Case Report, J Liver Disease Transplant Vol: 13 Issue: 1
Primary Hepatic Amyloidosis Associated with Chronic Hepatitis C Infection: A Case Study
Pratim Sengupta1*, Tapas Roy1, Atreyee Chaudhuri2 and Kavita Rathore3
1Department of Nephrology, ILS Hospital, West Bengal, India
2Nephrocare India Private Limited, West Bengal, India
3Ananta Institute of Medical Sciences and Research Centre, Rajasthan University of Health Science, Rajasthan, India
*Corresponding Author: Pratim Sengupta,
Department of Nephrology, ILS
Hospital, West Bengal, India
E-mail: pratim.sengupta@gmail.com
Received date: 22 January, 2024, Manuscript No. JLDT-24-125604;
Editor assigned date: 24 January, 2024, Pre QC No. JLDT-24-125604 (PQ);
Reviewed date: 07 February, 2024, QC No. JLDT-24-125604;
Revised date: 14 February, 2024, Manuscript No. JLDT-24-125604 (R);
Published date: 21 February, 2024, DOI: 10.4172/2325-9612.1000252
Citation: Sengupta P, Roy T, Chaudhuri A and Rathore K (2024) Primary Hepatic Amyloidosis Associated with Chronic Hepatitis C Infection: A Case Study. J
Liver Disease Transplant 13:1.
Abstract
Primary hepatic amyloidosis is an infrequent condition marked by the accumulation of amyloid protein within the liver. This case study presents a case of a 38-year-old male with chronic hepatitis C infection who presented with abdominal discomfort and hepatomegaly. Liver biopsy revealed amyloid deposits within the liver parenchyma, and Congo red staining confirmed the diagnosis of hepatic amyloidosis. The patient received antiviral treatment for hepatitis C along with additional supportive therapy, leading to an improvement in their liver enzyme levels. This case underscores the significance of including hepatic amyloidosis in the differential diagnosis for individuals with chronic hepatitis C, especially when presenting with hepatomegaly and elevated liver enzymes. The timely recognition and efficient handling are essential to avoid further harm to the liver and improve the outcomes for patients. Additional research is required to deepen our understanding of the connection between hepatitis C infection and hepatic amyloidosis.
Keywords
Hepatitis C; Genotype 1a; Primary hepatic amyloidosis; Chronic kidney disease; Hemodialysis
Introduction
A pathological process in which the fibrillar amyloid proteins that are resistant to catabolism, progressively accumulate in the extracellular and vessel walls and subsequently impact any organ individually or in tandem with other organs such as kidney, liver, spleen, heart, and gastrointestinal tract, is known as amyloidosis [1-3]. This process can manifest as an infiltrative process or as a focal lesion that resembles a tumor [4]. Systemic amyloidosis comprises two types, primary and secondary, characterized by the accumulation of serum amyloid A, immunoglobulin light chain, apolipoprotein A1, and transthyretin types of fibrils, within various organs [5] Among these, the deposition of immunoglobulin light chains including kappa or lambda chains in the target organ is the main pathogenic mechanism for primary amyloidosis [5]. On the other hand, chronic infections (Hepatitis B and C, tuberculosis, osteomyelitis, and bronchiectasis), immunodeficiency disorders (rheumatoid arthritis, hidradenitis suppurativa, and inflammatory bowel disease, and hereditary conditions like familial Mediterranean fever, cryopyrin-associated periodic syndrome) and neoplastic syndromes (tumour necrosis) are the major causes of secondary amyloidosis and are associated with serum amyloid amyloidosis [6]. Hepatic amyloidosis represents a rare occurrence of systemic amyloidosis affecting the liver leading to hepatomegaly in 33%-92% of patients accompanied by liver dysfunction, moderate to severe cholestasis, and, in severe cases, liver failure and mortality [7,8]. In addition to inducing liver diseases, Hepatitis C virus is linked to extrahepatic disorders [9], although very few literatures have reported cases of hepatitis C and serum amyloid amyloidosis [10-12]. In this case study, we present an uncommon instance of hepatitis C infection associated hepatic amyloidosis in a 38 year old male.
Case Presentation
A 38 year old male presented with bilateral lower limb weakness with difficulty walking, generalized weakness, low back pain, tingling sensation all over the body with intermittent episodes of persistent, nagging ache in the epigastrium and right hypochondriac region, accompanied by watery diarrhoea for the last 2 days. He had no prior occurrences of jaundice, blood vomiting and abdominal swelling, changes in bowel habits, upper gastrointestinal bleeding, or bleeding from any site. He was a known case of hypertension, type 2 diabetes, hypothyroidism, and chronic kidney disease on maintenance haemodialysis at thrice per week. He did not skip dialysis and was regularly taking it. He was hepatitis C positive earlier, and hepatitis C virus RNA was 96200 IU ml-1 a month ago, with the 1a genotype, and was on Sofosbuvir (300 mg) plus velpatasvir (90 mg) combination therapy Once Daily (OD). Although he had experienced acute viral hepatitis twice in the distant past, there was no record of joint pain, dermatological irritation, skin rigidity, mouth sores or ulcers, cough, or tingling in extremities. There was no history of tuberculosis, bronchiectasis, or chronic osteomyelitis and cancer in the past.
Methods
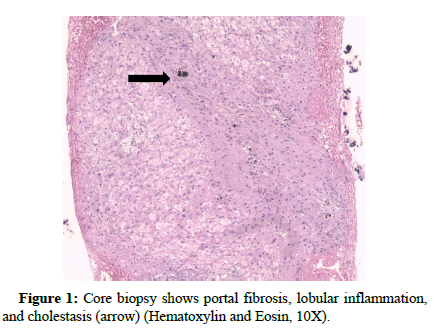
During clinical assessment in the emergency room, the patient was found to be conscious and fully oriented to time, place, and person, and exhibited no focal sensory or motor impairments. There were no signs of cyanosis or clubbing observed. Chest auscultation revealed bilateral basal scattered crepitation more on the left than the right side. Except for a mild tenderness in the right hypochondrium, his abdomen was soft and palpable with no other notable lumps or lymph nodes. His heart rate, respiratory rate, blood pressure, SPO2, and random blood glucose were 84 min-1, 20 min-1, 150/80 mm of Hg, and 92% in room air and 496 mg dl-1 respectively. After admission his initial blood biochemistry revealed his haemoglobin: 11.8 g l-1, total leucocyte count: 11100 cells μL-1, serum creatinine: 4 mg dl-1, urea: 57 mg dl-1, uric acid: 4.5 mg dl-1, calcium: 8.6 mg dl-1, sodium: 139 mmol L-1, potassium: 5.7 mmol L-1, phosphorous: 5.5 mg dl-1. His liver enzymes were elevated; Alanine transaminase (SGPT): 107 U L-1, Aspartate aminotransferase (SGOT): 193 U L-1, and Gamma- Glutamyl Transferase (GGT): 3966 U L-1, total bilirubin: 3.5 mg dl-1, direct bilirubin: 3.3 mg dl-1, and indirect bilirubin: 0.2 mg dl-1. Except for a slight rise in erythrocyte sedimentation rate, C3 and C4 were within the normal range. His blood clotting profile was normal with an international normalized ratio of 1.02. Rheumatoid factor and cryoglobulins were negative. C reactive protein was 157 mg L-1. The results indicated negative results for Human Immunodeficiency Virus (HIV) antibodies, hepatitis B surface antigen, and autoimmune markers, including anti-smooth muscle antibodies, anti-nuclear antibodies, and anti-Liver Kidney Microsome type 1 antibodies. Abdominal ultrasound revealed a liver of normal size (13.4 cm) and a portal vein diameter within the normal range. The portal vein Doppler examination revealed no anomalies in blood circulation, and there were no observed additional irregularities. No thrombus was observed in the inferior vena cava or the hepatic vein. His direct Coombs test also yielded a negative result. Prophylactic antibiotic coverage with Piperacillin and Tazobactum (intra venous injection with 4.5 gms loading dose and 2.25 gms maintenance dose three times a day was initiated along with other supportive therapy for managing diabetes and hypertension. He complained of shortness of breath on the next day and urgent dialysis was done with expanded hemodialyzer-theranova. He complained of discomfort in the right upper abdominal region. A follow-up liver biochemistry showed total bilirubin, direct bilirubin, and indirect bilirubin were 4 mg dl-1, 3.9 mg dl-1, and 0.1 mg dl-1 respectively. SGOT was 152 U L-1, SGPT was 93 U L-1, GGT was 3166 U L-1 and alkaline phosphatase was 2100 U L-1. Serum samples for the detection of IgM antibodies to Hepatitis A and E Viruses (HAV IgM and HEV IgM) and Leptospira were done and reported negative. On day 5 of admission, a repeat liver biochemistry showed further elevated liver enzymes, SGOT: 203 U L-1, SGPT: 109 U L-1, GGT: 2622 U L-1, and alkaline phosphatase: 1797 IU L-1, The total bilirubin level was 4.4 mg dL-1, with direct bilirubin at 4.2 mg dL-1 and indirect bilirubin at 0.2 mg dL-1. He continued to have pain in the right hypochondrium. A gastroenterologist opinion was sought who opined to do a Magnetic resonance cholangiopancreatograph and findings showed enlarged liver (15.8 cm) with no obvious focal lesion. There was minimal perihepatic fluid. Itrahepatic biliary duct was not dilated. Confluence of both ducts visualized at porta with the normal cystic duct forming the common bile duct. The gallbladder showed partial distension without any signs of calculi. Common bile duct was normal in calibre with no stricture. The pancreas exhibited ordinary size and shape, and the pancreatic duct demonstrated a normal course and caliber, without any signs of stricture. His follow-up coagulation profile came deranged with an international normalized ratio of 1.97. Vitamin K 30 mg stat and 10 mg intravenous once daily was advised. On day 9th his liver enzymes markedly increased with total bilirubin: 8.5 mg dL-1, direct bilirubin: 7.2 mg dL-1 and in direct bilirubin: 1.3 mg d L-1 , Alkaline phosphatase: 1723 IU L-1, SGOT: 141 U L-1, SGPT: 49 U L-1, GGT: 1743.7 U L-1. Serum ammonia came in 93.8 μmol L-1 and Rifaximin (550 mg Tablet BD (bis in die)) was added. In view of persistent pain in right upper abdomen, elevated liver enzymes with normal cholangiography a liver biopsy was advised to understand the exact etiology. Ultrasound-guided liver biopsy was performed. Liver biopsy in multiple linear cores showed porto-portal fibrosis with the formation of incomplete nodules as highlighted by MT stain (Figure 1).
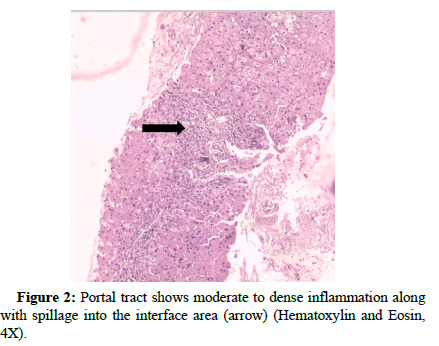
Examinations were conducted concerning both interface hepatitis and lobular inflammation (Figure 2).
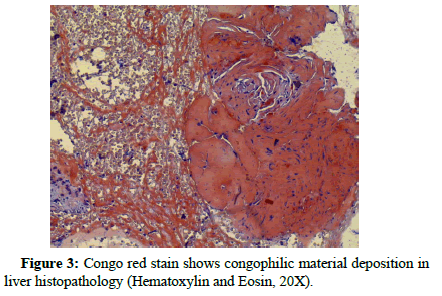
Polymorph and lymphocyte infiltration is noted around the hepatocyte at several foci. The modified Hepatic Activity Index (m- HAI) and fibrosis staging were (5/18) and (3/6) respectively. One area of core biopsy showed amyloid deposition within hepatic lobules that were positive for congo red stain (Figure 3).
His electrocardiogram was normal, an echocardiogram revealed a left ventricular ejection fraction of 47%, concentric left ventricular hypertrophy, generalized wall hypokinesia, and enlarged left atrium. Liver immunohistochemistry with anti-kappa and anti-lambda antibodies was omitted, given the normal results of the serum free light chain assay and serum protein immunofixation electrophoresis. To rule out any other foci for amyloid deposition bone marrow biopsy was done but did not confirm any amyloid deposit. The kidney biopsy could not be done because of the already shrunken-sized kidney. Beta 2 microglobulin was done and the report was normal (0.7 mcg ml-1). He was given colchicine (0.5 mg tablet BD) per day and another supportive therapy was given to the patients with thrice per week maintenance haemodialysis. After ten days, liver enzymes started to decline slowly, the pain subsided and he became hemodynamically stable. He was discharged on request in a hemodynamic steady condition and asked to follow up every 15 days at the outpatient clinic. His liver biochemistry report including the follow-up visits is summarized in Table 1.
| Day 1 | Day 2 | Day 5 | Day 7 | Day 9 | Day 14 | Day 21 | |
|---|---|---|---|---|---|---|---|
| Aspartate transaminase SGPT (U L-1) | 107 | 93 | 109 | 98 | 49 | 32 | 13 |
| Aspartate aminotransferase SGOT (U L-1) | 193 | 152 | 203 | 173 | 141 | 144 | 106 |
| Total Bilirubin (mg dL-1) | 3.5 | 4 | 4.4 | 7 | 8.5 | 8.6 | 12.7 |
| Direct Bilirubin (mg dL-1) | 3.3 | 3.9 | 4.2 | 6.8 | 7.2 | 7.5 | 10.7 |
| Indirect Bilirubin (mg dL-1) | 0.2 | 0.1 | 0.2 | 0.2 | 1.3 | 1.1 | 2 |
| GGT (U L-1) | 3966 | 3166 | 2622 | 2474 | 1744 | 1594 | 874 |
| Alkaline Phosphatase (IU L-1) | - | 2100 | 1797 | - | 1723 | - | - |
Table 1: Periodic records of liver function test
Results and Discussion
While amyloidosis is commonly perceived as a systemic disease, 10%-20% of cases may exhibit localized manifestations [1]. The involvement of the liver in amyloidosis is a rare complication that can occur in both primary and secondary forms. However, according to the previous literature the clinical appearance of hepatic amyloidosis spans from mild cases with marginal abnormal functioning of liver and hepatomegaly to more severe forms leading to portal vein hypertension, liver failure, and, in rare instances, spontaneous rupture [2]. In this particular case, the patient poses a diagnostic dilemma similar to the scenario described by Sonthalia et al., [3]. The manifestation of tenderness in the right hypochondrium, elevated liver enzymes, and exclusive lower limb weakness was observed in the patient who was undergoing maintenance hemodialysis. The patient was previously hepatitis C positive and on anti-HCV therapy. However, his liver enzymes continued to rise and no distinct indication was uncovered during the radiological assessment. The observation was in correlation with the study by Wang et al., where he stated that diagnosis of amyloidosis can pose challenges due to its symptoms, which may closely resemble those of various other conditions [4]. He also reported that in individuals presenting with hepatomegaly but no concurrent heart failure, elevated serum alkaline phosphatase without abnormal imaging findings should raise suspicion of hepatic amyloidosis. In addition to this, Wang et al., referred to congo red staining of the amyloid fibrils as ‘the gold standard’ for diagnosing amyloidosis [4]. The deposits in hepatic amyloidosis are typically found in the liver parenchyma and may cause atrophy of hepatocytes, resulting in a fatty liver appearance upon sectioning [1]. In our study also, liver biopsy revealed amyloidosis evident by Congo red positive staining. Katzmann et al., referred to the significance of serum and urine immunofixation electrophoresis as the most extensive and encompassing tool to screen monoclonal gammopathies [5]. Amyloid deposits may also be observed in a bone marrow biopsy [3,6]. Consequently, in order to exclude the possibility of amyloid deposition in other systemic organs, a bone marrow analysis was conducted, along with immune-fixation studies on blood and urine; however, all outcomes fell within the normal spectrum. No involvement was observed in the cardiac, renal, or nervous systems and no evidence of tuberculosis, rheumatoid arthritis, Systemic Lupus Erythematosus (SLE), Crohn’s disease, or evidence of other common inflammatory diseases. Patients with hepatic amyloidosis face a grim prognosis, with a median survival of 10-14 months from the time of diagnosis [7] and an average of 8-9 months if remain untreated [4,8]. Dialysis-related amyloidosis involving beta 2 microglobulin deposition and autosomal dominant systemic amyloidosis like familial amyloidotic polyneuropathy including genetically variant transthyretin deposition are the other less common forms besides primary and secondary amyloidosis [1,9]. Beta 2 microglobulin was found to be normal in our patient. This case underscores the necessity for a heightened degree of vigilance in diagnosing isolated hepatomegaly attributed to amyloidosis. Son et al., and Ghazanfar et al., clarified the hepatic involvement in amyloidosis with liver Space Occupying Lesions (SOL) in the setting of plasma cell disorder and with primary hepatic amyloidosis respectively [10,11].
Our case study is thus the first case presenting chronic hepatitis C virus-induced primary amyloidosis. There is no specific treatment for primary hepatic amyloidosis, but liver transplantation is the only option. The patient was given colchicine (0.5 mg BD) as it is evident in literature that it prevents febrile attacks, and long-term use can arrest the progression of primary amyloidosis [12]. The individual received conservative care, including supportive medications and undergoing maintenance hemodialysis three times a week. Subsequently, they were discharged in a hemodynamically stable state.
Conclusion
In summary, our case underscores the uncommon manifestation of primary hepatic amyloidosis as a complication of chronic hepatitis C infection. The present case report highlights the significance of considering hepatic amyloidosis as a potential complication in individuals with prolonged hepatitis C infection who presented with elevated liver enzymes in addition to hepatomegaly. Our patient's clinical presentation was marked by hepatic dysfunction, conclusively attributed to the deposition of amyloid proteins in the liver. Diagnosing hepatic amyloidosis demands a keen suspicion, and liver biopsy with congo red staining remains the benchmark for diagnosis. While the treatment options for hepatic amyloidosis in the context of chronic kidney disease are limited. Early diagnosis and appropriate management can help to prevent the progression of the disease and improve patient outcomes. Additional research is required to gain a deeper understanding of the pathophysiology and identify optimal management strategies for this uncommon complication associated with chronic hepatitis C infection.
References
- Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D (2016) Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol 8(6): 307-321.
- Urban BA, Fishman EK, Goldman SM, Scott WW, Jones B, et al. (1993) CT evaluation of amyloidosis: spectrum of disease. Radiographics 13: 1295-1308.
- Sonthalia N, Jain S, Pawar S, Zanwar V, Surude R, et al. (2016) Primary hepatic amyloidosis: A case report and review of literature. World J Hepatol 8(6): 340-344.
- Wang YD, Zhao CY, Yin HZ (2015) Primary hepatic amyloidosis: A mini literature review and five cases report. Ann of hepatol 11(5): 721-727.
- Katzmann JA, Kyle RA, Benson J, Larson DR, Snyder MR, et al. (2009) Screening Panels for Detection of Monoclonal Gammopathies. Clin Chem 55 (8): 1517–1522.
- Leung N, Nasr SH, Sethi S. (2012) How I treat amyloidosis: the importance of accurate diagnosis and amyloid typing. Blood 20(16): 3206-3213.
- Park MA, Mueller PS, Kyle RA, Larson DR, Plevak MF, et al. (2003) Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Medicine (Baltimore) 82: 291-298.
- Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, et al. (2011) Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc 86: 12-18.
- Sarkar C, Sharma MC, Nayak A, Ralte AM, Gupta V, et al. (2005) Primary AL (kappa-light chain) amyloidosis manifesting as peripheral neuropathy in a young male without increase in serum and urine immunoglobulin load: a diagnostic challenge. Clin Neuropathol 24: 118-125.
- Son RC, Chang JC, Choi JH (2011) Primary hepatic amyloidosis: Report of an unusual case presenting as a mass. Korean J Radiol 12: 382-385.
- Ghazanfar H, Khaja M, Haider A, Yapor L, Kandhi S, et al. (2022) Hepatic Amyloidosis as a Rare Cause of Liver Failure: A Case Report. Cureus 14(7): e27274.
- Leung YY, Yao Hui LL, Kraus VB (2015) Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 45(3): 341-350.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi