Research Article, J Soil Sci Plant Health Vol: 3 Issue: 1
Phytotoxicity of Bismuth Nitrate and Bismuth Citrate on Germination and Growth of Lolium Perenne Exposed on Filter Paper and on Artificially Contaminated Soil
Zohra Omouri1,3*, Jalal Hawari2,3, Michel Fournier1 and Pierre Yves Robidoux1
1INRS-Institut Armand Frappier, 531 boulevard des Prairies, Laval, Quebec, H7V 1B7, Canada
2Ecole Polytechnique de Montreal, Departement des genies civil, Geologique et des mines, 2900 boul, Edouard-Montpetit, Montreal, Quebec, H3T 1J4, Canada
3National Research Council of Canada, 6100 Avenue Royalmount, Montreal, Quebec, H4P 2R2, Canada
*Corresponding Author : Zohra Omouri
INRS-Institut Armand Frappier, 531 boulevard des Prairies, Laval, Quebec, H7V 1B7, Canada
Tel: 1-514-627-2417
E-mail: zohra.omouri@hotmail.com
Received: February 25, 2019 Accepted: April 15, 2019 Published: April 24, 2019
Citation: Omouri Z, Hawari J, Fournier M, Robidoux PY (2019) Phytotoxicity of Bismuth Nitrate and Bismuth Citrate on Germination and Growth of Lolium Perenne Exposed on Filter Paper and on Artificially Contaminated Soil. J Soil Sci Plant Health 3:1.
Abstract
Phytotoxicity of Bi citrate and Bi nitrate on perennial ryegrass were evaluated using standardized filter paper and soil tests. Endpoints included seeds germination, and root and shoot growth. The results showed that Bi nitrate seemed to be more toxic than Bi citrate, and root growth more sensitive than seeds germination and shoot growth. Filter paper test indicated that Bi nitrate significantly decreased root elongation (IC50=60.34 mg/L) at tested concentrations ≥ 30.35 mg/L
and seedling germination at 485 mg/L. Bi citrate decreased significantly root elongation (IC50=139.5 mg/L) and seedling germination at concentration ≥ 99.52 mg/L and at 398.08 mg/L, respectively. Data from OECD artificial soil spiked with Bi nitrate indicated a significant reduction on root mass at 485 mg/kg soil but no significant effect was observed on shoot mass and germination at concentration ≤ 485 mg/kg soil. In natural soil, Bi nitrate reduced significantly root mass and root
elongation at concentration ≥ 4.8 and 48.5 mg/kg soil, respectively. No effect was however observed on seeds germination and shoots mass. Natural soil spiked with Bi citrate showed no significant decrease in seed germination and root growth at concentrations ≤ 398 mg/kg. The toxicity of Bi salts on perennial ryegrass varied with the matrix in the following order: filter paper>natural soil>OECD artificial soil. We assume that Bi phytotoxicity is related to Bi bioavailability which in turn is controlled by solubility of the Bi salt in addition to the physicochemical properties of the tested matrix.
Keywords: Bismuth salts; Soil contamination; Lolium perenne L; Germination; Growth
Introduction
Bismuth (Bi) is a rare metal; it is about twice as abundant as gold in the earth’s crust [1]. According to Bowen [2] average concentrations of Bi in uncontaminated soils and in edible plants grown in uncontaminated soils are 0.2 μg/g and<0.06 μg/g dry soil, respectively. Although, Bi salts have been used for over 300 years, e.g. in the treatment of skin lesions and syphilis [3]. Bi remains one of the least understood and least studied element in the periodic table [4]. Some of the most widely used Bi salts in various industrial and medicinal applications include Bi nitrate and Bi citrate. Many studies reported that Bi nitrate has been used in the treatment of chronic diarrhea and cholera [5] in the anticancer treatment [6] and as a catalyst [7]. On the other hand, Bi citrate was used in the treatment of Helicobacter pylori infection hair dye [8] and cosmetics. Thus, the increased use of Bi based compounds in various industrial applications could lead to an increase in the concentrations of this metal in terrestrial and aquatic ecosystems. Indeed, Amneklev et al. [9] reported an increase of 300% in Bi measured at water treatment plants in Stockholm in 2007 compared to 2006, due to excessive use of Bi salts in cosmetic products. Furthermore, this study showed that the spreading of sewage sludge in agricultural soils in Europe and in the United States constitutes an important source of Bi contamination of soils. In addition, phosphate fertilizers used for soil amendment could be considered as another potential source of Bi contamination of soils [10]. Recently, some studies reported that ashes and dust from the incineration of industrial and hospital waste, could be another entry route for Bi into the environment [11]. According to Zhao et al. [12] in China Bi concentration ranging between 1.1 and 489 mg/kg have been found in ash samples collected in hospital waste incinerators. Xiong [11] reported that atmospheric dust samples from six Chinese cities contained high Bi concentrations (0.9 – 4.6 mg/kg) compared to the background concentration of Bi in soils (0.4 mg/kg). Old metal mining and smelters can also act as a potential source of Bi contamination of soil and water resources [13]. Jung et al. [14] reported an average total Bi concentration of 436 mg/ kg in soil samples from a mining dump sites in Korea and 21 mg/ kg in household garden soils around this area. Despite documented increasing levels of Bi concentrations in soil environments, toxicity and environmental fate of Bi towards plants remains relatively unknown. Lolium perenne L. (L. perenne), commonly called perennial ryegrass is one of the most important forage and turf grasses in the world [15]. It is widely used due to its high quality forage yield, rapid germination, long season production, and ability to adapt too many climatic conditions and to its remarkable resistance to grazing and trampling [16]. Perennial ryegrass is used as a model species in standardized toxicity assessment methods [17,18]. In general, plants can tolerate and bioaccumulate soil contaminants such as heavy metals. However, exposure to high concentrations of metals can cause inhibitory effects on growth and germination of plants such as perennial ryegrass. Some metals such as copper, nickel, manganese, lead, cadmium decrease root growth of ryegrass [19]. Bonnet et al. [20] Showed that zinc affects the detoxification enzyme activity (e.g. ascorbate peroxidase, superoxide dismutase). Chromium decreases the growth of Lolium perenne L. and causes the loss of leaves pigmentation [21]. Currently, despite the wide use of Bi in several industries, little data is available on the effects of Bi based compounds on terrestrial plants exposed to the metal through contaminated water, contaminated soil and soil fertilization. In this study, we investigated the toxic effects of Bi nitrate and Bi citrate on germination, root elongation and growth of the L. perenne plant, using filter paper and contaminated soil exposure tests. We also compared the toxicity data form Bi nitrate and Bi citrate to gain some insights on Bi bioavailability to L. perenne for each tested salt.
Materials and Methods
Chemicals and reagents
Bismuth (III) citrate (C6H5BiO7), Bismuth (III) nitrate pentahydrate (Bi (NO3)3∙5H2O), cadmium chloride hemi (pentahydrate) (CdCl2*2.5H2O) were purchased from Sigma. ASTM Type II water was obtained using a Millipore Super-Q water purification system or Zenopure Mega-90. Glassware and polyethylene containers were washed with acetone, soaked in nitric acid solution (10%, v/v), and rinsed with deionized water.
Soil characterization and samples preparation
The natural sandy soil used in this study was collected from a site located in a non-contaminated area from the Canadian Forces Base in Valcartier (QC, CAN). After collection, soil was passed through a 2 mm sieve to remove rocks, roots, and other large fragments. Preliminary toxicity tests (i.e. plant germination, earthworm lethality test) were carried out to confirm that the background level was not toxic. Physical and chemical characteristics of this soil are 0.7% clay, 2.0% total organic carbon, 97.6% sand, 1.6% silt,5.96 pH, 0.05 mg/kg bismuth,<5 mg/kg arsenic,<0.5 mg/kg Cadmium, 2 mg/kg cobalt, 180 mg/kg magnesium,<0.02 mercury,<5 mg/kg lead and 31 mg/kg Zinc. Soil was spiked by adding bismuth nitrate or bismuth citrate to soil to obtain the selected nominal concentrations. A negative control (soil without Bi nitrate/Bi citrate) was prepared by adding Type II water only to the soil. Spiked soils were mixed for 20 ± 2 h in a rotary mixer to obtain a homogeneous distribution of the metal. The soil was then rehydrated to 60% of its water holding capacity (WHC). The WHC was determined by saturating the soil with Type II water and by measuring the water content as described previously by [22]. Water content was determined by measuring the loss of soil weight after drying for 18 h at 105°C in an oven. After hydration, the soil samples were then mixed overnight in a rotary mixer, and kept at room temperature (20 ± 2°C) for 2 weeks for aging. Soil aliquots were taken at the beginning and at the end of the experiments to determine the moisture content, and pH. The pH of the soil samples was measured using a 1:5 (v:v) soil/water suspension [23]. In addition, an artificial soil was prepared by mixing 70% sand, 20% kaolin clay, and 10% de sphagnum peat as described by OECD (1984). The pH of the mixture was adjusted to 6.5 ± 0.5 by addition of calcium carbonate (CaCO3).
Plant toxicity tests
Filter paper and soil tests were used to assess toxicity of Bi nitrate and Bi citrate on perennial ryegrass plant. Perennial ryegrass seeds used in this study were obtained from Pickseed Canada Inc. (St-Hyacinthe, Quebec, Canada).
Seed germination and root elongation test on filter paper: The effects of Bi nitrate and Bi citrate on seed germination and root elongation of perennial ryegrass were assessed following method described for lettuce root elongation [24]. In the present study we replaced lettuce by Lolium perenne L. Three replicates were carried out for each test concentration and six replicates for the negative control (deionized water without Bi nitrate or Bi citrate). Briefly, five seeds were placed in a glass petri dish (100 × 15 mm) containing a filter paper (Whatman grade 3) impregnated with 4 ml of Bi nitrate or Bi citrate aqueous solution or deionized water for the negative control. Petri dishes were than incubated in the dark at 24 ± 2°C for 5 days. After exposure, the percentage of germination was calculated and the root elongation was measured. Bi nitrate and Bi citrate concentrations were prepared separately using a series of dilutions from a stock solution of 485 and 398.01 mg/L nominal concentration, respectively, in order to use identical Bi concentrations. The cadmium (as CdCl2* 2.5H2O) was used as reference toxicant with concentrations ranging from 1.56 to 110 mg Cd/L.
Germination and growth test in contaminated soil: Toxicity assays in natural and artificial contaminated soil were carried out according to the recommendations of [18] and ASTM (1999) standardized protocols. Soil assays were performed in 3 or 4 triplicate, including controls. Perennial ryegrass seeds were sown in pots containing 200 g of soil. Each pot was placed in a sealed plastic bag to maintain soil moisture, and then incubated in a growth chamber (Conviron Inc., Winnipeg, Manitoba, Canada) at 24 ± 2°C in the dark. After 48 h, the exposure conditions were changed and a photoperiod of 16 h light (5000 ± 500 lux; 25 ± 2°C): 8 h dark (20°C ± 2°C) was used. Seedling emergence, root elongation and wet mass of shoot and root were measured after 7 days of exposure. Artificial soil (OECD) and natural sandy soil were spiked with nominal Bi nitrate concentrations ranging from 15.5 to 485 mg/kg and from 4.8 to 485 mg/kg dry soil, respectively. The Bi citrate was tested on natural sandy soil only with nominal concentrations ranging between 3.98 to 398 mg/kg dry soil. Negative control containing deionized water only was performed for each test experiment.
Data analysis
All data were expressed as the average ± standard deviation (SD). Toxicity endpoints such as inhibitory effect concentration (e.g. IC25 and IC50), were determined using the ToxCalc (Version 5.0; Tidepool Scientific Software, McKinleyville, CA). Program JMP IN (v 4.04, SAS Institute Inc.) was used to run an ANOVA followed by a test multiple comparisons in order to identify treatments with significant differences compared to the control (Dunnett’s; p<0.05).
Results
Filter paper assays
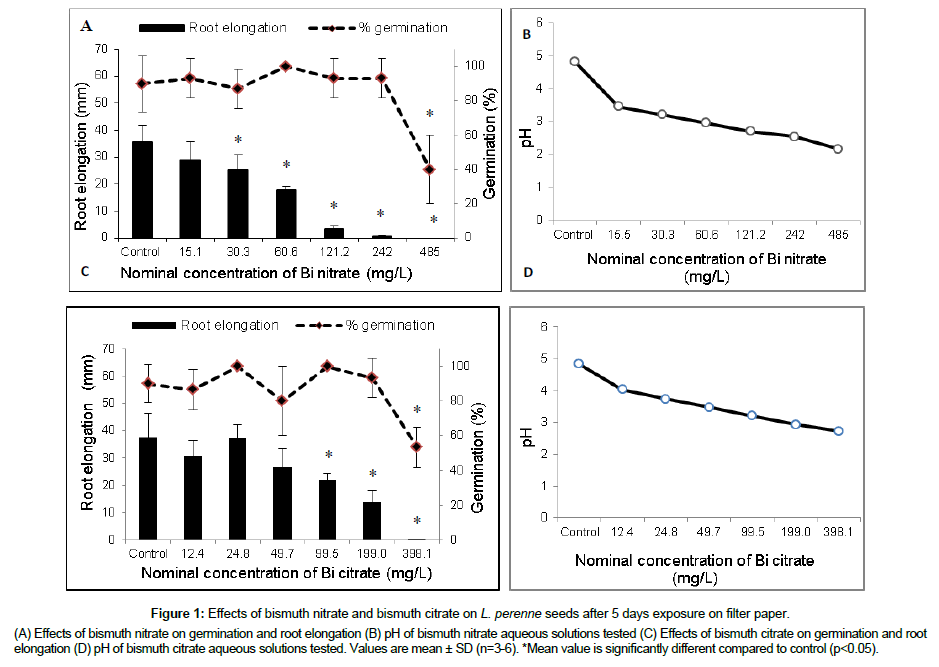
The effects of Bi nitrate and Bi citrate on seed germination and root elongation of the ryegrass after 5 days of exposure on filter paper are shown in Figure 1. Bi nitrate decreased significantly (p<0.05) the root elongation of ryegrass at tested concentrations ≥ 30.3 mg/L and completely inhibited growth at 485 m/L (Figure 1A). However, seed germination is less sensitive to Bi nitrate. The percentage of germination decreased significantly only at the highest concentration tested 485 mg Bi nitrate/L with 40% germination compared to 100% germination in the control. In addition, Figure 1B shows that the pH of Bi nitrate decreased as a function of Bi nitrate concentrations up to reach 2.18 at the highest tested concentration 485 mg Bi nitrate/L. Figure 1C presents the effects of Bi citrate on germination and root elongation of ryegrass. The results showed also that root growth was more sensitive to Bi citrate than seeds germination. The percentage of germination decreased significantly (p<0.05) compared to control at 398.1 mg Bi citrate/L with a 53.3% germination, whereas, root elongation decreased significantly (p<0.05) at tested concentration ≥ 99.52 mg Bi citrate/L. Figure 1D shows the pH decrease of Bi citrate following the increase of Bi citrate concentrations. The pH decrease of Bi citrate was slightly less compared to pH decrease of Bi nitrate.
Figure 1: Effects of bismuth nitrate and bismuth citrate on L. perenne seeds after 5 days exposure on filter paper.
Soil assays
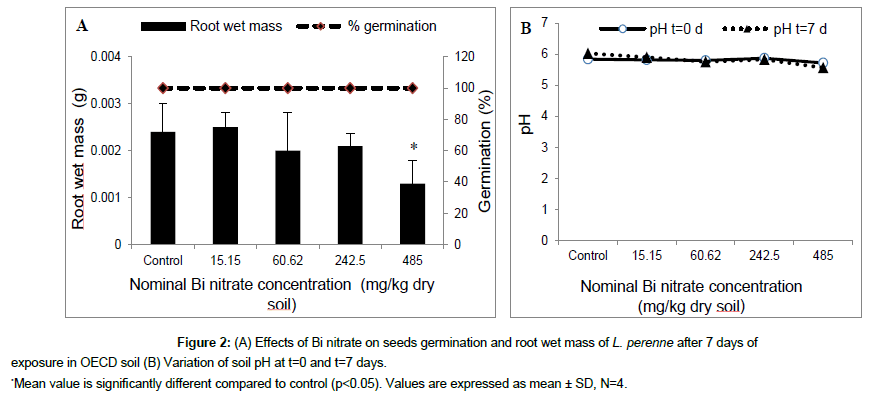
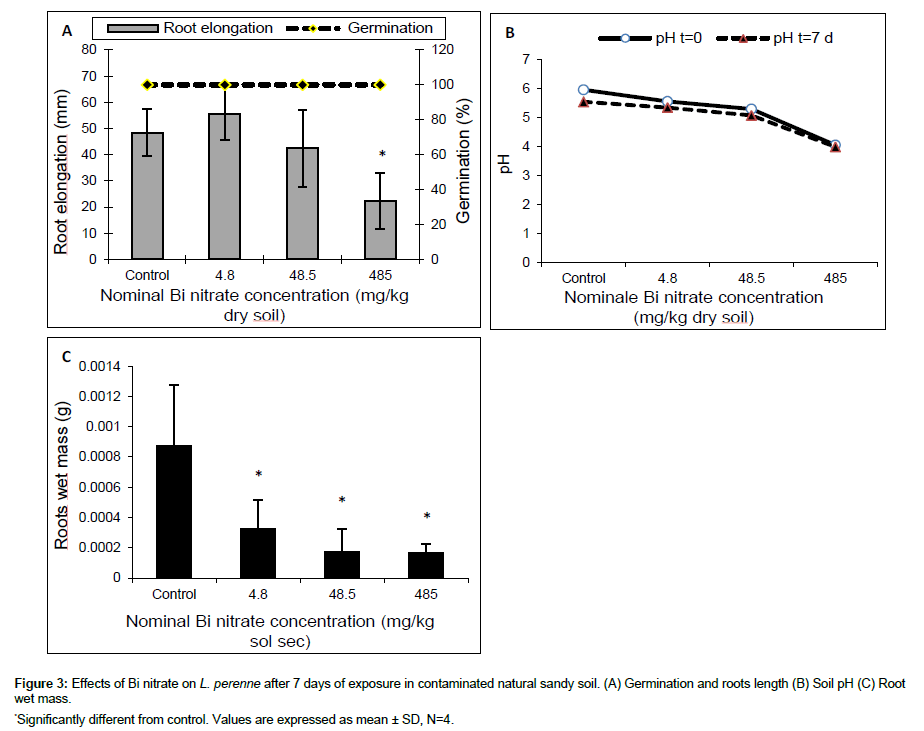
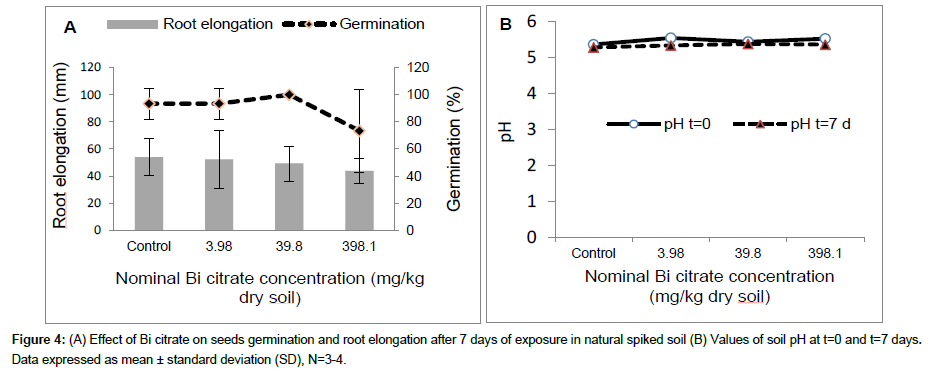
The results obtained after 7 days of exposure of ryegrass seeds in artificial (OECD) soil contaminated with Bi nitrate are shown in Figure 2. At concentrations ≤ 485 mg/kg dry soil, Bi nitrate did not seem to affect ryegrass seeds germination (Figure 2A), while the root wet mass was reduced significantly at 485 mg Bi nitrate/kg dry soil compared to control (OECD soil without Bi nitrate). The root wet mass decreased from 2.4 ± 0.6 mg in control to 1.3 ± 0.5 mg at 485 mg Bi nitrate/kg dry soil. However, no significant effect was observed using the shoot wet mass (data not shown). At the beginning of experiment (t=0), soil pH decreased slightly and not significantly with the rise of Bi nitrate in soil, from 5.85 in control to 5.72 at 485 mg Bi nitrate/kg dry soil (Figure 2B). Similar values were observed at the end of the experiment (t=7 days). The results of experiment carried out in natural sandy soil contaminated with Bi nitrate are presented in Figure 3. After 7 days of exposure, Bi nitrate did not affect seedling germination of ryegrass at tested concentration ≤ 48.5 mg/kg dry soil (Figure 3A), whereas, a significant decrease (p<0.05) in root elongation was observed at tested concentration of 485 mg Bi nitrate/kg dry soil compared to control (Figure 3A). Indeed, average root elongation reduced from 48.5 mm in control to 22.5 mm at 485 mg/kg dry soil. The root biomass was the most sensitive endpoint using Bi nitrate (Figure 3C). Root wet mass decreased significantly (p<0.05) at all tested concentration (≥4.8 mg Bi nitrate/kg dry soil). Average root biomass decreased from 0.000875 g in control to 0.000167 g at 485 mg Bi nitrate/kg dry soil. At t=0 d, soil pH decreased as a function of the Bi nitrate concentration in soil from 5.95 to 4.05 for control and 485 mg Bi nitrate/kg dry soil, respectively. Furthermore, the same pattern was observed in soil pH at the end of experiment (t=7 d). In addition, no significant difference was observed in the pH values measured at the beginning (t=0) compared to those measured at the end (t=7 d) of the test experiment (Figure 3B). Results obtained from L. perenne seeds exposed 7 days in natural soil contaminated with Bi citrate are summarized in Figure 4. Root elongation slightly decreased but not significantly as function of Bi citrate soil concentration. Similar results were observed for root wet mass (data not shown). At t=0 soil pH was 5.52 at 398 mg Bi citrate/kg dry soil compared to 5.36 for the control (Figure 4B). After 7 days, the pH values were similar to those obtained at the beginning of the test experiment (t=0).
Discussion
This study shows that bismuth phytotoxicity depends on the type of bismuth salt used (Bi nitrate or Bi citrate), the type of exposure test (filter paper vs soil test experiments) and the type of substrate (artificial or natural sandy soil) employed.
Filter paper exposure
The data from paper filter exposure indicate that for the same Bi metal nominal concentrations (6.5, 13.1, 26.1, 52.2, 104.4, 208.9 mg Bi/L deionized water); Bi nitrate was more toxic than Bi citrate (Figure 1). IC25 and IC50 were largely lower for Bi citrate than Bi nitrate (Table 1). This effect may be explained in part by lower water solubility and thence lower bioavailability of Bi citrate than Bi nitrate. Indeed, some authors reported that Bi nitrate was relatively more water soluble compared to Bi citrate [25,26]. Similarly, Cespedes et al. [27] reported that growth inhibitory and cytotoxic effects of three derivatives of Bi (III) on three different plants (Lactuca sativa L., Lolium multiflorum and Trifolium pretense) exposed on a filter paper increased when the Bicontaining compound was more soluble in water. On the other hand, the drastic decrease of soil pH in Bi nitrate aqueous solutions compared to Bi citrate aqueous solutions could contribute to the observed effects. L. perenne can tolerate a wide range of soil pH ranging from 4.5 to 8.4 [28].The pH of the aqueous Bi nitrate and Bi citrate solutions tested at high concentration was more acidic than the tolerated limit (Figure 1B and 1C), up to 2.2 and 3, respectively. In this case, the decrease in pH caused by Bi salts could affect growth and germination of plant. Thus, the effects caused by acidic pH can interfere with the effects caused by bioavailable Bi. Results of this study indicated also that seeds germination was the less sensitive endpoints affected by Bi nitrate and Bi citrate compared to the root elongation. As shown in Figure 1, germination was inhibited in a significant manner (p<0.05) only at the highest concentrations tested i.e. 485 and 398.08 mg/L for Bi nitrate and Bi citrate, respectively. Similarly, Nagata (2015) reported that Bi nitrate (Bi (NO3)2) affected more Arabidopsis thaliana root growth than shoot growth and seeds germination. The authors showed also that Bi accumulated in root was 7-fold higher compared to the shoot, suggesting the decrease of Bi transport mechanism to reduce transport of Bi to shoot. Furthermore, Nagata [29] suggested that Bi might perturb Fe homeostasis by inducing Fe accumulation in Arabidopsis thaliana root. The lower sensibility of seed germination compared to root and shoot growth in several plant species exposed to metals had been widely reported [30,31]. However, despite the large number of studies on the inhibitory effects of several metals on the germination and growth of plants, the mechanisms involved remain poorly understood. Cespedes et al. [27] suggested that inhibition of mitochondrial respiration and interaction in cell proliferation process or energetic pathways might be the mechanisms by which bismuthine derivatives inhibit seedling growth. Other recent studies reported that inhibition of specific enzymatic reactions is one of the main mechanisms behind metal toxicity on seed germination [29,32].
| Bi treatment | Root elongation (mm) | |
|---|---|---|
| IC25 (95% CI) (mg/L) | IC50 (95% CI) (mg/L) | |
| Bi nitrate | 23.79 (5.9 - 46.0) | 60.34 (45.88 - 77.115) |
| Bi citrate | 45.22 (24.55 - 100.92) | 139.5 (70.67 -238.07) |
| Cd chloride (reference toxicant) | 6.22 (2.35 – 11.16) | 21.91 (13,07 - 29,14) |
IC50 : estimated concentration (linear interpolation) that inhibit growth by 50%.
Table 1: Toxicity of Bi nitrate and Bi citrate in filter paper using ryegrass root elongation after 5 days of exposure.
Soil exposure
The results indicated that Bi nitrate was more toxic in natural sandy soil compared to artificial (OECD) soil. Data showed also that root growth was the most sensitive endpoint to Bi nitrate exposure compared to germination and shoot growth. IC25 and IC50 for root wet mass in natural soil and OECD soil were 1.01 and 292.8 and 3.05 and>485 mg/kg respectively (Table 2). Differences in toxicity may be explained by higher Bi bioavailability to L. perenne in natural sandy soil than in artificial soil due to variations in the physicochemical and biological properties of the two soils. The artificial soil used in this study contained less sand (70%) with high content of clay (20%) and sphagnum peat (10%), while the natural sandy soil (97.6% sand) used had low clay content (0.7%) and low total organic carbon (2%). Thereby, soil texture and composition may partly influence Bi bioaccessibility and phytotoxicity. It has been reported that bioavailability and uptake of Bi by plant from soil could be influenced by several soil factors such as organic matter content and soil texture [14]. Hou et al. [34] reported that the amount of Bi (added as Bi nitrate) retained in different samples of soil depends on surface area, on organic matter and aluminum content of soil. In another study, Berthelot et al. [35] found that total Bi concentration and clay content enhanced Bi bioaccessibility in soil but total organic carbon and amorphous aluminium oxide had an inverse effect on Bi bioaccessibility. However, total carbon content seems to be the most important soil parameter to influence soil Bi bioaccessibility [35]. Moreover, the results of recent studies showed that activities associated with soil organisms such as earthworms increased considerably bioaccessibility and bioavailability of Bi [36,37]. Results from Bi nitrate spiked soil showed a decrease in soil pH in natural sandy soil, whereas pH does not change in artificial soil. However, soil pH did not decrease drastically such as observed in the case of filter paper assays. Soil is a very complex matrix and multiple factors could influence soil pH such as mineral content, acid and base-forming ions in soil and soil texture. Indeed, it has been reported that soils with high clay and organic matter content have a greater buffering capacity and have generally the capacity to resist to a drop or rise in pH compared to sandy soils [38]. Berthelot et al. [35] investigated a site highly contaminated with mixture of metals and explosive concluded that Bi bioaccessibilty was not affected by the pH ranging from 5.35 to 7.96. Conversely, Hou et al. [39] reported that the mobile fraction of Bi in soil increased with increasing pH. Soils organisms may play a role in the release of Bi into the aqueous phase of the soil thus increasing its availability and uptake by plants. Tsang et al. [40] reported that Bi released from non-sterile soil contaminated with Bi was much higher compared to sterile soil, suggesting that soil microorganisms influence the release of Bi in soil. Thereby, differences in biological properties between the natural soil and the artificial soil used in this study could affect Bi availability and hence Bi plant toxicity. Thus, it can be concluded that the availability and toxicity of Bi on ryegrass depend on the combination of various factors such as Bi salt solubility and soil physicochemical and biological characteristics.
| Bi salt treatment/substrat | Root elongation (mm) | Root wet mass (g) | ||
|---|---|---|---|---|
| IC25 (mg/kg) | IC50 (mg/kg) | IC25(mg/kg) | IC50 (mg/kg) | |
| Bi nitrate/ OECD soil | nd | nd | 292.8 | > 485 |
| Bi nitrate/ Natural soil | 71.7 | 315.1 | 1.01 | 3.05 |
| Bi citrate /Natural soil | nd | nd | nd | nd |
IC50 : estimated concentration (linear interpolation) that inhibit growth by 50%.
nd : Not Determined.
Table 2 : Toxicity of Bi nitrate and Bi citrate in spiked artificial (OECD) and natural soil using ryegrass after 7 days of exposure.
Conclusion
The present study compares the toxic effects of Bi nitrate and Bi citrate to ryegrass using paper filter, artificial and natural sandy soil. The results showed that Bi phytotoxicity varies depending on the substrate physicochemical properties, type of Bi salt used, bismuth route of exposure and type of assays employed, i.e. filter paper and type of soil assays employed. In addition, both filter paper and soil toxicity tests results indicated that Bi nitrate was more toxic to ryegrass than Bi citrate, probably due in part to relatively higher Bi nitrate water solubility and hence Bi bioavailability. Furthermore, phytotoxic effects showed that root growth was the more sensitive to Bi salts than seedling germination and shoot growth. However, the mechanisms of Bi toxicity leading to the inhibition of germination and growth require more investigations.
Acknowledgment
We thank Sabine Dodard of the NRC Canada, Montreal for technical assistance. We are also grateful to Drs Sonia Thiboutot and Guy Ampleman from Defence Research and Development Canada–Valcartier (Canadian Ministry of National Defence) for their support of this project.
References
- Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, et al. (2008) Uncommon heavy metals, metalloids and their plant toxicity: A review. Env Chem Lett 6: 189-213.
- Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London, UK.
- Lambert JR, Midolo P (1997) The actions of bismuth in the treatment of helicobacter pylori infection. Aliment Pharmacol Ther 1: 27-33.
- Yang N, Sun H (2011) Bismuth Environmental Pollution And Health Effects: 414-420.
- Roscoe HE, Schorlemmer C (1890) A treatise on chemistry. D Appleton and Company, New York.
- Yang Y, Zhou S, Ouyang R, Yang Y, Tao H, et al. (2016) Improvement in the anticancer activity of 6-mercaptopurine via combination with bismuth(III). Chem Pharm Bull 1539-1545.
- Mansoor SS, Aswin K, Logaiya K, Sudhan SPN (2016) Bismuth nitrate as an efficient recyclable catalyst for the one-pot multi component synthesis of 1,4-dihydropyridine derivatives through unsymmetrical hantzsch reaction. J Saudi Chem Soc 20: 100-108.
- Sampathkumar K, Yesudas S (2009) Hair dye poisoning and the developing world. J Emerg Trauma Shock 2: 129-131.
- Amneklev J, Augustsson A, Sorme L, Bergbäck B (2016) Bismuth and silver in cosmetic products: A source of environmental and resource concern. J Ind Eco 20: 99-106.
- Eriksson J (2001) Concentrations of 61 trace elements in sewage sludge, farmyard manure, mineral fertiliser, precipitation and in oil and crops, Swedish environmental protection agency, Sweden.
- Xiong QL, Zhao WJ, Guo XY, Shu TT, Chen FT, et al. (2015) Dustfall heavy metal pollution during winter in North China. Bull Environ Contam Toxicol 95: 548-554.
- Zhao L, Zhang FS, Wang K, Zhu J (2008) Chemical properties of heavy metals in typical hospital waste incinerator ashes in China. Waste Management 29: 1114-1121.
- Li X, Thornton I (1993) Arsenic, antimony and bismuth in soil and pasture herbage in some old metalliferous mining areas in England. Environ Geochem Health 15: 135-144.
- Jung MC, Thornton I, Chon HT (2002) Arsenic, Sb and Bi contamination of soils, plants, waters and sediments in the vicinity of the Dalsung Cu-W mine in Korea. Sci Total Environ 295: 81-89.
- Soto-Barajas MC, Gomez J, Vazquez de Aldana BR, Zabalgogeazcoa I (2013) Incidence and identification of endophytes Epichloe/Neotyphodium in wild populations of Lolium perenne (Deutsche Phytomedizinische Gesellschaft e.V. Verlag, Braunschweig) 33-38.
- Hoffman L, DaCosta M, Ebdon JS, Zhao J (2012) Effects of drought preconditioning on freezing tolerance of perennial ryegrass. Environmental and Experimental Botany 79: 11-20.
- ASTM (1999) Standard Guide for Conducting Terrestrial Plant Toxicity Tests. E-1963-98, American Society for Testing and Materials, Philadelphia, Pennsylvania.
- US EPA (1982) Early Seedling Growth Toxicity Test. in Number EG-13. Office of Toxic Substances, Ofiice of Pesticides and Toxic Substances, Washington, DC.
- Wong MH, Bradshaw AD (1982) A comparison of the toxicity of heavy metals, using root elongation of Rye Grass, Lolium perenne. The New Phytologist 91: 255-261.
- Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant d (Lolium perenne L. cv Apollo). J Exp Bot 51: 945-953.
- Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68: 1563-1575.
- Robidoux PY, Svendsen C, Caumartin J, Hawari J, Ampleman G, et al (2000) Chronic toxicity of energetic compounds in soil determined using the earthworm (Eisenia andrei) reproduction test. Environmental Toxicology and Chemistry 19: 1764-1773.
- ISO (1994) Soil Quality-Determination of pH. ISO 10390.) International Organization for Standardization CP 401-1214 Vernier, Geneva, Switzerland.
- US EPA (1989) Protocols for Short Tem Toxicity Screening of Hazardous Wast Sites. in EPA/600/3-88/029.
- Briand GG, Burford N (1999) Bismuth compounds and preparations with biological or medicinal relevance. Chem Rev 99: 2601-2658.
- Larranaga MD, Lewis RJ, Lewis RA (2016) Hawley's Condensed Chemical Dictionary. Wiley.
- Cespedes CL, Lemus A, Salazar JR, Cabrera A, Sharma P (2003) Herbicidal, plant growth inhibitory, and cytotoxic activities of bismuthines containing aromatic heterocycles. J Agric Food Chem 51: 2923-2929.
- Alberta Agriculture and Forestry (2004) Perennial Ryegrass Seed Production in Western Canada. p: 15.
- Nagata T (2015) Growth inhibition and IRT1 induction of Arabidopsis thaliana in response to bismuth. Journal of Plant Biology 58: 311-317.
- Kang IM, Kong IC (2016) Effects of properties of metal-contaminated soils on bacterial bioluminescence activity, seed germination, and root and shoot growth. Springer Plus 5: 272.
- Kapustka LA, Lipton J, Galbraith H, Cacela D, Lejeune K (1995) Metal and arsenic impacts to soils, vegetation communities and wildlife habitat in southwest montana uplands contaminated by smelter emissions: II. Laboratory phytotoxicity studies. Environmental Toxicology and Chemistry 14 : 1905-1912.
- Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. Journal of Natural Science, Biology, and Medicine 4: 272-275.
- OECD (1984) Test No. 207 Earthworm, Acute Toxicity Tests.
- Hou H, Takamatsu T, Koshikawa MK, Hosomi M (2005) Migration of silver, indium, tin, antimony, and bismuth and variations in their chemical fractions on addition to uncontaminated soils. Soil Science 170: 624-639.
- Berthelot Y, Trottier B, Robidoux PY (2009) Assessment of soil quality using bioaccessibility-based models and a biomarker index. Environ Int 35: 83-90.
- Omouri Z, Hawari J, Fournier M, Robidoux PY (2017) Acute toxicity of bismuth to the earthworm Eisenia andrei. International Journal of Ecotoxicology and Ecobiology 2: 125-135.
- Omouri Z, Hawari J, Fournier M, Robidoux PY (2018) Bismuth citrate to earthworm Eisenia andrei exposed to natural sandy soil. Ecotoxicology and Environmental Safety 147: 1-8.
- McCauley A, Jones C, Olson-Rutz K (2009) Soil pH and Organic Matter, Module 8. Nutrient management. Extension service. Montana State University.
- Hou H, Takamatsu T, Koshikawa MK, Hosomi M (2006) Concentrations of Ag, In, Sn, Sb and Bi, and their chemical fractionation in typical soils in Japan. European Journal of Soil Science 57: 214-227.
- Tsang KW, Dugan PR, Pfister RM (1994) Mobilization of Bi-ion, Cd-ion, Pb-ion, Th-ion, and U-ion from contaminated soil and the influence of bacteria on the process. Emerging Technologies in Hazardous Waste Management IV, ACS Symposium Series 554: 78-93.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi