Research Article, J Clin Image Case Rep Vol: 6 Issue: 7
Post Hepatitis B Vaccination SeroConversion among Health Care Workers in a Referral Hospital Tanzania: A Cross Sectional Study
Gembe A1*, Bilaro E1, Mrosso L1, Mhina S1, Komba P2, Jumanne J2,Nzota L3, Mwelela A3, Joseph J3, Mashiku L3 and Sikira B3
1Department of Internal Medicine, Tumbi Regional Referral Hospital, Tumbi, Tanzania
2Department of Environmental Health, Tumbi Regional Referral Hospital, Tumbi, Tanzania
3Department of Laboratory, Tumbi Regional Referral Hospital, Tumbi, Tanzania
*Corresponding Author: Gembe A
Department of Internal Medicine,
Tumbi Regional Referral Hospital,
Tumbi,
Tanzania,
Tel: +255716009131;
E-mail: dr.adamgembe@gmail.com
Received date: 30 May 2022, Manuscript No. CICR-22-65336; Editor assigned date: 03 June 2022, PreQC No. CICR-22-65336 (PQ); Reviewed date: 17 June 2022, QC No. CICR-22-65336; Revised date: 29 July2022, Manuscript No. CICR-22-65336 (R); Published date: 05 August 2022, DOI: 10.4172/CICR.1000229.
Citation: Gembe A, Bilaro E, Mrosso L, Mhina S, Komba P, et al. (2022) Post Hepatitis B Vaccination Sero-Conversion among Health Care Workers in a Referral Hospital Tanzania: A Cross Sectional Study. J Clin Image Case Rep 6:7.
Abstract
Background: Hepatitis B Virus (HBV) vaccine is known to offer protection against transmission of HBV infection. Health care workers are required to have this vaccination as part of their occupational health safety measure. Post vaccination immunological response data for HCWs in our setting is not available. This study therefore aimed at evaluation of anti-HBs titer levels after hepatitis B vaccination among HCWs from tumbi regional referral hospital.
Methodology: A cross-sectional study involving 246 HCWs was conducted. Five (5 mls) of blood sample was collected from each study participant and the serum was used for HBV immunological anti-HBs quantification by ELISA test. Data was analyzed using SPSS version 20.0 and a P value of ≤ 0.05 was considered significant. Anti-HBs titers of>10 mIU/ml was considered protective and good compliance to vaccine schedule was defined as receiving all the three doses of vaccine at recommended time intervals.
Results: The mean age of study participants was 40 ± 10.8 years where 146 (67.9%) were males. Majority of study participants were nurses 103 (47.9). Smoking was uncommon among the study participants compared to alcohol intake 29 (13.9%). 89.3% health care workers developed protective antibodies after hepatitis vaccination, age below 55 years (OR 2.98, 95% CI, 1.02-8.89) and good compliance to vaccine schedule (OR 2.75, 95% CI 1.02-7.43) were the significant predictors of achieving sero-protection.
Conclusion: The seroconversion rate post HBV vaccination is high among HCWs in TRRH and it’s comparable to findings elsewhere. Owing the fact that HCWs are at high risk of HBV infection, it is of paramount importance for HCWs to be tested for protective titers post completion of vaccination schedule. Booster doses of vaccine should be provided to individuals with BMI ≥ 30 and those who fail to comply with vaccination schedule.
Keywords: Seroconversion; Enzyme Linked Immunosorbent Assay (ELISA)
Abbreviations
ELISA: Enzyme Linked Immunosorbent Assay; HBV: Hepatitis B virus; TRRH: Tumbi Regional Referral Hospital; HBsAg: Hepatitis B Surface Antigen; Anti HBs: Hepatitis B Surface Antigen Antibodies; HBeAg: Hepatitis B e Antigen; HCW: Health Care Worker; SPSS: Statistical Package for Social Sciences; WHO: World Health Organization; HCWs: Health Care Workers
Introduction
Background: Hepatitis B Virus (HBV) infection is a life threatening infection caused by hepatitis b virus. The global prevalence of HBV infection is alarming. Chronic infection with HBV is associated with high mortality and morbidity due to hepatocellular carcinoma and cirrhosis.
The World Health Organization (WHO) 2015 factsheet reported 255 million people were diagnosed with chronic hepatitis b infection whilst 887,000 deaths due to cirrhosis and hepatocellular carcinoma. In Sub Saharan Africa HBV infection is endemic and accounts for around 6.1% despite universal vaccination program [1,2].
Post hepatitis B vaccination seroconversion is variable across the globe. In Pakistan, a study involving 666 HCWs found a seroconversion prevalence of 86%. On the other hand, a higher seroconversion rate was reported among 258 HCWs in India with a prevalence of 96.5% [3]. Similarly, in Sri Lanka a seroconversion rate of 90.1% was reported. Furthermore, another study in India has shown a decline in seroconversion with time 99.9% one year post vaccination, 80.96% five years post vaccination and 46.16% ten years post vaccination [4]. In Africa, Ghana, a study involving 711 HCWs revealed a seroconversion rate of 91.8% after completion of 3 doses of anti HBs vaccine [5]. Whether these findings can be replicated in our setup remains to be an important research question.
Despite HBV infection being a major public issue in both community and healthcare settings in Tanzania, there is no comprehensive data regarding sero-conversion after HBV vaccination among healthcare workers in the country. Furthermore, little is known on the efficacy of the vaccine, the sero-conversion rate and duration of immunity among vaccinated HCWs in Tanzania at large. This ominous lack of post vaccination surveillance programs in Tanzania makes the detection and management of vaccine non-responders difficult.
In view of their heightened exposure and risk to infection, the HCW population constitutes a rational starting point for efforts to decrypt HBV vaccine failure and non-respondents. This study therefore aimed to evaluate the sero-conversion rate after HBV vaccination among HCWs from Tumbi regional referral hospital.
The study objectives were as follows:
• To determine the post hepatitis B vaccination sero-conversion among health care workers in TRRH.
• To determine the factors associated with seroconversion among HCWs in TRRH.
Methods
Study design
This was a cross sectional study carried out at TRRH with a capacity of 230 beds. This Hospital is situated about 35 Km from Dares- Salaam along Morogoro road. It is a referral centre for 6 districts of Pwani region. It serves a population of 1,110,917 people.
The hospital has 350 HCWs in departments of internal medicine, surgery (General, pediatric and orthopedic surgery), intensive care unit, obstetrics and gynecology, pediatrics and neonatal care, social welfare and environmental department. As of current, during inscription of this proposal, 246 HCWs had received HBV vaccination.
Inclusion criteria: All who consented to participate in the study; All HCWs who received any dose of hepatitis B vaccine.
Exclusion criteria: HCWs who refute consent.
Consecutive sampling was employed in which all TRRH HCWs on their respective stations were approached, introduced to study subject and participants were recruited until a desirable sample size was obtained.
A pretested structured questionnaire was filled by respective HCW under assistance form a research assistant/principal investigator. Each HCW basic information such as age, gender, occupation, source of HBV vaccination, how long ago was the vaccination, HBV dose received, vaccination interval, comply with dose interval, smoking and alcohol history were enquired.
Five (5 mls) of blood was collected from each study participant into EDTA vacutainer tubes and transported to Tumbi regional referral hospital main laboratory for processing and testing. Samples were separated by centrifugation and the serum used for HBV immunological profile testing following the manufacturers’ protocol and the rest were kept at -20˚C and later used to estimate HBV antibody titer quantification.
The presence of anti-HBs was determined using the anti-HBs quantitative ELISA kit (Fortress Diagnostics Limited, Northern Ireland, United Kingdom). Each test was performed in duplicates according to the manufacturer’s instructions; the plate was read on ELISA plate reader Emax (Molecular Devices LLC, USA).
Anti-HBs titer was used to evaluate the efficacy of hepatitis B vaccine and titers of >10 mIU/ml was considered protective.
Compliance to vaccine schedule was defined as receiving all the three doses of vaccine at recommended time intervals (0, 4 and 24 weeks).
Data analysis: The data obtained from the questionnaires was entered in to Statistical Package of Social Sciences (SPSS) version 20 for Windows for data analysis. Cross checking of filled questionnaires after data collection was done for quality control of data.
The qualitative variables such as: gender, occupation, source of HBV vaccination, comply with dose interval, smoking and alcohol history were summarized using frequency distribution tables.
Quantitative variables in the study included; age, duration since vaccination, HBV vaccine doses received, these were summarized by frequency distribution. Mean and standard deviation was employed for age.
Comparison of categorical variables between groups was performed using chi-square tests or Fisher exact t-test. Multivariate analysis was used to determine factors associated with development of protective antibodies. Variables with unadjusted P value of less than 0.2 were entered into multivariate analysis. The level of significance used was P=0.05.
Ethical consideration
Permission was sought from TRRH administration. Informed consent was obtained from all HCWs that a vein puncture was to be done to obtain a blood sample that will be used for this study only.
Anticipated outcomes such as pain at the needle prick site were explained to the study participants and no long-term complications are anticipated. Similarly, since the study involves blood sample collection from a vein, there is a risk of introducing infection as a result, prior to sample collection the site was cleansed by an alcohol swab.
The potential benefit of the study that is establishing if HCWs are protected against HBV infection following vaccination will be addressed.
Results
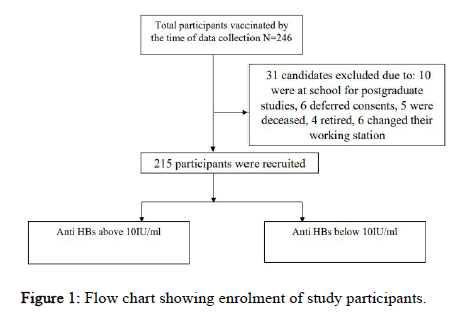
A total of 246 staffs were eligible for the study, 31 staffs were excluded from the study as stipulated in the Figure 1 below. The mean age of study participants was 40 ± 10.8 years where 146 (67.9%) were males. Majority of study participants were nurses 103 (47.9). Smoking was uncommon among the study participants compared to alcohol intake 29 (13.9%). Overweight was a significant finding in this study 88 (40.9%). Most staffs had more than 10 years of working experience and they were vaccinated from the government source of vaccine. Among the vaccinated staff, many complied with three doses schedule and only 6 (2.8%) had taken a booster dose. Completion of three doses of vaccination was noted more among doctors followed by nurses and lab technicians, at 90%, 82% and 75% respectively. Furthermore, completion rate was higher among individuals with less than five years of practice than those with more than 10 years of practice as shown in Table 1 below.
| Characteristics | N (%) | |
|---|---|---|
| Age | Mean age years ± SD | 40 ± 10.8 |
| Age group | 20-40 | 107 (49.8) |
| 41-60 | 108 (50.2) | |
| Sex | Female | 69 (32.1) |
| Male | 146 (67.9) | |
| Occupation | Doctors | 52 (24.2) |
| Nurses | 103 (47.9) | |
| Laboratory technicians | 9 (4.2) | |
| Others | 51 (23.7) | |
| Alcohol | Ever | 29 (13.9) |
| Smoking | Ever | 1 (0.5) |
| BMI | <18.5 | 1 (0.5) |
| 18.5-24.9 | 65 (30.2) | |
| 25-29.9 | 88 (40.9) | |
| >30 | 61 (28.4) | |
| Years of practice | >10years | 101 (47) |
| Source of HBV vaccination | Government facility | 191 (88.8) |
| HBV vaccine dose received | 3 doses | 170 (79.1) |
| Complied with dose interval | Ever | 168 (78.1) |
| Booster dose taken since | Ever | 6 (2.8) |
| schedule completion | ||
| Completion of three doses | Doctors | 45 (90) |
| Nurses | 83 (82.2) | |
| Laboratory technician | 6 (75) | |
| Others | 37 (72.5) | |
| Completion of three doses | <5 years of practice | 43 (82.7) |
| 5-10 years of practice | 48 (81.4) | |
| > 10 years of practice | 80 (80.8) | |
| Completion of three doses | Doctors | 45 (90) |
| Nurses | 83 (82.2) | |
| Laboratory technician | 6 (75) | |
| Others | 37 (72.5) | |
Table 1: Demographic characteristics of study participants N=215.
The post hepatitis B vaccination sero-conversion among health care workers in TRRH
The post hepatitis B vaccination sero-conversion among health care workers in TRRH was 89.3% (Table 2).
| Anti HBs titers | N (%) |
|---|---|
| Anti HBs <10 mIU/l | 23 (10.70) |
| Anti HBs <10 mIU/l | 192 (89.3) |
Table 2: The post hepatitis B vaccination sero-conversion among health care workers in TRRH.
The factors associated with post hepatitis B vaccine seroconversion among HCWs in TRRH
The level of post hepatitis B seroconversion was similar among males and females (94% vs. 87%) respectively, P Value 0.155, similarly, there was no statistically significant difference in levels of immunologic response between participants of different cadres, and those who received the vaccine from government or nongovernment facilities.
Participants, who received the last vaccine less than 5 years ago, were more likely to develop protective antibodies than those who received the vaccine more than five years ago, but this association was not statistically significant.
We also noted a decreasing trend towards immunologic response as BMI increases. Alcohol intake did not significantly affect Hepatitis B vaccine sero-protection rate as shown in Table 3 below.
| Anti-HBs Titers | P value | |||
|---|---|---|---|---|
| <10 mIU/l | >10 mIU/l | |||
| Age | ≥ 55 Years | 7 (25) | 21 (75) | 0.017 |
| <55 Years | 16 (8.6) | 171 (91.4) | ||
| Gender | Male | 4 (5.8) | 65 (94.2) | 0.15 |
| Female | 19 (13) | 127 (87) | ||
| Occupation | Doctor | 4 (7.7) | 48 (92.3) | 0.3 |
| Nurse | 15 (14.6) | 88 (85.4) | ||
| Lab tech | 0 | 9 (100) | ||
| Others | 4 (7.8) | 47 (92.2) | ||
| Source of vaccine | Private facility | 1 (4.3) | 22 (95.7) | 0.25 |
| Government facility | 22 (11.5) | 169 (88.5) | ||
| Last vaccine | <5 years | 5 (6.5) | 72 (93.5) | 0.29 |
| 5-10 years | 17 (13.5) | 109 (86.5) | ||
| >10 years | 1 (12.5) | 7 (87.5) | ||
| Dosage received | Less than three doses | 6 (15.4) | 33 (84.6) | 0.23 |
| Three doses | 17 (9.9) | 154 (90.1) | ||
| BMI | <18.5 | 0 (0.0) | 1 (100) | 0.11 |
| 18.5-24.9 | 3 (4.6) | 62 (95.4) | ||
| 25-29.9 | 9 (10.2) | 79 (89.8) | ||
| ≥ 30 | 11 (18) | 50 (82) | ||
| Alcohol | Yes | 3 (10.3) | 26 (89.7) | 1 |
| No | 20 (10.8) | 166 (89.2) | ||
| Years of practice | <5 Years | 9 (17.3) | 43 (82.7) | |
| 5-10 Years | 11 (18.6) | 48 (81.4) | ||
| >10 Years | 19 (19.2) | 80 (80.8) | ||
Table 3: Univariate analysis of factors associated with post hepatitis B vaccine seroconversion among HCWs in TRRH.
Participants below 55 years were nearly three times more likely to develop significant immunologic response compared to those above 55 years, (OR 2.99, 95% CI 1.02-8.79), likewise participants who complied with dose schedule of the three vaccine were also more likely to mount more immunologic response compared to those who failed to comply. OR 2.75, 95% CI 1.02-7.43). These associations were statistically significant as shown in Table 4 below.
| P | AOR | 95% C.I. | ||
|---|---|---|---|---|
| BMI ≥ 30 | 0.077 | 0.44 | 0.17 | 1.09 |
| Age <55 Years | 0.04 | 2.98 | 1.02 | 8.89 |
| Gender (Female) | 0.3 | 0.54 | 0.17 | 1.74 |
| Years since last vaccine (<5) | 0.36 | 1.66 | 0.56 | 4.86 |
| Good compliance to dose schedule | 0.04 | 2.75 | 1.02 | 7.43 |
Table 4: Multi-variate analysis of factors associated with post hepatitis B vaccine seroconversion among HCWs in TRRH.
Discussion
The level of protective antibodies was similar to studies conducted in other areas, signifying the long-term persistence of protective antibodies. Our findings are consistency with studies done in Asia-Sri Lanka, West Africa-Ghana and Northern Africa-Egypt where the seroconversion rate were found to be 91.8%, 83.2% and 91% respectively [6].
Immunologic repose to hepatitis B vaccine has been shown to decline with increase in age at vaccination [7-9]. This is similar to our findings in which individuals aged 55 years and above had significantly less odds of mounting protective antibodies, theory for this finding includes waning immune response with age, Indeed the sero-protection rate was 92.2%, 90.5% and 71.5% among individuals aged 20-39 years, 40-55 years and above 55 years respectively, this imply that immunization should be done at earlier age and that we should consider addition of booster dose of vaccine in individuals above 55 years [10].
In addition the odds of developing protective antibodies was significantly more among participants who complied with hepatitis dose schedule, this is similar to studies conducted in India, within the group of participants who failed to comply with vaccination schedule, the administrative staffs were more prominent followed by laboratory technicians and nurses similar to studies conducted in other parts [11]. Previous studies conducted among health care workers indicated main reason for non-compliance to be negligence; this together with our findings underscores the importance of continued education to health care workers on risk of their occupational activities and impact of hepatitis infections [12].
The odds of achieving protective antibodies have been shown to be higher among patient with normal BMI, this is similar to studies conducted by Rabie, et al. and Abdolsamadi, et al. [13]. Some of the concepts put forward are as follows; high BMI is associated with decrease in number and activity of naïve CD8-T cells and NK cells from the thymus. In addition, some literature reports decrease in proliferation of B cells and T cells in obese individuals. On the other hand, obese individuals have a low grade chronic inflammatory state that may predispose to low seroconversion rate post HBV vaccination [14].
The rate of seroconversion was not affected by gender in this study. Similarly, studies conducted in other areas there was no difference in protective antibodies formed among males and females [15]. Contrary, studies done in Asia showed a high seroconversion rate in females compared to males and this difference was statistically significant [16].
Conclusion
The seroconversion rate post HBV vaccination is high among HCWs in TRRH and it’s comparable to findings elsewhere. Owing the fact that HCWs are at high risk of HBV infection, it is of paramount importance for HCWs to be tested for protective titers post completion of vaccination schedule. This study shows the need for booster dose of vaccine to old age individuals and those who fail to comply with hepatitis vaccine dose schedule.
References
- Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, et al. (2017) Series Viral hepatitis in sub-Saharan Africa 1 Hepatitis B in sub-Saharan Africa : Strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol 2:900–909.
[Crossref] [Googlescholar] [Indexed]
- Kilonzo SB, Gunda DW, Mpondo BCT (2018) Hepatitis B Virus Infection in Tanzania: Current Status and Challenges. J Trop Med 2018:4239646.
[Crossref] [Googlescholar] [Indexed]
- Basireddy P, Avileli S, Beldono N, Gundela SL (2018) Evaluation of immune response to hepatitis B vaccine in healthcare workers at a tertiary care hospital. Indian J Med Microbiol 36:397–400.
- Mahawal BS, Bhai N, Kataria VK, Gulati N, Chandola I (2013) Estimation of Anti Hbs antibody titer in adults during 5-10 years period following three doses of vaccine. IOSR J Pharm Biol Sci 7:20–23.
- Id DO, Awuku YA, Adjei G, Cudjoe O, Benjamin H, et al. (2019) Post Hepatitis B vaccination sero-conversion among health care workers in the Cape Coast Metropolis of Ghana. PLoS One 14:e0219148.
- El Bahnasy R, Abu Salem M, El Shazly H, Morad W, Moaz E (2016) Predictors of poor response to the hepatitis B vaccine among healthcare workers at the National Liver Institute Hospital. Menoufia Med J 29:131.
- Yang S, Tian G, Cui Y, Ding C, Deng M, et al. (2016) Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep 6:1-12.
- Fisman DN, Agrawal D, Leder K (2002) Effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis 35:1368-1375.
- Zeeshan M, Jabeen K, Ali AN, Ali AW, Farooqui SZ (2007) Evaluation of immune response to Hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: an observational prospective study. BMC Infect Dis 7:1-6.
[Crossref] [Googlescholar][Indexed]
- Van Der Meeren O, Crasta P, Cheuvart B, De Ridder M (2015) Characterization of an age-response relationship to GSK's recombinant hepatitis B vaccine in healthy adults: an integrated analysis. Hum Vaccin Immunother 11:1726-1729.
[Crossref] [Googlescholar] [Indexed]
- Ziglam H, El-Hattab M, Shingheer N, Zorgani A, Elahmer O (2013) Hepatitis B vaccination status among healthcare workers in a tertiarycare hospital in Tripoli, Libya. J Infect Public Health 6:246-251.
[Crossref] [Googlescholar][Indexed]
- Aaron D, Nagu TJ, Rwegasha J, Komba E (2017) Hepatitis B vaccination coverage among healthcare workers at nationalhospital in Tanzania: how much, who and why?. BMC Infectious Dis 17:1-7.
- Abdolsamadi HR, Vaziri PB, Abdollahzadeh SH, Kashani KHM, Vahedi M (2009) Immune response to hepatitis B vaccine among dental students. Iran J Public Health 38:113–118.
- Fan W, Chen XF, Shen C, Guo ZR, Dong C (2016) Hepatitis B vaccine response in obesity:A meta-analysis. Vaccine 34:4835–4841.
[Crossref] [Googlescholar] [Indexed]
- Joukar F, Mansour-Ghanaei F, Naghipour MR, Asgharnezhad M (2017) Immune response to hepatitis B vaccine among north iranian healthcare workers and its related factors. J Infect Dev Ctries. 11:501–507.
- Chathuranga LS, Noordeen F, Abeykoon AMSB (2013) Immune response to hepatitis B vaccine in a group of health care workers in Sri Lanka. Int J Infect Dis 17:e1078–1079.
[Crossref] [Googlescholar][Indexed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi