Research Article, J Spine Neurosurg Vol: 9 Issue: 3
Posterior Lumbar Interbody Fusion Using a Porous PEEK Implant and Bone Marrow Concentrate
Paul R Waldrop1, Christopher R Rehak2 and J Kenneth Burkus3*
1University of Alabama School of Medicine, Birmingham, Alabama, USA
2Intern, Spine Service, The Hughston Clinic, Columbus, Georgia, USA
3Staff Physician, Spine Service, The Hughston Clinic, Columbus, Georgia, USA
*Corresponding Author: J Kenneth Burkus
The Hughston Clinic, 6262 Veterans Parkway
Columbus, Georgia 31908-9517, USA
Tel: +1 706-494-3239
Fax: +1 706-494-3102
E-mail: jkb66@knology.net
Received: May 06, 2020 Accepted: May 13, 2020 Published: May 21, 2020
Citation: Paul Waldrop R, Rehak CR, Kenneth Burkus J (2020) Posterior Lumbar Interbody Fusion Using a Porous PEEK Implant and Bone Marrow Concentrate. J Spine Neurosurg 9:4.
Abstract
Objective: We report the 1-year results of a prospective, singlecenter clinical study in which a posterior lumbar interbody fusion (PLIF) was performed using a novel porous polyether ether ketone (PEEK) implant and pedicle screw fixation in patients with symptomatic single-level degenerative lumbar disc disease.
Methods: Thirty consecutive patients with single-level symptomatic degenerative lumbar disc disease were enrolled in the study. All patients underwent a single level PLIF procedure using a porous PEEK implant with pedicle screw fixation and bone marrow concentrate applied to a ceramic carrier. Patients were assessed preoperatively and at 1.5, 3, 6, and 12 months after surgery. Standardized outcome measures were used to evaluate the patient’s condition before and after surgery. Plain radiographs were used to assess fusion, sagittal plane angulation, bony ingrowth, subsidence, and migration of the implant.
Results: Three patients were lost to follow up after their 3-month follow-up visits and were removed from the study; 27 patients were followed for a minimum of 12 months. At their last follow up, Oswestry disability index (ODI) scores and back and leg pain scores showed improvement in all patients. All patients showed radiographic fusion with no motion across the instrumented interspaces on dynamic flexion-extension lateral radiographs. Segmental lordosis at the surgical site improved from -1° to an average of -7° (0° to -9°) with no evidence of implant migration or subsidence. Average disc space height increased 6 mm. No patient showed radiographic evidence of a pseudarthrosis. No patient developed radiolucency at the implant interface with the host bone.
Conclusion: We found that used of a porous-surface PEEK implant with bone marrow concentrate on a ceramic carrier is a clinically viable alternative for improving the osseointegration and fusion rates in patients undergoing single-level PLIF surgery for degenerative disc disease in the lumbar spine.
Keywords: Porous PEEK; Posterior lumbar interbody fusion; Lumbar arthrodesis; Degenerative lumbar disc disease; Bone marrow concentrate
Introduction
In posterior lumbar interbody fusion (PLIF) surgery, intradiscal implants are used to restore displace height, sagittal alignment and facilitate de novo bone growth across the vertebral interspace [1-4]. Porous PEEK implants have a surface architecture designed to promote bone in-growth while maintaining the favorable biomechanical and imaging properties [5-7]. The implant surface mimics bone with its highly porous interconnected architecture. The increased surface area and wicking capability of the porous surface improves host tissue to implant contact compared with traditional interbody implants. The mechanical properties of porous PEEK material have been well established. Porous PEEK retains the strength and durability of smooth PEEK [5]. It maintains more porosity under physiologic loads and withstands impacted insertion testing [8]. In addition, porous PEEK more effectively shares load with interlocking bone than porous titanium implants.
Autologous bone from the iliac crest is the gold standard for most types of bone graft procedures because of its osteogenic properties. Iliac crest bone grafts (ICBG) are effective, but they are associated with a number of problems, such as pain and infection at the donor site. There is also a limited amount of graft available. Bone marrow aspiration and concentrate (BMC) is a less invasive procedure than harvesting ICBG. The use of BMC, containing osteogenic cell precursors and cytokines, has been shown to be as effective as iliac crest bone graft in promoting posterolateral spinal fusion [9]. The use of BMC also has been shown to have a therapeutic benefit in treating long bone nonunions [10-12] and osteonecrosis [13]. Hydroxyapatite (HA) carriers, when combined with BMC, becomes a moldable, threedimensional structure capable of conforming to the full geometry of the recipient site and providing a scaffold for bone growth.
Preclinical studies show porous PEEK implants resulting in more robust lumbar interbody fusion in an ovine model compared to rough titanium implants [8]. Clinical studies assessing outcomes using porous PEEK in anterior cervical fusions show that consistent improvements were observed as early as 6 weeks, and maintained out to 12 months [14,15]. These early improvements may reflect the stabilization created by this unique surface architecture.
We enrolled 30 patients in this prospective study to investigate the efficacy of bone marrow concentrate with a porous PEEK implant used in a single-level PLIF lumbar surgery.
Materials and Methods
Inclusion criteria
All patients had clinical complaints of low back pain and radiating leg pain that was unresponsive to a minimum of 8 weeks of nonoperative treatment that included immobilization, traction, modalities, medications, and physical therapy. In addition to recurrent or persistent complaints of pain, all patients had an objective neurologic deficit that included 1 or more of the following: an asymmetric deep tendon reflex, a sensory deficit in a dermatomal pattern, or motor weakness. All patients had a correlative neuroradiographic study. We recorded patients’ smoking status and presence of diabetes, osteoporosis, and obesity. Patients were excluded from the study if they had a medical condition that required medication, such as steroids or nonsteroidal anti-inflammatory medications, that could interfere with fusion.
Patients were included in this study if their plain radiographic findings documented single-level disc disease, and they had undergone at least 1 additional confirmatory neuroradiographic study, such as MRI or CT-enhanced myelography. Patients with up to a Grade spondylolisthesis were also included.
Patient demographics
Between July 2017 and February 2018, 30 consecutive patients with single-level degenerative disc disease of the lumbar spine and associated radiculopathy were enrolled in the study and were treated by a posterior lumbar interbody fusion (PLIF) procedure using a porous PEEK implant, pedicle screw fixation and bone marrow concentrate on a ceramic carrier. Three patients were lost to followup between their 1.5- and 3-month follow-up examinations and were excluded from the study.
Of the remaining 27 patients, there were 12 men (44%) and 15 women (56%) with an average age of 65 years (range, 41-87 years) in the study (Table 1).
Table 1: Clinical characteristics of 27 patients with more than 12 months of follow up.
| Variable | Value |
|---|---|
| Demographics | |
| Mean age (range) | 65 years (41-87) |
| Sex | |
| Female, n (%) | 15 (56) |
| Male, n (%) | 12 (44) |
| BMI, kg/m2 (range) | 29.4 (15.6-42.1) |
| Comorbidities | |
| Tobacco use, n (%) | 11 (41) |
| Diabetes, n (%) | 4 (15) |
| Osteoporosis, n (%) | 4 (15) |
| Diagnoses | |
| Stenosis, n (%) | 27 (100) |
| Spondylolisthesis, n (%) | 12 (44) |
| Degenerative disc disease, n (%) | 11 (44) |
| Adjacent segment degeneration, n (%) | 4 (15) |
BMI: Body Mass Index
Surgical technique
All patients had an open PLIF procedure; no out-patient surgeries were performed. We made a midline incision over the index level to expose the posterior spinal elements bilaterally and performed a unilateral or bilateral laminotomy. Next, the disc space was exposed and a discectomy was performed with removal of cartilaginous endplates and preservation of the bony endplates.
We used no autogenous bone grafts or local host bone reamings. Fifty-five to 60 mL of bone marrow was aspirated from the pelvis and processed to produce a concentration of stem cells. The bone marrow aspirate was concentrated in a centrifuge in the operating room using the FDA-approved ART BMC Plus device (Celling Biosciences, Austin, Texas, USA). The BMC was immediately applied to a ceramic HA carrier and packed into the disc space and the interbody implant. A single porous PEEK interbody implant was used in all patients (COALESCE, NuVasive, San Diego, California, USA) and was impacted obliquely into the prepared vertebral interspace. Pedicle screws were inserted bilaterally.
Clinical and radiographic follow-up
Patients were examined at 1.5, 3, 6, and 12 months. Functional outcomes were assessed by the Oswestry Disability Index questionnaire (ODI), which was used to measure the effects of back pain associated with activities of daily living [16]. Numeric rating scales were used to assess back and leg pain intensity. Patients rated both back and leg pain duration and intensity from 0 to 10 on visual analog scales, with a score of 0 representing "no pain" and a score of 10 representing "pain as bad as it could be." Neurological success was defined as maintenance or improvement in 3 objective clinical findings: sensory, motor, and reflex testing.
Neutral anteroposterior and lateral radiographs were obtained at each visit. Dynamic flexion-extension lateral radiographs were taken at 6 and 12 months. Sagittal plane angulation was measured on neutral lateral radiographs and determined by Cobb’s criteria. Intradiscal distraction and subsidence was measured by assessing the vertical distance between the midpoints of the adjacent vertebral endplates. Intradiscal motion and implant migration was assessed on the dynamic radiographs. CT scans were obtained in patients with persistent or unresolved clinical symptoms of back or leg pain. Fusion was defined as bridging bone connecting the adjacent vertebral bodies either through the implant or around the implant, less than 5° of angular motion, less than or equal to 3 mm of translation, and an absence of radiolucent lines around the implant.
Adverse events
All patients were monitored for the presence of adverse events during their procedure and during routine or unanticipated office visits. All complications were documented, including subsequent surgical interventions.
Results
The 27 patients in the study had a minimum follow up of 12 months.
Surgical data
Treated levels are shown in Table 2. Average length of surgery was 133 minutes (range, 110 – 156 minutes) and average blood loss was 310 mL (range, 190 – 475 mL). Average hospitalization was 2.8 days (range, 2 – 4 days).
Table 2: Disc levels treated in 27 patients.
| Level | N (%) | |||
|---|---|---|---|---|
| L2-L3 | 1 (4%) | |||
| L3-L4 | 6 (22%) | |||
| L4-L5 | 13 (48%) | |||
| L5-S1 | 7 (26% ) | |||
Clinical outcomes
Preoperative ODI scores averaged 50.8 points (Table 3). At last follow up examination, ODI scores averaged 27.1 points for a mean overall ODI score improvement of 23.7 points when compared with preoperative scores. After surgery, patients’ mean back and leg pain scores showed improvement from preoperative scores (Table 4). At last follow up, average leg pain improved to 3.3 from a preoperative average score of 7.2. Similarly, back pain showed improvement from a preoperative average of 7.4 to a postoperative average of 4.1 points at last follow up. Neurological success was seen in all study patients with no patient showing a loss in neurological functioning for the duration of the study.
Table 3: Oswestry Disability Index (ODI) scores over time.
| Follow up Interval | No. of patients | ODI Score (%) |
|---|---|---|
| Preoperative | 27 | 50.8 |
| 1.5 months | 27 | 38.1 |
| 3 months | 25 | 32.0 |
| 6 months | 23 | 30.0 |
| 12 months | 27 | 27.1 |
Table 4: Mean visual analog scale back and leg pain scores over time.
| Follow up Interval | No. of patients | Back Pain | Leg Pain |
|---|---|---|---|
| Preoperative | 27 | 7.4 | 7.2 |
| 1.5 months | 27* | 5.1 | 4.3 |
| 3 months | 25 | 4.3 | 3.9 |
| 6 months | 23 | 4.5 | 3.4 |
| 12 months | 27 | 4.1 | 3.3 |
Radiographic outcomes
All patients showed radiographic evidence of fusion at 6 months after surgery; no patient showed motion across the fused interspace on dynamic flexion-extension lateral radiographs. No patient had lucency around the implant. There was no evidence of implant migration or hardware failure on postoperative radiographs in any patient throughout the duration of the study.
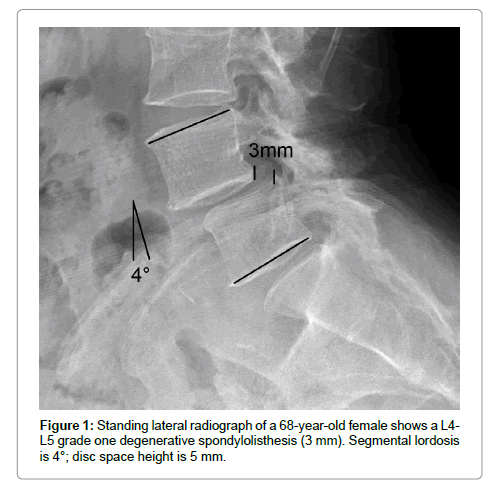
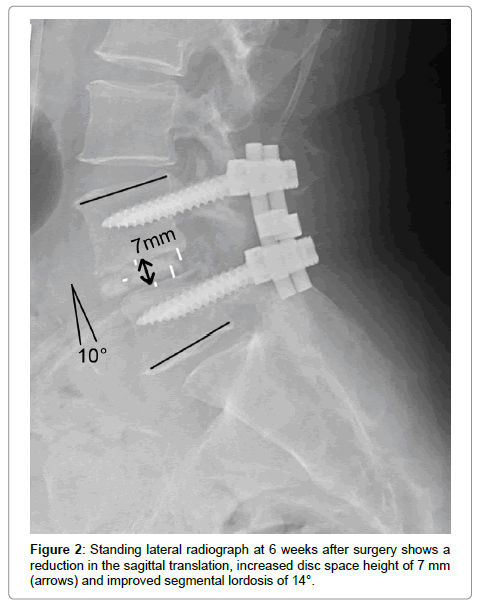
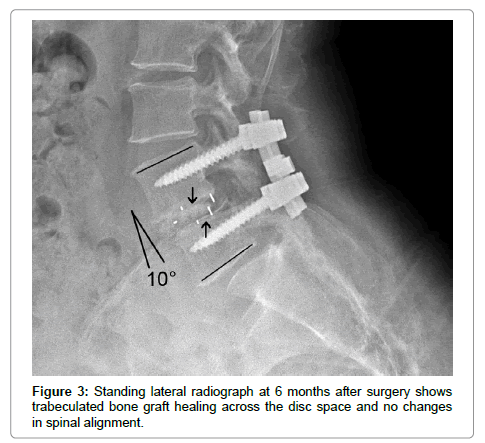
Postoperatively, the average disc space height was increased by 6 mm after surgery (Figures 1 and 2). The sagittal plane alignment was improved in all patients at surgery and was maintained at 12 months (Figure 3). The average preoperative sagittal plane angulation was -1° (lordosis) and ranged from +4° (kyphosis) to –3° (lordosis). Average sagittal plane angulation improved to an average of –7° (0° to –9° lordosis).
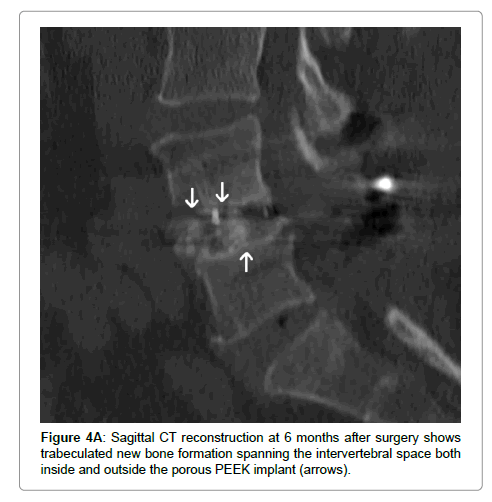
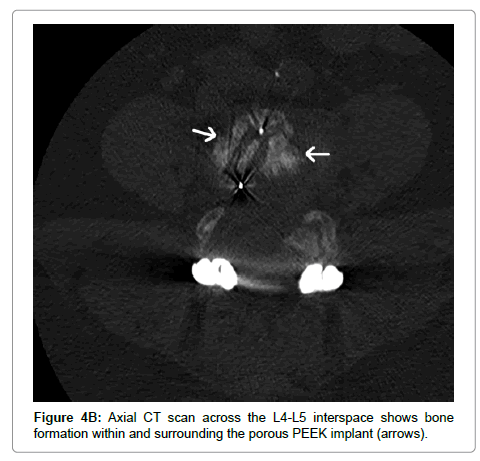
Computed tomography (CT) scans were performed in 8 patients at 6 months after surgery. All scans confirmed the presence of trabeculated new bone formation spanning the instrumented disc space (Figure 4).
Adverse events
No adverse events occurred at surgery or were identified during postoperative follow-up examinations. No patient underwent additional surgical procedures.
Discussion
There are a variety of different interbody device technologies available today. These implants can be compared in terms of their stiffness, structural integrity, and their imaging characteristics [4,17]. Importantly, they can also be evaluated for their osseointegration capability, the ability for bone to attach to the implant [1,2]. Surface topography plays a significant role in the osseointegration capabilities of an implant. Machined PEEK has been the market-leading material; however, it is lacking in its osseointegration capabilities [18]. Newer technologies, such as titanium-coated PEEK or roughened titanium, have made progress with improved osseointegration. Further differentiating surface technologies, such as nanocoated PEEK cages (Ti- and CaP-nanocoated polyetheretherketone cages), have been shown clinically to be safe and to improve rates of interbody fusion [19]. The use of nanocoated PEEK cages have been shown to have superior fusion rate when compared to uncoated PEEK cages. These improvements in fusion rates come at a cost to other features, such as imaging or structural integrity [20,21]. Porous PEEK retains the strength and durability of smooth PEEK. Importantly, it also maintains its porosity under physiologic loads and withstands impacted insertion testing whereas titanium-coated implants risk delamination.
Advanced interbody implants have surface architecture and microporosity that varies widely. The size of the surface microarchitecture can be an osteogenic factor [8]. Successful interbody fusion requires the addition of osteogenic cells or osteoinductive signals to form bone across the vertebral interspace. However, with an advanced biomaterial scaffold, no osteogenic cells or signals are needed to initiate boney ingrowth onto the surface of the implant [6,7]. The surface architecture has the power to initiate bone formation on its own.
In this study, the early integration of the porous implant to the adjacent vertebral endplates helped to stabilize the disc space and contributed to the high rates of fusion, preservation of disc space height, and sagittal contours. The porous PEEK implant surface technology promotes a superior osteogenic response and improves the osteointegration at the bone-implant interface [6-8]. The improved integration of the implant surface to the host bone facilitates fusion by stabilizing the vertebral motion segment and reducing the contact stresses at the graft-vertebral interface. Autogenous graft with its limitations and complications can be avoided. Bone marrow concentrate and a ceramic carrier can promote consistent fusions across the interspace. In addition, the deleterious effects of smoking, diabetes, osteoporosis, and obesity on rates of fusion may be overcome by utilizing this technique.
Conclusions
Our purpose was to assess the clinical outcomes and the bone healing response to a porous PEEK interbody implant used a single level PLIF surgery. Advanced surface technologies in PEEK implants have demonstrated the ability to initiate and support bone healing in interbody spinal fusions. Porous PEEK is designed for osseointegration while retaining the mechanical and radiographic properties of smooth PEEK. This unique porous architecture can lead to improved outcomes for patients through the ability to initiate and support bone healing in spinal fusions. Autogenous bone grafting and the morbidities and complications associated with this second surgery can be eliminated with this advanced interbody technology. Poroussurface PEEK is a clinically viable alternative for improving clinical outcomes and the osseointegration of interbody implants in patients undergoing single-level PLIF surgery.
References
- Hasegawa T, Ushirozako H, Shigeto E, Ohba T (2020) The titanium-coated peek cage maintains better bone fusion with the endplate than the PEEK cage 6 months after PLIF surgery-A multicenter, prospective, randomized study. Spine (Phila Pa 1976).
- Jiya TU, Smit T, van Royen BJ, Mullender M (2011) Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-L-lactide-co-D,L-lactide fusion devices. Clinical outcome at a minimum of 2-year follow-up. Eur Spine J 20:618-622.
- Kettler A, Wilke HJ, Dietl R, Krammer M, Lumenta C, et al. (2000) Stabilizing effect of posterior lumbar interbody fusion cages before and after cyclic loading. J Neurosurg 92:87-92.
- Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, et al. (1998) Interbody cage stabilization in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br 80:351-359.
- Evans NT, Torstrick FB, Lee CS, Dupont KM, Safranski DL, et al. (2015) High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater 13:159-167.
- Torstrick FB, Evans NT, Stevens HY, Gall K, Guldberg RE (2016) Do surface porosity and pore size influence mechanical properties and cellular response to PEEK? Clin Orthop Relat Res 474:2373-2383.
- Torstrick FB, Safranski DL, Burkus JK, Chappuis JL, Lee CSD (2017) Getting PEEK to stick to bone: the development of porous PEEK for interbody fusion devices. Tech Orthop 32:158-166.
- Carpenter RD, Klosterhoff BS, Torstrick FB, Foley KT, Burkus JK, et al. (2018) Effect of porous orthopaedic implant material and structure on load sharing with simulated bone ingrowth: a finite element analysis comparing titanium and PEEK. J Mech Behav Biomed Mater 80: 68-76.
- Johnson RG (2014) Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine (Phila Pa 1976) 39: 695-700.
- Gan Y, Dai K, Zhang P, Tang T, Zhu Z, et al. (2008) The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 29: 3973-3982.
- Galois L, Bensoussan D, Diligent J, Pinzano A, Henrionnet C, et al. (2009) Autologous bone marrow graft and treatment of delayed and non-unions of long bones: technical aspects. Biomed Mater Eng 19: 277-281.
- Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. J Bone Joint Surg 87: 1403-1437.
- Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 405: 14-23.
- Burkus JK (2018) Early outcomes of anterior cervical discectomy and fusion using a porous PEEK interbody fusion device. J Spine Neurosurg 7:2.
- Burkus JK, Rehak C (2019) Anterior cervical discectomy and fusion using porous PEEK Implants at levels adjacent to a previous fusion. J Spine Neurosurg 8:3.
- Fairbank JC, Couper J, Davies JB, O'Brien JP (1980) The Oswestry low back pain disability questionnaire. Physiotherapy 66: 271-273.
- Rapoff AJ, Ghanayem AJ, Zdeblick TA 1997 Biomechanical comparison of posterior lumbar interbody fusion cages. Spine (Phila Pa 1976) 22:2375-2379.
- Elifky TA, Patil ND, Allam Y, Ragab R (2020) Endplate changes with polyetheretherketone cages in posterior lumbar interbody fusion. Asian Spine J 14:229-237.
- Willems K, Lauweryns P, Verleye G, VAN Goethem JA (2019) Randomized controlled trial of posterior lumbar interbody fusion with Ti- and CaP-nanocoated polyetheretherketone cages: comparative study of the 1-year radiological and clinical outcome. Int J Spine Surg 13:575–587.
- Lebhar J, Kriegel P, Guillin R, Chatellier P, Ropars M, et al (2020) Role of MRI in the assessment of interbody fusion with tantalum intervertebral implant. Orthop Traumatol Surg Res 106:285-289.
- Seo DK, Kim MJ, Roh, SW, Jeon SR (2017) Morphological analysis of interbody fusion following posterior lumbar interbody fusion with cages using computed tomography. Medicine (Baltimore) 96(34):e781618.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi