Review Article, J Clin Exp Oncol Vol: 6 Issue: 4
Roles of NAD (P) H-Quinone Oxidoreductase 1 (NQO1) On Cancer Progression and Chemoresistance
Pimradasiri Srijiwangsa1 and Kesara Na-Bangchang1,2*
1Chulabhorn International College of Medicine, Thammasat University, Pathum Thani, Thailand
2Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Chulabhorn International College of Medicine, Thammasat University, Pathum Thani, Thailand
*Corresponding Author : Kesara Na-Bangchang
Chulabhorn International College of Medicine, Thammasat University (Rangsit Campus), Pathum Thani 12121, Thailand
Tel: 1800/662564-4400
E-mail: kesaratmu@yahoo.com
Received: July 03, 2017 Accepted: July 11, 2017 Published: July 18, 2017
Citation: Srijiwangsa P, Na-Bangchang K (2017) Roles of NAD (P) H-Quinone Oxidoreductase 1 (NQO1) On Cancer Progression and Chemoresistance. J Clin Exp Oncol 6:4. doi: 10.4172/2324-9110.1000192
Abstract
NAD(P)H-Quinone Oxidoreductase 1 (NQO1), originally referred to as DT-diaphorase, is a xenobiotic metabolizing/antioxidant enzyme that detoxifies chemical stressors, providing cytoprotection in normal tissues. NQO1 catalyzes obligatory two-electron reduction of several endogenous and environmental quinones to hydroquinone that are ready for further conjugation and excretion. The enzyme requires NADH or NADPH as an electron donor for enzymatic activity. High-level of NQO1 expression has however, been correlated with numerous human malignancies, suggesting its role in cancer progression and chemoresistance. This adaptation renders the cancer cells to survive in relatively high oxidative stress condition compared to normal cells, as well as protects cancer cells from toxic action of chemotherapeutic agents. Inhibitors of NQO1 enzyme have been found to improve anticancer activities of conventional chemotherapeutic agents. The review provides a perspective on a molecular basis of NQO1-mediated cancer cells progression and the suppression and possible strategy to improve chemosensitivity and to overcome chemoresistance of NQO1 inhibitors when used in combination with chemotherapeutic drugs.
Keywords: NAD(P)H-quinone oxidoreductase 1 (NQO1); Cancer progression; Chemoresistance
Introduction
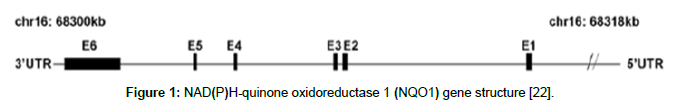
Maintenance of redox homeostasis in living cells is essential in cancer prevention. In aerobic conditions, cells are frequently exposed to the generation of reactive oxygen species (ROS) that can affect proteins, lipids and DNA functions. These events play important pathological role in the development of various human diseases such as cancer [1]. Subsequently, cells develop endogenous resistance components in order to neutralize oxidative stress and to maintain ROS at low physiological levels. NAD (P) H-quinone oxidoreductase 1 (NQO1) is a cancer preventer/detoxifying enzyme which is a key catalyst essentially in the protection of the structure and the functioning of normal healthy cells [2]. Recent evidences have demonstrated the dark side of NQO1. Constitutive up-regulation of NQO1 has been found in many types of cancers including breast, uterine cervix carcinoma, lung, and Cholangiocarcinoma cells [3-6]. Cancer cells, differently from normal cells, show an increased rate of ROS generation as by-products of their metabolism and, as “masters” of adaptation. They take advantage of the over-activation of antioxidant defenses, in particular Nrf2-dependent antioxidant genes such as NQO1 [7]. This capacity to adapt and survive under conditions of electrophilic, oxidative and inflammatory stress is strongly dependent on the expression of a complex network genes induced by Nrf2 encoding proteins with different antioxidant and cytoprotective functions [7]. Overexpressed NQO1 results in development favorable position to growth cells by shielding those cells from oxidative stress and chemotherapeutic agents leading to cancer progression and chemo resistance. This review focuses on the malicious properties of NQO1 and its role in cancer cell progression chemoresistance development (Figure 1).
Figure 1: NAD(P)H-quinone oxidoreductase 1 (NQO1) gene structure [22].
NQO1: structure and regulation by the Keap1/Nrf2/ARE pathway
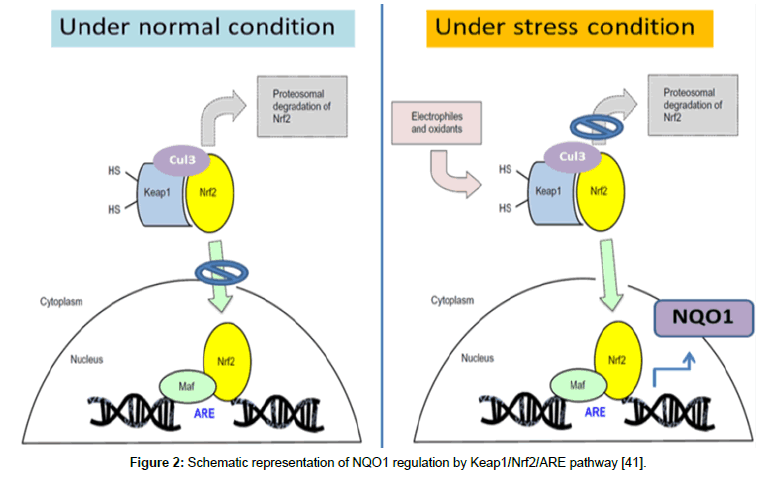
NAD (P) H-quinone oxidoreductase (NQO1), originally referred to as DT-diaphorase (EC 1.6.99.2), was the first member of the NAD (P) H dehydrogenase family discovered by Professor Ernster in the late 1950s. It is a flavoenzyme that plays an important role in protection of cells against endogenous and exogenous quinones by catalyzing two- or four-electron reductions of these substrates [2]. The human NQO1 gene (formerly called DIA4) is located on chromosome 16q22.1. NQO1 gene consists of five introns and six exons with an approximate length of 20 kb [8]. NQO1 is expressed in various tissues [9] and induced by a wide array of chemical inducers, oxidative stress, H2O2, hypoxia and heavy metals. In addition, it is also induced by polycyclic aromatic hydrocarbons such as those found in combustion processes (e.g. cigarette smoke and urban smog). The induction is mediated through the Keap1/Nrf2/ARE pathway. The NQO1 gene has been shown to be activated together with other Nrf2- induced detoxifying enzyme genes such as HO1 (heme oxygenase 1), GST (glutathione S-transferase) and GCL (glutamate-cysteine ligase) [7,10]. Two cellular events are important for the mechanisms by which the transcription of NQO1 gene battery is regulated (Figure 2):
Figure 2: Schematic representation of NQO1 regulation by Keap1/Nrf2/ARE pathway [41].
a) Under normal or unstressed condition, Nrf2 (nuclear factor erythroid 2-related factor 2), a basic leucine zipper transcription factor of the “cap-‘n collar” family, is kept in the cytoplasm by a cluster of proteins. The Kelch like-ECH-associated protein 1 (KEAP1) and Cullin 3 (Cul3) protein degrade Nrf2 by ubiquitination. Cul3 ubiquitinates Nrf2, while Keap1 is a substrate adaptor protein that facilitates the reaction. Once Nrf2 is ubiquitinated, it is transported to the proteasome, where it is degraded and its components recycled.
b) Under oxidative stress or inflammatory condition, critical cysteine residues in Keap1 are disrupted, and thereby disrupting the Keap1-Cul 3 ubiquitination system. When Nrf2 is not ubiquitinated, it builds up in the cytoplasm, and translocate into the nucleus. In the nucleus, it combines (forms a heterodimer) with one of small Maf proteins (MAFF, MAFG, and MAFK) and binds to the antioxidant response element (ARE) in the upstream promoter region of many antioxidative genes and initiates their transcription and protein expression including NQO1.
NQO1: A marker of “cell protector”
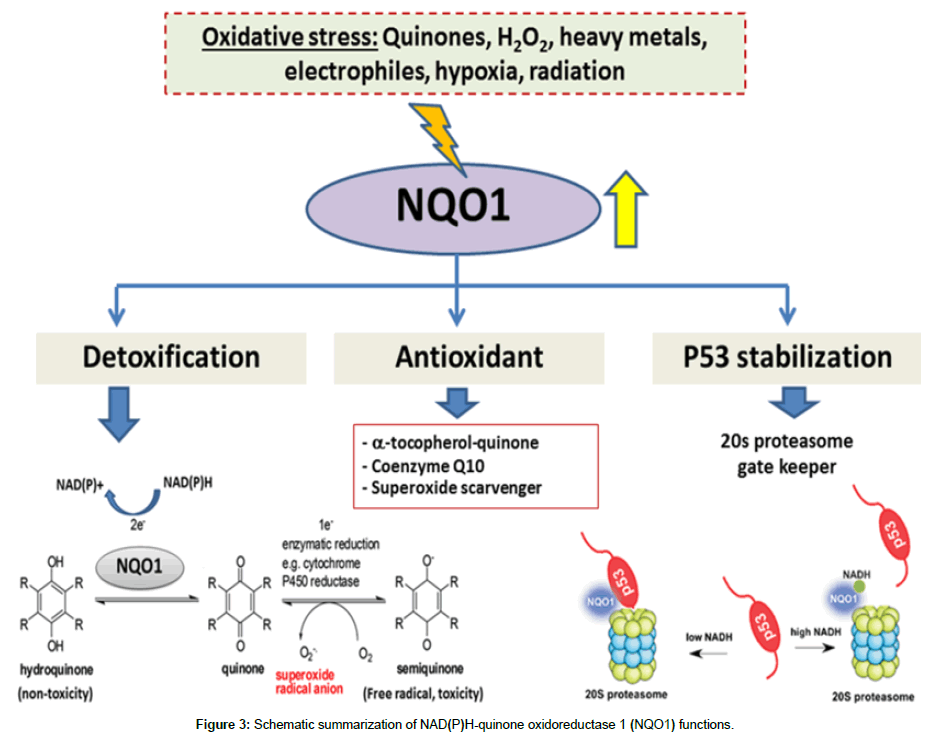
The approach to prevent cell damage or cancer development is suppression of the carcinogenic metabolic activation and preventing the production of ultimate carcinogens and oxidative stress. NQO1 has attracted interest over the years as an enzyme involved in the detoxification of xenobiotics such as quinones and quinoneimines, and as an enzyme associated with protection against mutagenesis and carcinogenesis. It is an obligate two-electron reductase that is characterized by its capacity for utilizing either NADH or NADPH as a reducing cofactor, and by its activity inhibition by dicoumarol [11]. Thus, the induction of NQO1 enzyme correlates with inhibition against chemical-mediated tumorigenesis. The multiple roles of NQO1 as a “cell protector” are summarized in three categories as shown in Figure 3.
Detoxification enzyme: The classical direct detoxification role of NQO1 is inherent in its catalytic mechanism, the enforced twoelectron reduction of a wide range of quinones to their corresponding hydroquinones by using either NADPH or NADH as the hydride donor [11]. Quinones are widely distributed in nature in human bodies (e.g. vitamin K) and in natural environments (e.g. automobile exhaust, cigarette smoke, and urban air particulates or diets). Quinones are highly reactive molecules that can induce cancers and neurodegenerative diseases. They readily undergo either one- or twoelectron reduction [12]. NQO1 distracts quinone electrophiles from participating in reactions that could lead to either sulfhydryl depletion, or to one-electron reductions which can generate semiquinones and various reactive oxygen intermediates as a consequence of redox cycling. In addition, the hydroquinone products of the NQO1 reaction can be further metabolized to glucuronide and sulfate conjugates to facilitate their excretion [2]. The two-electron reduction activity catalyzed by NQO1 is of benefit to the cells as it prevents generation of free radicals by redox cycle. The detoxification of redoxcycling quinones by NQO1 therefore, protects the cells from oxidative stresses and prevents carcinogenesis.
Antioxidant enzyme: Apart from its reducing action, NQO1 additionally scavenges superoxide to shield against oxidative stress actuated by cytotoxic substances. This action could provide additional cytoprotection and could especially be effective in tissues with low level of SOD expression [13]. Along these lines, in cardiovascular cells where SOD level is low and NQO1 level is high, induction of NQO1 has been shown to be correlated with enhancing superoxide scavenging action, while its suppression correlated with a decrease in superoxide scavenging activity [14]. Therefore, NQO1 protects the cell from unwanted oxidative damage by maintaining the reduced form of endogenous antioxidants. It is thought to help preserve certain endogenous antioxidants in their reduced and active forms. Particularly, both ubiquinone (coenzyme Q) and α-tocopherolquinone, the two essential lipid-soluble antioxidants, are substrates for NQO1 in vitro [2].
Tumor suppressor p53 protein stabilizer: Tumor suppressor p53 plays an important role in protection of genome against internal and external stresses. It prevents cell transformation and tumor formation through transcriptional dependent and independent mechanisms [15]. Under normal condition, p53 protein is rapidly degraded through its interaction with Mdm-2 that induces ubiquitination. In response to a variety of stress signals, the p53 protein is stabilized and activated as a sequence specific transcription factor. This leads to cell cycle arrest, senescence, or apoptosis. NQO1 is physically associated with the 20S proteasomes and it can also bind and protect a subset of short-lived proteins from 20S proteasomal degradation. This suggests that NQO1 may function as a gatekeeper of the 20S proteasomes in the cells to regulate the proteasomal degradation of p53 protein [16]. Recently, a model is proposed whereby some short-lived proteins (such as p53, p73 and ODC) are inherently unstable and degraded “by default” by the 20S proteasomes in cells unless stabilized by a stabilizer including NQO1. This degradation by default mechanism is distinct from the current “modification to destabilization” mechanism that is mediated by polyubiquitination. The nascent protein manages to escape 20S proteasomal breakdown matures and is engaged in larger functional protein complexes. At this stage, the degradation of the protein can occur mostly via ubiquitin-dependent 26S proteasomal degradation [17].
NQO1 and chemoprevention
It is well recognized that NQO1 plays a key role in the cellular adaptation and protection against oxidative stress. The properties of NQO1 as detoxifying/antioxidants enzyme and p53 stabilizer, are crucial for reducing electrophiles and ROS, thus decreasing DNA damage and mutations and preventing genomic instability in normal cells. The critical roles of NQO1 in chemical-induced toxicity and carcinogenesis have been proven by using NQO1 knock-out mice model. Those NQO1 knock-out mice showed increased sensitivity to menadione-induced hepatic toxicity [18] benzo[a]pyrene and 7,12-dimethylbenz[a] anthracene-induced skin carcinogenesis [19] and benzene-induced toxicity [20]. Moreover, the protective role of NQO1 against carcinogenesis is highlighted by results from various studies in humans on single-nucleotide polymorphism (SNP) in the promoter region of the NQO1 gene. The most prominent and frequent variant of NQO1 is a C to T substitution at nucleotide position 609 of the NQO1 cDNA (rs1800566), also known as NQO1*2. This nucleotide alteration results in a proline to serine amino acid change at position 187 (P187S) that is accompanied with a reduction of enzyme activity due to instability of the protein product. The activity of the homozygous variant genotype (NQO1*2/*2 or T/T) enzyme is therefore, substantially undetectable, whereas NQO1*1/*2 or C/T heterozygotes exhibit moderate activity between the homozygous SNP genotype and wild type (NQO1*1/*1 or C/C). The NQO1 T/T mutation has been shown to be associated with increased risk of benzene poisoning and thus, increased risk of developing several types of cancers such as gastrointestinal cancer, lung cancers, and colon cancer [21-23].
Double-edged swords of NQO1 in cancer cells
NQO1 activation and hallmark of cancer progression: Basically, NQO1 is present in almost all types of normal human tissues [9] and is induced along with a battery of defensive genes to provide protection against different stresses. In addition, it also prevents various organs from carcinogen-induced tumorigenesis. Despite the fact that absent or low NQO1 activity has been associated with susceptibility to cancers (the role as cell protector), a marked up-regulation of NQO1 has been found in many inflammatory conditions and tumorous tissues [12]. Accumulating evidences suggest that NQO1 protein and mRNA were abnormally elevated (>60%) in several solid tumors, e.g., breast cancer, gastric cancer, small cell lung cancer, ovarian carcinoma, pancreatic ductal adenocarcinoma, as well as cholangiocarcinomacompared with normal tissues (Table 1). This may provide cancer cells a growth advantage over normal cells.
| Cancers | Number of cancer patients | NQO1 protein expression rates (%) | References |
|---|---|---|---|
| Gastric adenocarcinoma | 203 | 75.86 | [26] |
| Breast cancer | 176 | 84.70 | [42] |
| Small cell lung cancer | 115 | 70.43 | [4] |
| Serous ovarian carcinoma | 160 | 85.60 | [5] |
| Squamous cell carcinoma of the uterine cervix | 177 | 80.23 | [6] |
| Head and neck squamous cell carcinoma | 162 | 84.00 | [43] |
| Pancreatic ductal adenocarcinoma | 126 | 83.30 | [44] |
| Cholangiocarcinoma | 43 | 94.5* | [3] |
(nmol/min/mg protein)
Table 1: Overexpression of NQO1 protein in various human cancer tissues.
Recently, a close correlation between NQO1 enzyme activity and protein expression has been reported in cancers. Mikami et al. demonstrated high NQO1 expression in both colon cancer cell lines and colorectal tumor tissues [24]. In addition, the level of NQO1 was shown to be significantly high in tumors with nodal metastases compared with those without metastasis. In addition, NQO1 overexpression in breast cancer, gastric adenocarcinoma, lung, and uterine cervix carcinoma was markedly associated with increased tumor size, severity of histology of tumors, clinical stage, and lymph node metastasis [4,6,25,26]. In contrast, Gen et al. reported higher expression of NQO1 protein in lower-grade and superficial bladder tumors compared with high-grade and invasive tumors [27]. The strong association between high NQO1 expression and cancer progression has been supported by results of a study showing that the overexpression of NQO1 significantly induced cell cycle progression via the up regulation of cyclin A2, B1 and D1 which led to the melanoma cells proliferation [28]. Furthermore, NQO1 siRNA mediated knockdown Cholangiocarcinoma cells were shown to effectively diminish colony formation capacity, induce cell cycle arrest, and exhibit increased expression levels of p21 and decreased cyclin D1 protein expression-mediated suppression of CCA cells migration [29]. Altogether, these findings indicate that high NQO1 expression might be related to tumorigenesis and malignant status of cancer cells and complex molecular pathways are involved in the regulation of NQO1 gene expression.
Accumulating evidences have suggested that increased NQO1 expression is associated with poor prognosis and short survival in cancer patients. Burarat et al. reported a significant association between high level of NQO1 expression and overall survival time of cholangiocarcinoma patients. This observation raises the exciting possibility of using NQO1 as a tumor marker [30]. Furthermore, high level of NQO1 protein expression in small cell lung cancer, gastric adenoma and uterine cervix adenoma was shown to be significantly correlated with lower disease-free survival and 5 year survival rates, particularly early-stage cancer patients [4,6,26]. Hence, NQO1 plays an important role in cancer progression and reduction of survival time. The enzyme may potentially be used as a biomarker as well as therapeutic target for cancers. In addition, the application of NQO1 inhibitors in potentiating anticancer efficacy of conventional drugs has been a growing interest.
NQO1 modulation as a strategy in chemotherapy: The role of NQO1 in protecting cells by overcoming environmental stresses has already been demonstrated and compounds that are able to modulate this activity are of great interest as potential drug candidates particularly anticancer. The activation of NQO1 in normal cells can prevent tumor formation, while its inhibition can improve therapeutic efficacy of chemotherapeutic drugs. Reasonably, up-regulated NQO1 in cancer cells is thought to be responsible for chemoresistance by decreasing oxidative toxicity of chemotherapeutic agents and making more reduced environmental stress for tumor growth. This would provide the possibility of pharmacological and genetic strategies targeting NQO1 for exerting chemotherapeutic effects or potentiating pharmacodynamics activity of the co-administered chemotherapeutic drugs (Table 2). As far as enhancement of cancer chemotherapy is concerned, modulation of NQO1 may confer increased chemosensitivity of several cancers relying on NQO1. Dicoumarol is often used as a pharmacological NQO1 inhibitor to investigate the role of this enzyme in cancer cells. Dicoumarol affects a structure change in NQO1 resulting in inhibitory effect on NQO1 activity. However, the action of dicoumarol is compromised by its extensive protein binding [31] which can interfere with the interpretation of the function of NQO1 in cells. An example is where dicoumarol potentiates cell apoptosis by blocking JNK and NF-κB pathways and induces TNF-α in HeLa cell [32]. The enhancement of efficacy of conventional chemotherapeutic agents by dicoumarol has been demonstrated with doxorubicin or cisplatin induced apoptosis in urogenital cancer cells [33,34]. The inhibition of NQO1 by dicoumarol was suggested to stimulate formation of superoxide, overt oxidative stress, subsequent suppress of cell growth, and induce apoptosis in pancreatic cancer cells [35,36]. In cholangiocarcinoma (CCA), NQO1 plays roles in modulating sensitivity of the cancer cells to gemcitabine when given in combination with dicoumarol. Dicoumarol enhances gemcitabine cytotoxicity in high NQO1 activity CCA cells. The mechanism of dicoumarol-induced cell killing may not only be mediated via disruption of mitochondrial function and formation of ROS, but may also be related to suppression of the pro-survival response to the chemotherapy. The increased p53 expressions, together with decreased Bcl-XL protein expression, have been shown to be related to enhanced cytotoxicity of gemcitabine [30]. The pharmacological inhibitory activity on NQO1 has recently emerged as a promising approach for cancer therapy.
| NQO1 inhibition | Cell line | Chemotherapeutic agents | Effects | ||
|---|---|---|---|---|---|
| Dicoumarol [36] | Pancreatic cancer cells | None | Inhibition of NQO1 with dicumarol increases apoptotic cell death mediated with cytochrome c release and poly (ADP-ribose) polymerase cleavage and also increase oxidative stress | ||
| Dicoumarol [35] | Pancreatic cancer cells | None | Inhibition of NQO1 increases intracellular O2 production and inhibits pancreatic cancer cells growth | ||
| NQO1 siRNA [45] | Mouse skin tumors | None | NQO1 siRNA abolishes activation of NF-kB, c-Jun, Akt, p38, p44/42 MAPK and potentiates cell apoptosis | ||
| NQO1 siRNA [29] | CCA cells | None | NQO1 siRNA impaired colony formation capacity, induced cell cycle arrest at the G1 phase, suppressed migration, increased expression levels of p21 and decreased cyclin D1 protein expression levels. | ||
| Dicoumarol [33] | Urothelial cancer cell | Doxorubicin | Dicoumarol could enhance the cytotoxicity of doxorubicin in urothelial cancer cells with WT-p53 through the p53/p21/p38 MAPK pathways | ||
| Dicoumarol [34] | Urogenital cancer cells | Cisplatin | Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in WT-p53 urogenital cancer cells | ||
| Dicoumarol [30] | CCA cells | Gemcitabine | Inhibition of NQO1 with dicumarol enhanced sensitivity of gemcitabine by increase p53 and decrease Bcl-xL protein expression | ||
| NQO1 siRNA [40] | CCA cells | Gemcitabine, 5-Fluorouracil, Doxorubicin | NQO1 siRNA enhanced sensitivity of chemotherapeutic agents by increase p53 and Bax protein expression | ||
Table 2: The effect of NQO1 modulation on cancer cells.
Likewise, the tumor suppressor p53 is one of the master regulators for cell cycle and death. Increased p53 level following exposure of cells to noxious stimuli including chemotherapeutic agents, mediates growth inhibition. It has been suggested that p53- mediated apoptosis mechanisms are both transcriptional activitydependent and independent. P53 level is regulated by the rate of its degradation which is known to be mediated by the Mdm-2-ubiquitinproteasome degradation pathway [37]. The role of NQO1 on p53 regulation has recently been reported in two respects. Inhibition of NQO1 results in p53 degradation and on the other hand, inhibition of NQO1 by chemotherapeutic agents enhances p53-related cell apoptosis. Restoration of p53 function by modulation of NQO1 would therefore, be one strategy promoting cell killing effect of cancer chemotherapeutic agents. NQO1 inhibition was shown to decrease p53 expression [38] and regulate the stability of the tumor suppressor WT p53. While NQO1 binds and stabilizes WT p53, NQO1 inhibitors including dicoumarol, other coumarins and flavones induce ubiquitinindependent proteasomal p53 degradation and thus, inhibiting p53- induced apoptosis. Tsvetkov et al. showed that curcumin, a natural phenolic compound found in the spice turmeric, induced ubiquitinindependent degradation of WT p53 and inhibited p53 induced apoptosis in normal thymocytes and myeloid leukemia cells [39]. The inhibition of NQO1 mediated p53 has been reported to contribute to the increase in chemosensitivity leading to cell killing in human tumor cells. Results from a previous study suggested that inhibition of NQO1 by dicoumarol suppressed p53 protein levels and induced cell death [38]. In contrast, dicoumarol at non-cytotoxic concentrations but sufficient to inhibit NQO1 enzyme activity, was shown to enhance p53 protein levels [30]. Zeekpudsa et al. showed that the suppression of NQO1 by si-RNA increased p53 expression which was associated with increased p21 and Bax levels [40]. Both p21 and Bax are p53-dependent downstream gene products. In addition to the roles of NQO1 for indirect detoxification of quinones and in antioxidant defense, the interaction of NQO1 with p53 may represent an additional mechanism that contributes to chemosensitivity of NQO1 leading to augment cancer cells death [41-45].
Various inhibitors of NQO1 have been developed based on the modulatory effect of dicoumarol on potentiating anticancer effect of conventional chemotherapeutics through NQO1 activity. Detailed investigations of their selective activities on cancer cells are needed prior to the next steps of pre-clinical studies.
Conclusion
The NQO1, a xenobiotic metabolizing enzyme, plays important roles both in protecting normal cells from toxic effects of chemical stressors and oxidants and in promoting progression and development of chemoresistance in cancer cells. Thorough understanding of molecular mechanisms by which it exert these pathological effects is essential for exploiting as targets for cancer chemotherapy through suppression of the enzyme activity in order to improve therapeutic efficacy and overcome chemoresistance of cancer cells to conventional drugs. For such promising approach to become realistic, a number of steps of pre-clinical and clinical studies are required to confirm its therapeutic potential.
References
- Gupta SC, Hevia D, Patchva S, Park B, Koh W, et al. (2012) Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention and therapy. Antioxid Redox Signal 16: 1295-1322.
- Dinkova-Kostova AT, Talalay P (2010) NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116-123
- Buranrat B, Chau-in S, Prawan A, Puapairoj A, Zeekpudsa P, et al. (2012) NQO1 expression correlates with cholangiocarcinoma prognosis. Asian Pac J Cancer Prev 13: 131-136.
- Cui X, Jin T, Wang X, Jin G, Li Z, et al. (2014) NAD(P)H:quinone oxidoreductase-1 overexpression predicts poor prognosis in small cell lung cancer. Oncol Rep 32: 2589-2595.
- Cui X, Li L, Yan G, Meng K, Lin Z, et al. (2015) High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer 15: 244.
- Ma Y, Kong J, Yan G, Ren X, Jin D, et al. (2014) NQO1 overexpression is associated with poor prognosis in squamous cell carcinoma of the uterine cervix. BMC Cancer 14: 414.
- Krajka-Kuzniak V, Paluszczak J, Baer-Dubowska W (2017) The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacological reports 69: 393-402.
- Jaiswal AK (1991) Human NAD(P) H:Quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry 33: 10647-10653.
- Siegel D, Ross D (2000) Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med 29: 246-253.
- Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Samadi N (2017) Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair 54: 13-21.
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, et al. (2000) NAD(P)H:quinone oxidoreductase 1 (NQO1): Chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129: 77-97.
- Jaiswal AK (2000) Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med 29: 254-262.
- Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, et al. (2004) NAD(P)H:Quinone oxidoreductase 1: Role as a superoxide scavenger. Mol Pharmacol 65:1238-1247.
- Zhu H, Li Y (2012) NAD(P)H: quinone oxidoreductase 1 and its potential protective role in cardiovascular diseases and related conditions. Cardiovasc Toxicol 12:39-45.
- Shu KX, Li B, Wu LX (2007) The p53 network: p53 and its downstream genes. Colloids Surf B Biointerfaces 55: 10-18.
- Gong X, Kole L, Iskander K, Jaiswal AK (2007) NRH:quinone oxidoreductase 2 and NAD(P)H:Quinone oxidoreductase 1 protect tumor suppressor p53 against 20s proteasomal degradation leading to stabilization and activation of p53. Cancer Res 67: 5380-5388.
- Asher G, Shaul Y (2006) Ubiquitin-independent degradation: lessons from the p53 model. Isr Med Assoc J 8: 229-232.
- Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, et al. (1998) Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem 273: 7382-7389.
- Long DJ, Waikel RL, Wang XJ, Roop DR, Jaiswal AK (2001) NAD(P)H:Quinone oxidoreductase 1 deficiency and increased susceptibility to 7,12-dimethylbenz[a]-anthracene-induced carcinogenesis in mouse skin. J Natl Cancer Inst 93: 1166-1170.
- Bauer AK, Faiola B, Abernethy DJ, Marchan R, Pluta LJ, et al. (2003) Genetic susceptibility to benzene-induced toxicity: role of NADPH:Quinone oxidoreductase-1. Cancer Res 63: 929-935.
- Kolesar JM, Dahlberg SE, Marsh S, McLeod HL, Johnson DH, et al. (2011) The NQO1*2/*2 polymorphism is associated with poor overall survival in patients following resection of stages II and IIIa non-small cell lung cancer. Oncol Rep 25:1765-1772.
- Yu H, Liu H, Wang LE, Wei Q (2012) A functional NQO1 609C>T polymorphism and risk of gastrointestinal cancers: A meta-analysis. PloS ONE 7: e30566.
- Zhou JY, Shi R, Yu HL, Zheng WL, Ma WL (2012) Association of NQO1 Pro187Ser polymorphism with the risks for colorectal cancer and colorectal adenoma: a meta-analysis. Int J Colorectal Dis 27: 1123-1124.
- Mikami K, Naito M, Ishiguro T, Yano H, Tomida A, et al. (1998) Immunological quantitation of DT-diaphorase in carcinoma cell lines and clinical colon cancers: Advanced tumors express greater levels of DT-diaphorase. Jpn J Cancer Res 89: 910-915.
- Glorieux C, Sandoval JM, Dejeans N, Ameye G, Poirel HA, et al. (2016) Overexpression of NAD(P)H:Quinone oxidoreductase 1 (NQO1) and genomic gain of the NQO1 locus modulates breast cancer cell sensitivity to quinones. Life Sci 145: 57-65.
- Lin L, Qin Y, Jin T, Liu S, Zhang S, et al. (2014) Significance of NQO1 overexpression for prognostic evaluation of gastric adenocarcinoma. Exp Mol Pathol 96: 200-205.
- Gan Y, Mo Y, Kalns JE, Lu J, Danenberg K, et al. (2001) Expression of DT-diaphorase and cytochrome P450 reductase correlates with mitomycin C activity in human bladder tumors. Clin Cancer Res 7: 1313-1319.
- Garate M, Wani AA, Li G (2010) The NAD(P)H:Quinone oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Radic Biol Med 48: 1601-1609.
- Butsri S, Kukongviriyapan V, Senggunprai L, Kongpetch S, Zeekpudsa P, et al. (2017) Downregulation of NAD(P)H:Quinone oxidoreductase 1 inhibits proliferation, cell cycle and migration of cholangiocarcinoma cells. Oncol lett 13: 4540-4548.
- Buranrat B, Prawan A, Kukongviriyapan U, Kongpetch S, Kukongviriyapan V (2010) Dicoumarol enhances gemcitabine-induced cytotoxicity in high NQO1-expressing cholangiocarcinoma cells. World J Gastroenterol 16: 2362-2370.
- Scott KA, Barnes J, Whitehead RC, Stratford IJ, Nolan KA (2011) Inhibitors of NQO1: Identification of compounds more potent than dicoumarol without associated off-target effects. Biochem Pharmacol 81: 355-63.
- Cross JV, Deak JC, Rich EA, Qian Y, Lewis M, et al. (1999) Quinone reductase inhibitors block SAPK/JNK and NF kappaB pathways and potentiate apoptosis. J Bio Chem 274: 31150-31154.
- Matsui Y, Watanabe J, Ding S, Nishizawa K, Kajita Y, et al. (2010) Dicoumarol enhances doxorubicin-induced cytotoxicity in p53 wild-type urothelial cancer cells through p38 activation. BJU Int 105: 558-564.
- Watanabe J, Nishiyama H, Matsui Y, Ito M, Kawanishi H, et al. (2006) Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in p53 wild-type urogenital cancer cell lines. Oncogene 25: 2500-2508.
- Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, et al. (2003) Dicumarol inhibition of NADPH:Quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res 63: 5513-5520.
- Lewis A, Ough M, Li L, Hinkhouse MM, Ritchie JM, et al. (2004) Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin Cancer Res 10: 4550-4558.
- Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88: 323-331.
- Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, et al. (2003) Interaction of human NAD(P)H:Quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. Biol Chem 278: 10368-10373.
- Tsvetkov P, Asher G, Reiss V, Shaul Y, Sachs L, et al. (2005) Inhibition of NAD(P)H:Quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci USA 102: 5535-5540.
- Zeekpudsa P, Kukongviriyapan V, Senggunprai L, Sripa B, Prawan A (2014) Suppression of NAD(P)H-quinone oxidoreductase 1 enhanced the susceptibility of cholangiocarcinoma cells to chemotherapeutic agents. J Exp Clin Cancer Res 33: 11.
- Gao B, Doan A, Hybertson BM (2014) The clinical potential of influencing Nrf2 signaling in degenerative and immunological disorders. Clin Pharmacol 6: 19-34.
- Yang Y, Zhang Y, Wu Q, Cui X, Lin Z, et al. (2014) Clinical implications of high NQO1 expression in breast cancers. J Exp Clin Cancer Res 33: 14.
- Yang Y, Jin T, Liu S, Chen L, Lin L, et al. (2014) Prognostic significance of NADPH quinine oxidoreductase 1 overexpression in head and neck squamous cell carcinoma. Zhonghua Bing Li Xue Za Zhi 43: 463-467.
- Ji M, Jin A, Sun J, Cui X, Yang Y, et al. (2017) Clinicopathological implications of NQO1 overexpression in the prognosis of pancreatic adenocarcinoma. Oncol Lett 13: 2996-3002.
- Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB (2006) Genetic deletion of NAD(P)H:Quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, Ikappa Balpha kinase, c-Jun N-terminal kinase, Akt, p38 and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem 281: 19798-19808.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi