Research Article, J Clin Exp Oncol Vol: 7 Issue: 5
Prognostic Factors for Survival in Patients with Hepatocellular Carcinoma Treated By Transarterial Chemoembolization Using Drug-Eluting Beads
Levasicc N1, Lesnik LA1, Garbajs M2, Dezman R2 and Popovic P2*
1Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
2Clinical Institute of Radiology, University Medical Centre, Ljubljana, Slovenia
*Corresponding Author : Popovic P
Clinical Institute of Radiology, University Medical Centre Ljubljana, Zaloska cesta, Ljubljana, Slovenia
Tel: +38615223415
E-mail: peter.popovic@kclj.si
Received: August 24, 2018 Accepted: September 12, 2018 Published: September 19, 2018
Citation: Levasicc N, Lesnik LA, Garbajs M, Dezman R, Popovic P (2018) Prognostic Factors for Survival in Patients with Hepatocellular Carcinoma Treated By Transarterial Chemoembolization Using Drug-Eluting Beads. J Clin Exp Oncol 7:5. doi: 10.4172/2324-9110.1000230
Abstract
Objective: Transarterial chemoembolization (TACE) is the most widely used therapeutic option in the treatment of intermediate hepatocellular carcinoma (HCC). Due to the heterogeneity of the intermediate stage patient population, survival rates are variable. The purpose of this retrospective study was to investigate prognostic factors in predicting overall survival in patients with HCC treated by transarterial chemoembolization using drug-eluting beads loaded with doxorubicin (DEBDOX TACE).
Methods: 119 patients with intermediate stage HCC who had undergone DEBDOX TACE between February 2010 and January 2017 were studied. All procedures were performed under Cone Beam Computed Tomography (CBCT) control. Survival was calculated from the date of the first procedure. The survival rates and curves were calculated using the Kaplan-Meier method. Survival curves were compared using the log-rank test.
Results: Overall, 362 procedures were performed (mean: 3.04 per patient). After a mean follow up of 24.5 ± 1.3 months, 83 patients had died and 36 survived. The average survival was 30.7 months ± 2.3 months (95% CI: 26.3-35.2 months). Median survival was 24.7 months. One-year, two-year and five-year survival rates were 84%, 47% and 3%, respectively. Independent survival prognostic factors were Child-Pugh class ,B (p=0.009), ascites (p=0.019), portal hypertension (p=0.024), bilirubin increase after procedure (p=0.002) and number of procedures (p=0.022).
Conclusion: The presence of ascites, portal hypertension, Child-Pugh class B, Child-Pugh score, a higher increase in bilirubin levels after the procedure and a lower number of DEBDOX TACE procedures are significant prognostic factors in overall survival.
Keywords: Hepatocellular carcinoma; Transarterial chemoembolization; Drug-eluting beads; Survival
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the second most common cause of cancerrelated death in the world [1,2]. In more than 80% of cases, HCC occurs in the setting of cirrhosis [1,2]. The prognosis is therefore determined not only by factors related to the tumor but also by factors related to cirrhosis. Survival is highly dependent on the stage of the disease. Early stage patients can be cured, but this group represents only 20-30% of all patients [3,4]. Curative methods are surgical resection, liver transplantation and radiofrequency ablation (RFA) [4-6]. However, because of its insidious nature, most HCC patients are diagnosed at intermediate or advanced stages.
Transarterial chemoembolization (TACE) is the therapeutic option most frequently used in the treatment of intermediate stage hepatocellular carcinoma (HCC) according to Barcelona Clinic Liver Cancer (BCLC) [1,5]. As classified, these are patients with multifocal disease without portal invasion and extrahepatic disease, with liver cirrhosis Child A or B and with a good performance status (1,2). Due to the heterogeneity of the intermediate stage patient population, survival rates are variable and scattered across the literature [3]. Therefore, not all patients with intermediate stage HCC will derive similar benefit from TACE. Some may benefit from other treatment options that are currently approved or being explored. These include different TACE modalities, such as conventional TACE, doxorubicineluting bead TACE (DEB-TACE), transarterial radioembolization (TARE), combined approaches with radiofrequency ablation (RFA) or sorafenib [4,7-11].
Current prognostic factors for determining survival in patients treated with TACE, such as clinical performance, patient status, number and size of tumors, presence of macrovascular invasion, extrahepatic spread and grade of hepatic damage, are mainly based on clinical assessment and are included in the BCLC classification [1]. Additional prognostic factors and indexes are therefore required for choosing the appropriate patient profile for the first intervention, as well as for subsequent treatments with TACE [12-15].
The purpose of this retrospective study was to investigate prognostic factors in predicting overall survival in patients with HCC treated with drug-eluting beads loaded with doxorubicin transarterial chemoembolization (DEBDOX TACE).
Materials and Methods
Patient selection
This single institution retrospective study included 119 patients (101 men and 18 women, mean age 67.3 ± 8.1 years) with intermediate stage HCC that were treated with DEBDOX TACE between February 2010 and January 2017. Clinical examination, laboratory evaluation and CT and/or MR imaging were performed in each patient at baseline at least one month before the TACE session. Treatment with chemoembolization was based on consensus at a Liver Multidisciplinary Team Meeting. The inclusion criteria were: intermediate stage HCC patients treated with DEBDOX-TACE; Child- Pugh score A or B (up to 8 points). Exclusion criteria were: liver transplantation combined HCC and cholangiocarcinoma, BCLC stage C disease and metastatic disease. Written informed consent of patients was obtained before the treatments.
The study was performed in accordance with the Helsinki Declaration ethical standards for biomedical studies on human beings, on the basis of patient charts held at the Clinical Department of Gastroenterology and Clinical Institute of Radiology, University Medical Centre Ljubljana. It was approved by the Republic of Slovenia National Medical Ethics Committee on the 18th of April 2017 (decision number 60/04/17).
Procedure
Patients were treated with one or more procedures performed in local anesthesia. DEBDOX TACE was performed with a super-selective catheterization of the tumor-feeding artery, followed by embolization with DC Beads (DEBs) (DC Bead®, Terumo Europe N.V, Belgium) with a diameter of 75-150, 100-300 and 300-500 μm or Tandem (Tandem®, Boston Scientific, Marlborough, Massachusetts) with a diameter of 100μm, loaded with 50-150 mg of doxorubicin. All embolizations were carried out under Cone Beam Computed Tomography (CBCT) control. Patients were tracked with a liver CT or MR after treatment, and the response to treatment was determined based on an assessment of the vitality of the lesions according to mRECIST criteria. A 64- and 16-cut multidetector CT (Siemens Medical Systems®, Erlangen, Germany) and a 3T MAGNETOM Trio, A Tim System MR scanner (Siemens Medical Systems®, Erlangen, Germany) were used.
Statistical analysis
Statistical analysis was performed using IBM® SPSS® Statistics 24 (International Business Machines Corp., Armonk, NY) for Windows. To determine the survival rates and curves based on the values of predictive factors, each variable was divided into 2 or up to 4 categories.
The survival rates and curves were calculated using univariate analysis using the Kaplan-Meier method. Differences were determined with a log-rank test. The following factors were chosen and analyzed: the presence and etiology of cirrhosis, presence of ascites, presence of portal hypertension, class and number of points on the Child-Pugh scale, location, number and size of lesions in the liver, increase in bilirubin after treatment (difference in bilirubin values before and after treatment), difference in liver aminotransferase (AST, ALT) values before and after treatment, AFP value, amount of chemotherapeutic (doxorubicin), type and size of chemoembolizing particles and the number of DEBDOX TACE procedures per patient.
All prognostic factors statistically significant in the univariate analysis were included in the Cox proportional hazards model to detect independent predictive survival factors.
The limit of statistical significance was set at p<0.05. Continuous variables were expressed as means ± standard deviation and categorical variables as frequencies and percentages.
Results
Patient characteristics
The baseline demographic, clinical, laboratory and imaging characteristics of the patients included in the analysis are summarized in Table 1. The AFP range among the studied patients was 1.0-12,809.8 kIE/l. Fifty-nine out of 83 patients had an elevated AFP level (>6.2 kIE/l). The other 36 patients had no data on their AFP level.
| Gender, n. (%) | |
|---|---|
| M | 101 (84.9) |
| F | 18 (15.1) |
| Age, years | |
| Median | 67.7 |
| Mean ± SD | 67.3 ± 8.1 |
| Imaging characteristics | |
| Bilobar, n. (%) | 44 (37) |
| Unilobar, n. (%) | 75 (63) |
| Right lobe, n. (%) | 61 (81.3) |
| Left lobe, n. (%) | 14 (18.7) |
| Number of lesions per patient, avg. ± SD | 3.2 ± 2.8 |
| Portal vein thrombosis, n. (%) | |
| Yes | 6 (5.0) |
| No | 99 (83.2) |
| No data | 14 (11.8) |
| Portal hypertension, n. (%) | |
| Yes | 54 (45.4) |
| No | 35 (29.4) |
| No data | 30 (25.2) |
| Cirrhosis, n. (%) | |
| Yes | 99 (83.2) |
| No | 17 (14.3) |
| No data | 3 (2.5) |
| Cirrhosis etiology, n. (%) | |
| Ethanol | 52 (52.5) |
| HBV | 13 (13.2) |
| HCV | 11 (11.1) |
| Primary biliary cirrhosis | 3 (3.0) |
| Other | 20 (20.2) |
| Child-Pugh score (av. points ± SD) | 5.8 ± 1 |
| Child-Pugh score (classes) | |
| A, n. (%) | 74 (62.2) |
| B, n. (%) | 27 (22.7) |
| No data | 18 (15.1) |
| Laboratory characteristics | |
| Albumin, [g/l] ± SD | 38.7 ± 4.9 |
| INR, ± SD | 1.19 ± 1.65 |
| Total bilirubin, [μmol/l] ± SD | 22.8 ± 14.2 |
| Creatinine, [μmol/l] ± SD | 78.14 ± 23.16 |
| AST, [μkat/l] ± SD | 1.31 ± 4.19 |
| ALT, [μkat/l] ± SD | 0.74 ± 0.57 |
| γGT, [μkat/l] ± SD | 2.39 ± 2.72 |
| AFP, [kIE/l] ± SD | 765.2 ± 2,381.5 |
| AFP, median [kIE/l] | 17 |
Table 1: Patient characteristics at baseline. SD: Standard Deviation; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; INR: International Normalized Ratio; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; γGT: Gamma Glutamyl Transpeptidase; AFP: Alpha Fetoprotein.
Procedure
Overall, 362 DEBDOX TACE procedures were performed in 119 patients. The mean number of procedures per patient was 3.04, in the range of 1 to 8 procedures.
Clinical prognostic factors and survival
Patients were followed on average for 24.5 ± 1.3 months. During this time, 83 patients died and 36 survived. The average survival was 30.7 months ± 2.3 months (95% CI: 26.3-35.2 months). Median survival was 24.7 months. One-year, two-year and five-year survival rates were 84%, 47% and 3%, respectively.
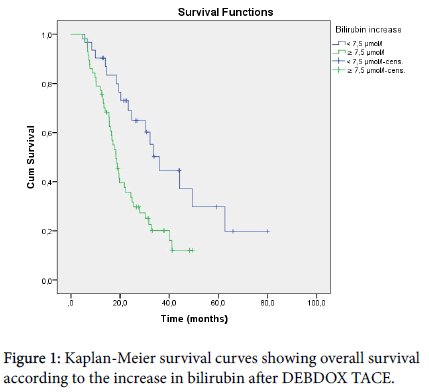
On univariate analysis (Table 2), ascites, portal hypertension, Child- Pugh score class and points, bilirubin increase after procedure (Figure 1) and number of DEBDOX TACE performed, showed statistically significant differences in overall survival.
| Factor | N. | Median survival | 95% CI | p value |
|---|---|---|---|---|
| Cirrhosis | 99 | 24.8 | 20.9-28.8 | 0.638 |
| No cirrhosis | 17 | 21.9 | 11.2-32.5 | |
| Alcoholic cirrhosis | 52 | 26.3 | 18.8-33.9 | 0.266 |
| HBV cirrhosis | 13 | 18.4 | 11.2-25.6 | |
| HCV cirrhosis | 11 | 40.8 | 25.9-55.7 | |
| Primary biliary cirrhosis | 3 | 18.7 | 0-37.5 | |
| Other | 20 | 19.6 | 13.7-25.4 | |
| Ascites | 28 | 16.7 | 12.4-21 | 0.019 |
| No ascites | 81 | 26.6 | 19.2-34 | |
| Portal hypertension | 54 | 19.5 | 16.3-22.7 | 0.024 |
| No portal hypertension | 35 | 32.4 | 27.3-37.5 | |
| Child-Pugh A | 74 | 31.7 | 21.1-42.2 | 0.009 |
| Child-Pugh B | 27 | 19.5 | 15.3-23.7 | |
| Child-Pugh 5 pt. | 52 | 31.7 | 18.7-44.6 | <0.0001 |
| Child-Pugh 6 pt. | 22 | 22.5 | 11.0-34 | |
| Child-Pugh 7 pt. | 20 | 19.7 | 19.2-20.2 | |
| Child-Pugh 8 pt. | 7 | 13 | 5.2-20.9 | |
| BCLC A | 31 | 22.2 | 13.1-31.3 | 0.665 |
| BCLC B | 88 | 24.7 | 20.3-29.1 | |
| Unilobar tumor | 75 | 24.3 | 19.2-29.5 | 0.547 |
| Bilobar tumor | 44 | 24.7 | 15.9-33.6 | |
| Unifocal tumor | 35 | 30.3 | 20.8-39.8 | 0.16 |
| Multifocal tumor | 81 | 23.3 | 16.3-30.3 | |
| Tumor size<3.95 cm | 58 | 24.8 | 14.3-35.4 | 0.43 |
| Tumor size ≥ 3.95 cm | 54 | 26.1 | 20.5-31.8 | |
| Bilirubin increase<7.5 μmol/l | 31 | 36 | 28.6-43.3 | 0.002 |
| Bilirubin increase ≥ 7.5 μmol/l | 57 | 18.4 | 15.6-21.1 | |
| AST increase<0.505 μkat/l | 33 | 32.7 | 22.9-42.5 | 0.29 |
| AST increase ≥ 0.505 μkat/l | 57 | 19.7 | 16-23.3 | |
| ALT increase<0.255 μkat/l | 39 | 24.3 | 13.3-35.4 | 0.868 |
| ALT increase ≥ 0.255 μkat/l | 51 | 19.7 | 13.5-25.8 | |
| AFP level<60.85 kIE/l | 52 | 24.8 | 19.4-30.3 | 0.176 |
| AFP level ≥ 60.85 kIE/l | 31 | 19 | 15-22.9 | |
| Doxorubicin<100 mg | 35 | 24.8 | 16.1-33.6 | 0.593 |
| Doxorubicin ≥ 100 mg | 76 | 24.7 | 20.1-29.3 | |
| Particle type - DC Beads® | 52 | 22.4 | 16.8-27.9 | 0.86 |
| Particle type- Tandem® | 26 | 30.2 | 6.8-53.6 | |
| Particle size ≤ 150 μm | 59 | 21.7 | 15.6-27.7 | 0.922 |
| Particle size>150 μm | 51 | 26.1 | 22-30.2 | |
| Number of DEBDOX TACE ≤ 3 | 77 | 21.9 | 17-26.8 | 0.022 |
| Number of DEBDOX TACE ≥ 4 | 42 | 32.7 | 20.9-28.5 |
Table 2: Univariate analysis of overall survival of patients. N: Number of patients in group; CI: Confidence Interval; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; AFP: Alpha Fetoprotein; TACE: Transarterial Chemoembolization.
Multiple Cox regression was performed on the terms found significant on univariate analysis. Presence of ascites, presence of portal hypertension, Child-Pugh class B, 8 points on the Child-Pugh score, increase in bilirubin after procedure of more than 7.5 μmol/l and three or more DEBDOX TACE performed, were independent variables associated with lower overall survival (Table 3).
| Factor | B | Exp (B) | 95% CI for Exp (B) | p value |
|---|---|---|---|---|
| No ascites | - | 1 | - | - |
| Ascites | 0.59 | 1.81 | 1.09–3.00 | 0.021 |
| No portal hypertension | - | 1 | - | - |
| Portal hypertension | 0.6 | 1.82 | 1.07–3.10 | 0.026 |
| Child-Pugh A | - | 1 | - | - |
| Child-Pugh B | 0.69 | 1.99 | 1.18–3.37 | 0.010 |
| Child-Pugh 5 pt. | - | 1 | - | - |
| Child-Pugh 6 pt. | 0.22 | 1.24 | 0.68–2.27 | 0.477 |
| Child-Pugh 7 pt. | 0.51 | 1.67 | 0.90–3.09 | 0.105 |
| Child-Pugh 8 pt. | 1.97 | 7.15 | 2.77–18.44 | <0.0001 |
| Bilirubin increase<7.5 μmol/l | - | 1 | - | - |
| Bilirubin increase ≥ 7.5 μmol/l | 0.9 | 2.46 | 1.37–4.41 | 0.003 |
| Number of DEBDOX TACE ≤ 3 | - | 1 | - | - |
| Number of DEBDOX TACE ≥ 4 | 0.53 | 1.7 | 1.07–2.70 | 0.024 |
Table 3: Multivariate analysis of overall survival of patients. The reference category is always listed as the first in the group. B: Beta Coefficient (0- no difference between groups; positive value- higher risk of death than in the reference group; negative value- lower risk of death than in the reference group); Exp(B): Risk ratio Between Groups; CI: Confidence Interval; TACE: Transarterial Chemoembolization.
Discussion and Conclusion
TACE is the treatment of choice for patients with intermediate-stage disease according to BCLC [1,6,16]. However, the patient population in this group is highly heterogenous, with significant variations in tumor characteristics, patient performance status and liver function. Due to the heterogeneity of the patient population, survival rates are variable. The median survival is expected to be around 11-16 months, whereas after chemoembolization the median survival is about 20 months, ranging widely from 14 to 45 months [1,3,17-22]. The average survival in our study was 30.7 months and the median survival was 24.7 months. This value is in line with other studies [23-25]. Longer survival was mainly reported in studies with a careful selection of patients, which does not provide a true picture of the treatment of patients with unresectable HCC [8,26]. The ideal goal is to identify the subclass of patients who will have poor prognosis despite TACE treatment. These patients could potentially benefit from other treatment possibilities, such as a combination of TACE and RFA, radioembolization with yttrium or systemic therapy with Sorafenib (as a monotherapy or combined with TACE) [12,17].
In the present study, pre-treatment parameters were used to determine whether they could be of use as prognostic factors for survival. Studies evaluating prognostic factors for survival of patients treated with DEBDOX TACE are scarce. Most of the current literatures dealing with prognostic factors for survival after chemoembolization are based on cTACE. The main prognostic factors for cTACE survival are hepatic impairment according to the Child-Pugh scale, tumor load and portal vein thrombosis [22,27-31].
One of the strongest prognostic factors in our study proved to be the Child-Pugh class. A statistically significant difference in survival was proven between patients in Child-Pugh A and B classes (p=0.010). On average, the survival of patients in Child-Pugh A class was 13 months longer than for class B. Similar results were reported by Malagari, et al showing a 1 year longer survival for such patients [26]. The Child- Pugh point score also proved to be a statistically significant prognostic factor (p<0.0001). The score is used to predict the prognosis of chronic liver diseases. A higher point score and a higher class represent more severe liver disease and, accordingly, worse survival. One- and 2-year survival of patients with Child A cirrhosis is 100% and 85%, Child B 85% and 57% and for Child C 45% and 35%, respectively [32]. An impact of the Child-Pugh score on the survival of patients is thus expected and has been reported in previous studies [22,26-31]. On the other hand, tumor burden and portal vein thrombosis did not prove to be statistically significant prognostic factors for survival. Nevertheless, median survival indicated a shorter survival of patients with a multifocal lesion with a tumor size of more than 3.95 cm and an AFP value of 60.85 kIE/l or more. Similarly, in the case of portal vein thrombosis, a shorter survival of patients with thrombosis was demonstrated, but the difference between the groups was not statistically significant.
In contrast to other malignant tumors, the outcome of HCC treatment depends not only on tumor stage but also on the liver function. Poor liver function manifests with portal hypertension and ascites. On multivariate analysis, both proved to be independent prognostic factors for survival after TACE (p=0.026 and p=0.021, respectively). 61% of patients had varices and their median survival was 19.5 months. 26% of patients had ascites and their median survival was 16.7 months. Since ascites is a complication of portal hypertension, a shorter survival of such patients was expected. Several mechanisms have been suggested to explain the impact of ascites on survival after TACE, but none of them has yet been completely clarified [27,33,34]. In one study, it was assumed that patients with HCC and ascites are more susceptible to ischemic damage after TACE and liver failure [27].
When considering the effectiveness of DEBDOX TACE, it was found that there is a temporary increase in the bilirubin level in patients after the treatment procedure. The highest point is reached 48 h after the procedure, after which the level starts to decrease [35]. Even though it is usually a temporary phenomenon, the range of increase varies from patient to patient. In our study, it was shown that an increase in bilirubin was one of the strongest prognostic factors of survival of the treated patients (p=0.003). Multivariate analysis showed a 2.46 times higher risk of death for patients with an increase in bilirubin level of 7.5 μmol/l or more. In the literature, only one study was found that showed a higher risk of death in patients with increasing bilirubin levels during follow-up after TACE. Furthermore, in this study, the statistical power of bilirubin as a time-fixed and as a time-dependent variable was compared. The study demonstrated statistically significant superiority of the time-dependent model over the time-fixed model in predicting survival [21]. This speaks in favor of our approach, since we assessed the dynamics of bilirubin in DEBDOX TACE and not only its basal level before the procedure. A possible explanation behind this finding is the ischemic cholangiopathy. Intraarterial chemotherapy, combined with embolization, damages the feeding arteries of the tumor. This occlusion can cause cholangiopathy, due to local ischemic damage to the biliary ducts [36]. However, no evidence of morphological changes due to bile duct ischemia was found on the control CT or MR scans of patients with a bilirubin increase of ≥ 7.5μmol/l. We therefore presume that these changes occur on the level of the microenvironment and are reflected in the elevated laboratory values of bilirubin. Greater ischemic damage means a greater bilirubin increase after TACE and, even though morphological changes are lacking, the survival in this group of patients is consequently shorter.
Another of the statistically significant predictive survival factors in our study was the number of DEBDOX TACE performed. The results showed that the median survival of patients treated with four or more TACE interventions was 32.7 months and for those treated with three or fewer treatments, survival was 21.9 months (p=0.024). This is consistent with a study by Xing and colleagues studying the healthrelated quality of life after DEBDOX TACE, which showed a longer median survival of patients who had received four or more treatments with TACE (39.1 months for those with four or more TACE interventions and 20.2 months for those treated with TACE three or more times) [37]. Additionally, the results of the study suggested that multiple DEBDOX TACE interventions not only improve the survival of patients with unresectable HCC but also allow long-term maintenance of an adequate quality of life of treated patients.
This study has some limitations. First, it is a retrospective study. Second, we included a limited number of patients. Future studies should aim at the inclusion of a larger group of patients, preferably in a multi-center context. Third, although four interventional radiologists performed DEBDOX TACE according to uniform protocols, minor variations are inevitable, including, for example, selectivity of catheterization. Fourth, the study did not test the impact of tumor histology, biochemical parameters of the liver and kidney state and the impact of newer CT perfusion prognostic factors [38].
In conclusion, our results indicate that the presence of ascites, portal hypertension, Child-Pugh class B, Child-Pugh score, a greater increase in bilirubin levels after the procedure and a lower number of DEBDOX TACE procedures are significant prognostic factors in overall survival. The data from this study and other similar studies can be used as a significant decision guide for clinicians in selecting treatment strategies.
References
- European Association for the Study of the Liver, European Organisation for Research and Treatement of Cancer (2012) EASL–EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 56: 908-943.
- Stewart BW, Wild CP (2014) World Cancer Report 2014. International Agency for Research on Cancer, Lyon.
- Popovic P (2016) Vloga radiologije v diagnostiki in zdravljenju jetrnoceliÄÂnega karcinoma. Medicinski Razgledi 55: 449-466.
- Crocetti L, Baere TD, Lencioni R (2010) Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 33: 11-17.
- Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: An update. Hepatol 53: 1020-1022.
- Waghray A, Murali AR, Menon K (2015) Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol 7: 1020-1029.
- Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, et al. (2015) Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 38: 352-360.
- Burrel M, Reig M, Forner A, Barrufet M, Lope CRD, et al. (2012) Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 56: 1330-1335.
- Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, et al. (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol 33: 41-52.
- Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, et al. (2010) Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with beadblock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 33: 541-551.
- Lencioni R, Chen XP, Dagher L, Venook AP (2010) Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: How can outcomes be improved? The Oncologist 15: 42-52.
- Bolondi L, Burroughs A, Dufour J, Galle P, Mazzaferro V, et al. (2012) Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 32: 348-359.
- Kim JH, Shim JH, Lee HC, Sung KB, Ko HK, et al. (2017) New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int 37: 1861-1868.
- Fatourou EM, Tsochatzis EA (2014) ART and science in using transarterial chemoembolization for retreating patients with hepatocellular carcinoma. Hepatobiliary Surgery and Nutrition 3: 415-418.
- Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, et al. (2015) Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol 62: 855-862.
- Bruix J, Sala M, Llovet JM (2004) Chemoembolization for hepatocellular carcinoma. Gastroenterology 127: S179-S188.
- Yamakado K, Hirota S (2015) Sub-classification of intermediate-stage (Barcelona Clinic Liver Cancer stage-B) hepatocellular carcinomas. World J Gastroenterol 21: 10604-10608.
- Popovic P, Stabuc B, Jansa R, Garbajs M (2016) Survival of patients with intermediate stage hepatocellular carcinoma treated with superselective transarterial chemoembolization using doxorubicin-loaded DC Bead under cone-beam computed tomography control. Radiol Oncol 50: 418-426.
- Llovet J, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 37: 429-442.
- Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T (2012) Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. European Journal of Radiology 81: 3985-3992.
- Cabibbo G, Genco C, Di Marco V, Barbara M, Enea M, et al. (2011) Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation: Predicting survival after TACE for hepatocellular carcinoma. Aliment Pharmacol Ther 34: 196-204.
- Lu W, Li Y, He X, Chen Y (2003) Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of two kinds of dosages of anticancer drugs and analysis of prognostic factors. Hepatogastroenterology 50: 2079-83.
- Kalva SP, Pectasides M, Yeddula K, Ganguli S, Blaszkowsky LS, et al. (2013) Factors affecting survival following chemoembolization with doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. J Vasc Interv Radiol 24: 257-265.
- Luz JHM, Luz PM, Martin HS, Gouveia HR, Levigard RB, et al. (2017) DEB TACE for intermediate and advanced HCC – Initial experience in a Brazilian Cancer Center. Cancer Imaging 17: 1-9.
- Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, et al. (2016) Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: Chemoembolization in hepatocarcinoma. J Gastroenterol Hepatol 31: 645-653.
- Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, et al. (2012) Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: Five-year survival analysis. Cardiovasc Intervent Radiol 35: 1119-1128.
- Hsin IF, Hsu CY, Huang HC, Huang YH, Lin HC, et al. (2011) Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: Incidence, risk factors, and prognostic prediction. J Clin gastroenterol 45: 556-562.
- Hu HT, Kim JH, Lee LS, Kim KA, Ko GY, et al. (2011) Chemoembolization for hepatocellular carcinoma: Multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol 22: 917-923.
- Zhang JW, Feng X, Liu H, Yao Z, Yu Y (2010) CT volume measurement for prognostic evaluation of unresectable hepatocellular carcinoma after TACE. World J Gastroenterol 16: 2038-2045.
- Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi H, et al. (2009) Risk factors for death in 224 cases of hepatocellular carcinoma after transcatheter arterial chemoembolization. Hepatogastroenterology 56: 213-217.
- Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, et al. (2000) A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer 88: 50-57.
- Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D, et al. (2005) Systematic review: The model for end-stage liver disease - should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 22: 1079-1089.
- Cho YK, Chung JW, Kim JK, Ahn YS, Kim MY, et al. (2008) Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer 112: 352-361.
- Hsu CY, Huang YH, Su CW, Chiang JH, Lin HC, et al. (2010) Transarterial chemoembolization in patients with hepatocellular carcinoma and renal insufficiency. J Clin Gastroenterol 44: e171-e177.
- Varela M, Real MI, Burrel M, Forner A, Sala M, et al. (2007) Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol 46: 474-481.
- Deltenre P, Valla D-C (2006) Ischemic cholangiopathy. J Hepatol 44: 806-817.
- Xing M, Webber G, Prajapati HJ, Chen Z, El-Rayes B, et al. (2015) Preservation of quality of life with doxorubicin drug-eluting bead transarterial chemoembolization for unresectable hepatocellular carcinoma: Longitudinal prospective study: HRQOL with DEB-TACE in unresectable HCC. J Gastroenterol Hepatol 30: 1167-1174.
- Popovic P, Leban A, Kregar K, Garbajs M, Dezman R, et al. (2017) Computed tomographic perfusion imaging for the prediction of response and survival to transarterial chemoembolization of hepatocellular carcinoma. Radiol Oncol 52: 14-22.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi