can activate both conventional and unconventional T cell subsets like MAIT cells, γδ T cells, and iNKT cells. MAIT cells were activated in a receptorindependent manner by IL-18 and IFN-α, increased levels of IL-18 cause CD69 expression on MAIT cells. IL-17 is a pro-inflammatory cytokine produced in the liver, skin, and colon epithelial cells involved in host defense and lung inflammation in SARS-CoV-2 infection. The homing receptor Chemokine C-X-C motif Receptor 3 (CXCR3) was normally expressed in lung homing but after the infection of SARS-CoV-2 shows increased expression of the early activation marker CD69 on MAIT cells and diminished expression of the homing receptor chemokine CXCR3. CD69 up regulation and CXCR3 down regulation patterns were more pronounced in MAIT cells compared to conventional CD4+ and CD8+ T cells, and CD69 expression was inversely linked with CXCR3 expression in MAIT cells. This circulating CD69+CXCR3- MAIT cell phenotype was reproduced in the airways where MAIT cells were also shown to be the main source of IL-17A. In COVID-19 patients MAIT cell frequency was higher than conventional T-cell subsets or other unconventional subsets. CD69 high phenotype MAIT cell activation was associated with CXCL10 and CX3CL1. The CXCR3 low phenotype was stable in mucosal scRNA seq. CD69 high CXCR3 low MAIT cell phenotype was associated with poor clinical outcomes among COVID-19 patients. In airways cRNAseq the MAIT cell displayed IL-17A biased profile with a pro-inflammatory response and less interferon expression. The reduction in MAIT cells can be recovered in convalescent COVID patients. However, the CXCR3 expression remains suppressed which can cause long-term consequences for immune defense against microbial disease.
MAIT cells in obesity
The inflammation in Visceral Adipose Tissue (VAT) caused by the accumulation of pro-inflammatory immune cells that include M1 macrophages, CD8+ T cells, Th17 CD4+ T cells, NK cells, and neutrophils in obesity and the reduction in the frequency of antiinflammatory immune cells such as M2 macrophages, Foxp3+ regulatory T cells (Treg), eosinophils, and type 2 Innate Lymphoid Cells (ILC2) which provides production against the local VAT inflammation. In obesity the change in the gut microbiota induce MAIT cells by recognizing MR1 which presents antigen from certain bacteria hence it cause MAIT cells to involve in the production of Transcription factor (T-bet), Chemokine/Cytokine Receptors (CCR6, IL-18R), and cytokines (TNFα and IL-17) which may cause inflammation in VAT. The production of IL-17 by the recognition of TCR-MR1 interaction, whereas production of TNFα production was MR1 independent. MAIT cells are less than 0.4% of T cells in obese patients than in normal people about 6% of total T cells. MAIT cells over expressed pro-apoptotic genes (Bax, cMyc, and Casp9) and down regulated the anti-apoptotic Bcl-2/BCL-2 gene and protein in Epi-AT and ileum of obese mice compared with lean controls shows that chronic inflammation in VAT cause apoptosis in MAIT cells.
MAIT cells in cardiovascular disease
In obesity and T2D there is a significant loss in the MAIT cell frequency compared to the healthy individual and also their frequency were decreased in later stages of disease progression CAD and CADCHF. The decreased MAIT cell frequency is correlated with heart dysfunction and increased level of pro-BNP as diagnostic marker for cardiac failure. MAIT cell frequency is inversely proportional to the level of Hb1A1c marker for the blood glucose level, increased Hb1A1c with lower MAIT cell frequency shows that MAIT cells are sensitive to the effect of long lasting high plasma glucose level. At high blood glucose level the MAIT cells shows higher proapoptotic propensity that is dependent on the transcription factor PLZF protein. Pro-inflammatory reaction and secretion of inflammatory molecules with deleterious metabolic consequences may contribute to enhance the proapoptotic signaling in MAIT cells; hence the frequency of MAIT cell is inversely propotional to the level of inflammatory cytokine IP-10. The MAIT cells express high levels of CD26/DPP IV which can cleave the chemokine. Thus high level of IP-10 shows the loss of MAIT cells in cardio metabolic diseases. Also CAD groups with elevated GGT and AST with altered liver function also have altered MAIT cell frequency which is associated with heart diseases.
Conclusion
MAIT cells are typically defined by their expression of an invariant T Cell Receptor (TCR) α-chain which recognizes vitamin B (Riboflavin), presented by the MHC class-I Related (MR1) protein. MAIT cell localization in the gut mucosa and their recognition of bacterial ligands might play a key role in sensing any modification of the microbiota composition, either commensal or pathogenic, which might reflect the infectious, metabolic, and general health status of the individual. In chronic pathological situations, including chronic viral infections and autoimmune, inflammatory, and metabolic diseases, MAIT cells can play a pathogenic role by sustaining inflammation and cytotoxicity. In the case of chronic diseases such as diabetes and cancer, it has both protective and pathogenic roles. Further studies should be performed to provide a better understanding of the functional plasticity of MAIT cells, the epigenetic regulation of MAIT cell function, the impact of their TCR repertoire, and their interactions with other immune and non-immune cells in physiological and pathological situations which may lead us to new therapeutic interventions.
Acknowledgements
The authors greatly acknowledge DST-FIST to Department of Biomedical Science, DST-RUSA 2.0 Biological Sciences, and DST-PURSE-2, to Bharathidasan University and DBT-BT/PR 12896/AGR/ 36/631, DBT-BT/PR13260/BRB/10/754, DST-SR/SO/ HS-6/2009, and DST-SERB/F/4461/2013-14 India (to SN).
Conflict of Interest
The authors have declared that no conflict of interest exists.
Informed Consent
Not applicable.
Ethical Approval
Approved by the Institutional Animal Ethical Committee of Bharathidasan University.
References
- Toubal A, Nel I, Lotersztajn S, Lehuen A (2019) Mucosal-associated invariant T cells and disease. Nat Rev Immunol 19:643-657.
[Crossref] [Google Scholar] [PubMed]
- Kumar BV, Connors TJ, Farber DL (2018) Human T cell development, localization, and function throughout life. Immunity 48:202-213.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Kong D, Wang H (2020) Mucosal-Associated Invariant T cell in liver diseases. Int J Biol Sci 16:460-470.
[Crossref] [Google Scholar] [PubMed]
- Gazali AM, Schroderus AM, Nanto Salonen K, Rintamaki R, Pihlajamaki J, et al. (2020) Mucosal-associated invariant T cell alterations during the development of human type 1 diabetes. Diabetologia 63:2396-2409.
- Wen X, Zhang X, Nian S, Wei G, Guo X, et al. (2021) Title of article: Mucosal-associated invariant T cells in lung diseases. Int Immunopharmacol 94:107485.
[Crossref] [Google Scholar] [PubMed]
- Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, et al. (2015) In vitro and in vivo analysis of the gram-negative bacteri derived riboflavin precursor derivatives activating mouse MAIT cells. J Immun 194:4641-4649.
[Crossref] [Google Scholar] [PubMed]
- Hinks TSC, Zhang X-W (2020) MAIT Cell Activation and Functions. Front Immunol 11:1014.
[Crossref] [Google Scholar] [PubMed]
- Dias J, Boulouis C, Gorin JB, van den Biggelaar RH, Lal KG, et al. (2018) The CD4- CD8- MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc Natl Acad Sci 115:E11513-E11522.
[Crossref] [Google Scholar] [PubMed]
- Garner LC, Klenerman P, Provine NM (2018) Insights Into Mucosal-Associated Invariant T Cell Biology From Studies of Invariant Natural Killer T Cells. Front Immunol 28;9:1478.
[Crossref] [Google Scholar] [PubMed]
- Gibbs A, Leeansyah E, Introini A, Paquin-Proulx D, Hasselrot K, et al. (2017) MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol 10:35-45.
[Crossref] [Google Scholar] [PubMed]
- Lee OJ, Cho YN, Kee SJ, Kim MJ, Jin HM, et al. (2014) Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol 49:47-54.
[Crossref] [Google Scholar] [PubMed]
- Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, et al. (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood, J Am Soc Hematol 117:1250-9.
[Crossref] [Google Scholar] [PubMed]
- Kurioka A, Jahun AS, Hannaway RF, Walker LJ, Fergusson JR, et al. (2017) Shared and distinct phenotypes and functions of human CD161++ Vα7.2+ T cell subsets. Front Immunol 8:281674.
[Crossref] [Google Scholar] [PubMed]
- de Lima Moreira M, Tsuji M, Corbett AJ, Araujo MS, Teixeira-Carvalho A, et al. (2017) MAIT-cells: A tailor-made mate in the ancient battle against infectious diseases?. Immunol Lett 187:53-60.
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, et al. (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. 422:164-169.
[Crossref] [Google Scholar] [PubMed]
- Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, et al. (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11:701-708.
[Crossref] [Google Scholar] [PubMed]
- Kurioka A, Walker LJ, Klenerman P, Willberg CB (2016) MAIT cells: New guardians of the liver. Clin Transl Immunology 5:e98.
[Crossref] [Google Scholar] [PubMed]
- Solders M, Gorchs L, Erkers T, Lundell AC, Nava S, et al. (2017) MAIT cells accumulate in placental intervillous space and display a highly cytotoxic phenotype upon bacterial stimulation. Sci Rep 7:6123.
[Crossref] [Google Scholar] [PubMed]
- Sobkowiak MJ, Davanian H, Heymann R, Gibbs A, Emgard J, et al. (2019) Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur J Immunol 49:133-143.
[Crossref] [Google Scholar] [PubMed]
- Hinks TS, Wallington JC, Williams AP, Djukanovic R, Staples KJ, et al. (2016) Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable Haemophilus influenzae infection. Am J Respir Crit Care Med 194:1208-1218.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi